Abstract
Objective
There is considerable evidence that maternal stress is associated with behavioural disturbances in offspring. The objective of this study was to examine whether there is an association between the severity of maternal stress during pregnancy and the severity of symptoms of attention-deficit hyperactivity disorder (ADHD). A second objective was to examine whether there is an association between maternal stress and children's response to methylphenidate (MPH).
Methods
Using the Kinney Medical and Gynecological Questionnaire, we assessed 203 children with ADHD, aged between 6 and 12 years, regarding maternal stress during pregnancy. We assessed symptom severity with the Child Behavior Checklist (CBCL) and Conners' Global Index for Parents (CGI-P) and Teachers (CGI-T). Subjects were recruited from the ADHD clinic and the day-treatment program of the Child Psychiatry Department of the Douglas Hospital, Montréal, Quebec. The quality of their therapeutic response was assessed in a double-blind, placebo-controlled randomized 2-week crossover trial of MPH.
Results
The most severe symptoms as assessed by the CBCL were found in the moderate stressor group, (p < 0.002), whereas, according to the CGI-P (emotional liability), the most severe symptoms were found in the severe stressor group (p < 0.029). There was no statistically significant difference between degree of response to MPH and level of maternal stress.
Conclusion
Children with ADHD whose mothers were exposed to moderate and severe stress during pregnancy tend to develop more severe symptoms than children with ADHD whose mothers were not exposed to prenatal stress. It is therefore important to minimize stress in pregnant women.
Medical subject headings: attention deficit disorder with hyperactivity, methylphenidate, stress, pregnancy
Abstract
Objectif
Il y a beaucoup de données probantes qui indiquent qu'il y a un lien entre le stress de la mère et les troubles de comportement chez ses enfants. Cette étude visait à déterminer s'il y a un lien entre la gravité du stress vécu par la mère au cours de la grossesse et celle des symptômes du trouble d'hyperactivité avec déficit de l'attention (THADA). L'étude visait aussi à déterminer s'il y a un lien entre le stress vécu par la mère et la réponse des enfants au méthylphénidate (MPH).
Méthodes
On a utilisé le questionnaire médical et gynécologique de Kinney pour évaluer 203 enfants âgés de 6 à 12 ans, qui avaient le THADA, en ce qui concerne le stress vécu par la mère au cours de la grossesse. Nous avons évalué la sévérité des symptômes au moyen de la liste de comportement pour les enfants (CBCL) et de l'indice global de Conners pour les parents (CGI-P) et les enseignants (CGI-T). On a recruté les sujets à la clinique de traitement du THADA et au programme de traitement de jour du Département de pédopsychiatrie de l'Hôpital Douglas de Montréal (Québec). On a évalué la qualité de leur réponse thérapeutique au cours d'un essai croisé, randomisé, contrôlé par placebo et à double insu d'une durée de deux semaines portant sur le MPH.
Résultats
On a trouvé les symptômes les plus sévères tels qu'évalués par la liste CBCL chez les membres du groupe des sujets modérément stressés (p < 0,002), tandis que, selon l'indice CGI-P (prédisposition émotionnelle), on a constaté les symptômes les plus sévères chez les sujets gravement stressés (p < 0,029). Il n'y avait pas de différence statistiquement significative entre le degré de réponse au MPH et le niveau de stress vécu par la mère.
Conclusion
Les enfants qui ont le THADA et dont la mère a été exposée à un stress variant de modéré à sévère au cours de la grossesse ont tendance à avoir des symptômes plus sévères que les enfants qui ont le THADA et dont la mère n'a pas été vécu de stress prénatal. C'est pourquoi il importe de minimiser le stress chez les femmes enceintes.
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a highly prevalent behavioural disorder characterized by increased levels of hyperactivity, impulsivity and inattention. It is the most commonly diagnosed behavioural disorder in children, with a prevalence of 8%–12% of all school aged children.1 Although its prevalence decreases with increasing age, symptoms persist throughout adulthood in about 50% of affected patients.2 If left untreated, the symptoms of ADHD can significantly affect a child's academic and social functioning.3,4 Moreover, these symptoms have been shown to predispose patients to accidents due to their inattentive state.
Although the etiology of ADHD remains unclear, family,5,6 twin7,8 and adoption9,10 studies have shown that this disorder has a highly genetic component11,12: up to 75%–80% of ADHD symptoms are due to genetic factors,13 and first-degree relatives of affected individuals have been shown to have a 2- to 8-fold increased risk for developing ADHD.14 The remaining causative factors are environmental and include various pre-, peri-and postnatal influences.15,16
Considerable evidence shows that maternal stress during pregnancy has profound effects on offspring. Wadhwa17 wrote an interesting review article highlighting how, in humans, prenatal stress has an effect in terms of increased spontaneous abortions and fetal malformations and decreased infant birth weight and length of gestation. Animal studies have shown that rats that experienced prenatal stress exhibit excessive neuroendocrine and behavioural responses to stress.18,19 Moreover, it has been shown that prenatal stress leads to shorter attention spans and delays in the neuromotor development of nonhuman primates.20 The mechanism by which maternal stress causes developmental problems in offspring is still unclear, but several hypotheses exist. It has been shown that activation of the sympathetic nervous system, which occurs during stress, causes an increase in uterine artery resistance that subsequently reduces blood flow to the fetus.21 This decrease in blood flow may impair the development of organs, including the brain. Moreover, it has been shown that, although 50%–90% of the cortisol released in the mother's blood in response to maternal stress is deactivated to cortisone by the enzyme 11β-hydroxysteroid-dehydrogenase, a certain amount of cortisol escapes this deactivation and reaches the fetus.22 By this mechanism, increased fetal cortisol can alter the developing nervous system and deregulate the hypothalamo-pituitary-adrenal (HPA) axis in the fetus, causing a deregulated HPA response to stress in the child postnatally.
It is hypothesized that ADHD symptomatology is related to the reduced amount of catecholamine in the synaptic cleft in particular brain regions, resulting in a decreased inhibition of frontal cortical activity on subcortical structures. The first-line drugs used in the treatment of ADHD are stimulants such as methylphenidate (MPH) and d-amphetamine (d-AMP).23 These drugs are thought to increase the neurotransmission of dopamine and norepinephrine by promoting the presynaptic release of catecholamines and blocking the reuptake of catecholamines into the presynaptic nerve endings. All these actions result in a greater amount of catecholamines, especially dopamine, in brain regions such as the cortex and basal ganglia.24 Unfortunately, only 70% of treated patients respond to MPH, and although other medications have also been used to treat the disorder, several side effects are associated with them.25
The goal of our research was to test whether there is an association between the severity of maternal stress during pregnancy and the degree of ADHD symptomatology and also whether the stage of pregnancy during which stress is experienced affects the severity of symptoms. Moreover, we examined the link between maternal stress and the children's response to treatment with MPH. We hypothesized that the severity of symptoms and their response to MPH would be moderated by the degree of maternal stress during pregnancy.
Methods
We sequentially recruited a total of 203 children (32 girls and 171 boys, aged between 6 and 12 y) from the Disruptive Behaviour Disorders Program (DBDP) and the children's outpatient clinics of the Douglas Mental Health University Institute, a psychiatric teaching hospital in Montréal, Quebec. The children were referred to the hospital's specialized care facilities by school principals, social workers and pediatricians. Ninety-five percent of eligible subjects agreed to participate in the trial.
The children were diagnosed with ADHD according to the DSM-IV-R.26 The diagnosis was based on a clinical evaluation with the family, observation of the child and a clinical interview of the parents that used the Diagnostic Interview Schedule for Children Version IV (DISC-IV),27 school reports and teacher interviews. Parents also filled out the Child Behavior Checklist (CBCL)28 and the Conners' Global Index for Parents (CGI-P).29 Teachers also completed the Conners' Global Index for Teachers (CGI-T).30 Exclusion criteria included a history of mental retardation characterized by an IQ < 70 as assessed by the Wechsler Intelligence Scale for Children-III (WISC-III).31 We also excluded children with a history of Tourette syndrome, pervasive developmental disorder, psychosis or any medical condition interfering with their capacity to participate in the program.
The Kinney Medical and Gynecological Questionnaire32 was the tool used to interview the mothers to assess pre-, peri-and postnatal complications, including stressful life events. The McNeil-Sjöstrom scale33 was used for scoring the questionnaire. Mothers were asked to describe any emotionally charged or psychologically stressful events that occurred during their pregnancy with the child in question. We then analyzed this information, and the mother's stress level was scored from 1 to 5 on the DSM-III and DSM-III-R axis IV scales, according to the highest level of stress experienced during the pregnancy. A score of 1 means that the mother had no stress factors during the pregnancy, and a score of 5 indicates that the mother suffered very serious psychological stress during the pregnancy. Subjects who scored 1 or 2 were placed in the “none” stressor group (these mothers had perhaps moved into a new home, had minor financial worries or had an argument with a friend during their pregnancy). Subjects who scored 3 were placed in the “moderate” stressor group (they had such stressors as important financial troubles or marital problems). Subjects who scored 4 or 5 were categorized into the “severe” stressor group (severe stressors include such things as repeated physical or sexual abuse, imprisonment of a spouse or the death of a very close relative during the pregnancy). The parental reports were complemented with obstetrical records from hospital files.
The children had their response to MPH assessed by participating in a double-blind, placebo-controlled, 2-week crossover randomized trial. The study was approved by the Research and Ethics Board of the Douglas Mental Health University Institute. Parents signed informed consent and all the children agreed to participate in the trial. Details of the evaluation of therapeutic response to MPH were published earlier.34 Briefly, baseline evaluations were completed after at least a 1-week washout period before the clinical trial. After baseline assessments, children randomly received either placebo or 0.5 mg/kg of MPH adjusted to body weight and divided in 2 equal dosages (morning and noon) during a 1-week period; they were crossed over during the second week. Although MPH takes effect shortly after administration, the children were kept on medication for 7 consecutive days to account for individually or environmentally caused day-to-day variability in behaviour. The pharmacist prepared both drug and placebo in colored gelatine capsules and was not involved in the clinical evaluations. Adherence to medication was evaluated by a weekly pill count. Evaluations used to assess the children's response to medication included the CGI-P, CGI-T and laboratory measures including the Restricted Academic Situation Scale35 and the Continuous Performance Task.36 These assessments, when combined, allowed the evaluation to include improvement of symptoms at home, at school and in the laboratory. After the 2-week trial was over, the research team met, discussed the cases and used the test scores to help determine the consensus clinical response regarding improvement in the children's symptoms during the active week in comparison with the placebo week. The children were grouped into 2 categories, poor responders (showing mild or no improvement) and good responders (showing improvement that was moderate to significant).
Statistical analysis
We used χ2 statistics and single-measure analysis of variance (ANOVA) to compare demographic characteristics. We used ANOVA to compare illness severity in patients exposed to different levels of maternal stress during pregnancy, with the independent factor being maternal stress during pregnancy (3 levels) and the dependent outcome measures being the CBCL scores and Conners' Global Index Parent and Teacher scores. Any significant result was followed by post hoc comparisons using the Tukey honestly significant difference (HSD) method to determine the strength of the contrast between the 3 levels of maternal stress. These analyses were conducted for the global level of stress experienced by the mother during the entire pregnancy; subsequently, analysis was restricted to the level experienced in each trimester to determine whether stress is more operant during specific phases of intrauterine development. Finally, we tested the effect of maternal stress on therapeutic response to MPH by contrasting the proportions of responders and nonresponders in the 3 groups of patients stratified according to maternal stress during pregnancy according to χ2 statistics.
Results
The demographic variables for children grouped in the none, moderate and severe maternal stressor groups are presented in Table 1. The groups are fairly consistent and there are no statistically significant differences between them other than that the no-stressor group has a slightly higher income and the severe-stressor group has a higher ratio of girls to boys. Also, maternal age at the child's birth was slightly younger in the high-stressor group. In every group, there is a much larger number of boys than girls, which is expected, considering the significantly higher prevalence of ADHD in boys.
Table 1
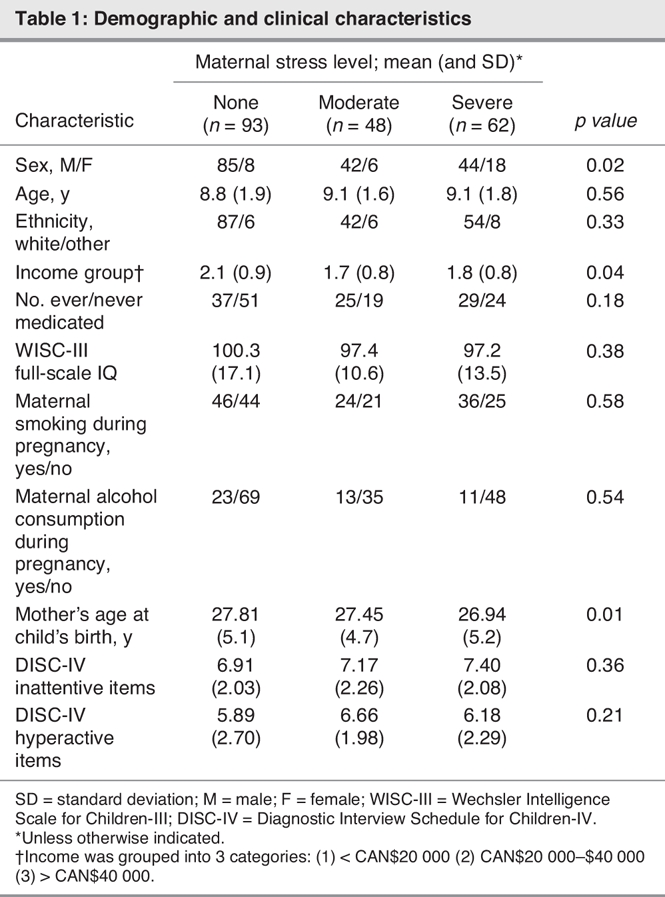
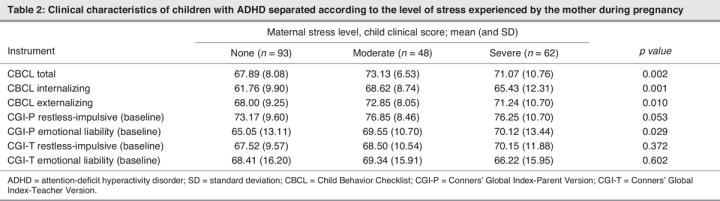
Table 2 shows the different clinical characteristics of the children in the 3 maternal stressor groups. When the 3 groups were compared, ANOVA revealed a statistically significant difference in the CBCL Total, Internalization and Externalization baseline scores as well as in the CGI-Parents Emotional Liability baseline scores (Table 2). Post hoc tests using the Tukey HSD method showed that the differences in CBCL Total, Internalization and Externalization scores are due to higher scores in the moderate-stressor group in comparison with the no-stressor group. However, differences in the CGI-Parent Emotional Liability baseline scores were due to the different scores in the no-stressor and severe-stressor groups. The remaining markers of symptom severity did not show any significant difference across the groups.
Table 2
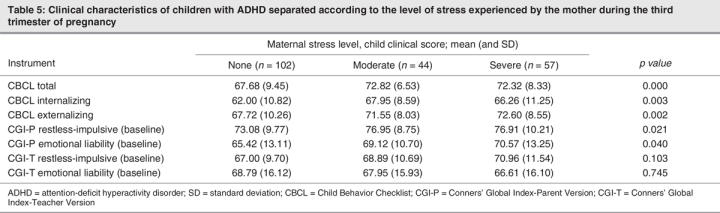
The separate analyses done for trimesters 1 and 2 showed similar results (Table 3, Table 4), with a significant difference in CBCL Total, Internalization and Externalization scores between the moderate-stressor and no-stressor groups. In trimester 3 (Table 5), post hoc analysis showed a significant difference between the no-stressor group and the moderate-stressor and severe-stressor groups.
Table 3
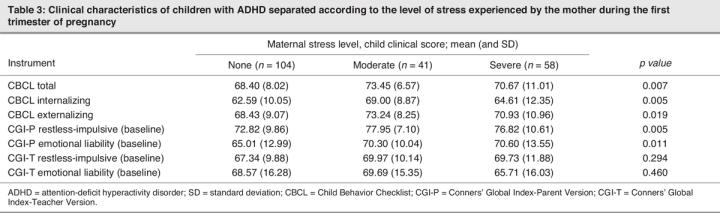
Table 4
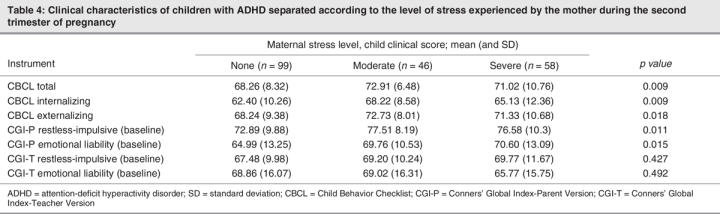
Table 5
Of all 203 patients, 137 were classified as good responders to MPH (66 in the no-stressor group, 32 in the moderate-stressor group and 43 in the severe-stressor group). Sixty-six of the children were poor responders (32 in the no-stressor group, 15 in the moderate-stressor group and 19 in the severe-stressor group). Chi-square analysis did not show a significant statistical difference between the response status of the 3 groups (χ2 = 0.206, p = 0.92).
Discussion
Although many studies have found a correlation between maternal stress and the subsequent development of ADHD symptoms in children,17 this study is unique in that it examines whether the severity of stress modulates symptom severity in the child, whether the trimester during which the mother experienced stress affects the severity of ADHD and whether stress affects the level to which the child responds to MPH.
The findings of this study show that there is indeed a relation between the severity of stress exposure during the pregnancy and ADHD symptoms in the child; this can be observed in the CBCL Total, Externalizing and Internalizing scores in Tables 2 to 5. Children of mothers who experienced moderate or severe stress have higher CBCL scores than their counterparts in the no-stressor group, confirming that prenatal stress does cause more severe ADHD symptomatology. It is also notable that these children experienced not only an increase in externalizing disorder (disruptive behaviours) but also internalizing disorders, including affective problems and anxiety. In the literature, it has been demonstrated that offspring of prenatally stressed animals develop impaired learning, cognitive function and affect (increased emotionality, fearfulness and depression).17
Conversely, we found that in general mothers who experienced severe stress during their pregnancy had children with lower CBCL scores than those who experienced moderate stress (see Table 2). However, when the trimesters are considered separately (Table 3, Table 4, Table 5), it is notable that the CBCL scores of the moderate-stressor group are higher than those of the severe-stressor group in the first trimester (Table 3), but as we progress to the second (Table 4) and third (Table 5) trimesters, the differences between CBCL scores become progressively less significant between these 2 groups.
A possible explanation for the higher CBCL scores in children whose mothers experienced moderate as opposed to severe stress is that mothers who experience more significant stress are the ones who most often seek help37–40 and, consequently, are better able to deal with their stress. Also, the help they receive while pregnant may continue after the child is born. It has been shown that even postnatal handling and care of the child can buffer the effects of increased maternal stress during pregnancy.41,42 For example, Lemaire and colleagues43 showed that prenatal stress reduced hippocampal cell proliferation in rats throughout life but that this could be counteracted by neonatal handling. Further, Weaver and colleagues44 showed that maternal behaviour produces stable alterations of DNA methylation and chromatin structure in rats, providing a mechanism for the long-term effects of maternal care on gene expression in the offspring. Therefore, it appears that the negative effects of prenatal stress on the child can be at least partly reversed with social help for the mother while she is pregnant or for the child during early stages of life.
When we compare the effects of stressors in each trimester on symptom severity, it becomes apparent that increased stress during the third trimester correlates with higher CBCL scores. As previously mentioned, not only does prenatal maternal stress affect the child, the early postnatal environment is also important in his or her development. We postulate that when a woman is in a stressful situation during the last trimester of her pregnancy, she may be in the same situation after her child is born and that this can affect the mother–child relationship. It has in fact been shown that early postnatal stress predisposes a child to developmental delays and behavioural problems.45,46
Another speculative explanation for why the children of mothers who suffer severe stress in the first trimester develop less severe ADHD symptoms than the children of those who suffer severe stress in the third trimester may be that more vulnerable fetuses are aborted in the first trimester and only the more resilient fetuses survive. Numerous studies have demonstrated the association between experiencing negative life events after conception and spontaneous abortion.17
In this study, no link was found between the level of maternal stress and the child's responder status to medication, possibly because the child's response to medication is not strongly related to early environmental factors or possibly because it is related to genetic and not environmental factors.47
Limitations
A few limitations should be considered when interpreting these research results. First, even though we had access to objective obstetrical records, most of the stressor data for this study was obtained through the Kinney Medical and Gynecological Questionnaire, which counts on the mother's memory to collect data regarding the pregnancy and therefore puts us at risk of encountering recall bias. Second, at 41%, 15% and 9%, respectively, the proportion of girls was much higher in the severe-stressor group than in the moderate-stressor or no-stressor groups. However, when we redid the calculations with only boys we got similar results, and therefore, sex did not affect symptom severity as related to stress. Third, as previously mentioned, we only examined the child's prenatal environment. A fourth possible limitation is that in this study we only looked at the mother's smoking and alcohol intake during pregnancy as possible confounding factors, given that in the literature these 2 factors are frequently noted. In our sample, there was no difference in the distribution of smoking and alcohol intake in the 3 stressor groups. However, other prenatal and perinatal factors that we did not explore may exist, and these may influence the development of certain symptoms in children. Note also that the association between increased stress and increased level of ADHD symptomatology may not be a causal relation. ADHD is a very heritable disorder. It may be that mothers who experience greater prenatal stress have a more significant history of ADHD themselves and confer a greater genetic risk for ADHD to their offspring. In our sample, mothers who experienced stressors were slightly younger than those who did not experience any stress during pregnancy. It would be interesting to further explore whether this younger age was related to an increased risk that these mothers possibly suffered from ADHD themselves.
Finally, it is important to note that this is a retrospective study focusing on children with ADHD. It would be interesting to follow a prospective cohort from conception to birth to latency age to examine how stress effects the development of various psychopathologies and also how maternal stress may influence the expression of selective symptoms in children who may be genetically predisposed to certain psychopathologies.
Clinical implications
To our knowledge, no studies have so far analyzed the correlation between the severity of maternal stress, the trimester during which the stress was experienced and the level of ADHD symptoms. Because the prenatal stage of a child's life seems to be very important in terms of his or her development, it is important to know the factors affecting the fetus during its growth. The results of this research have shown that it is extremely important to take care of and support pregnant women because this may decrease their child's chance of developing ADHD and other symptomatology.
Acknowledgments
The authors thank The Canadian Institutes for Health Research (CIHR), Fonds de la recherche en santé du Québec (FRSQ) and The Roaster's Foundation for their financial support. The authors thank Johanne Bellingham, Sandra Robinson and Nicole Pawliuk for their help in this study.
Footnotes
Contributors: Drs. Grizenko and Joober designed the study. Drs. Grizenko and Joober and Ms. Polotskaia and Ms. Ter-Stepanian acquired the data, which Dr. Grizenko, Ms. Shayan and Ms. Polotskaia analyzed. Dr. Grizenko and Ms. Shayan wrote the article, and Ms. Polotskaia, Ms. Ter-Stepanian and Dr. Joober revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Natalie Grizenko, Bond Pavilion, Douglas Mental Health University Institute, 6875 LaSalle Blvd., Montréal QC H4H 1R3; grinat@douglas.mcgill.ca
References
- 1.Faraone SV, Sergeant J, Gillberg C, et al. The worldwide prevalence of ADHD: Is it an American condition? World Psychiatry 2003;2:104-13. [PMC free article] [PubMed]
- 2.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet 2005;366:237-48. [DOI] [PubMed]
- 3.Merrell C, Tymms PB. Inattention, hyperactivity and impulsiveness: their impact on academic achievement and progress. Br J Educ Psychol 2001;71:43-56. [DOI] [PubMed]
- 4.Lee SS, Hinshaw SP. Severity of adolescent delinquency among boys with and without attention deficit hyperactivity disorder: predictions from early antisocial behavior and peer status. J Clin Child Adolesc Psychol 2004;33:705-16. [DOI] [PubMed]
- 5.Hechtman L. Families of children with attention deficit hyperactivity disorder: a review. Can J Psychiatry 1996;41:350-60. [DOI] [PubMed]
- 6.Samuel VJ, George P, Thornell A, et al. A pilot controlled family study of DSM-III-R and DSM-IV ADHD in African-American children. J Am Acad Child Adolesc Psychiatry 1999;38:34-9. [DOI] [PubMed]
- 7.Levy F, Hay DA, McStephen M, et al. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry 1997;36:737-44. [DOI] [PubMed]
- 8.Eaves LJ, Silberg JL, Meyer JM. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. J Child Psychol Psychiatry 1997;38:965-80. [DOI] [PubMed]
- 9.Cadoret RJ, Stewart MA. An adoption study of attention deficit/hyperactivity/aggression and their relationship to adult antisocial personality. Compr Psychiatry 1991;32:73-82. [DOI] [PubMed]
- 10.Sprich S, Biederman J, Crawford MH, et al. Adoptive and biological families of children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry 2000;39:1432-7. [DOI] [PubMed]
- 11.DiMaio S, Grizenko N, Joober R. Dopamine genes and attention-deficit hyperactivity disorder: a review. J Psychiatry Neurosci 2003;28: 27-38. [PMC free article] [PubMed]
- 12.Spencer TJ, Biederman J, Wilens TE, et al. Overview and neurobiology of attention-deficit/hyperactivity disorder. J Clin Psychiatry 2002;63:3-9. [PubMed]
- 13.Faraone SV, Perlis RH, Doyle AE, et al. Molecular Genetics of Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry 2005;57:1313-23. [DOI] [PubMed]
- 14.Biederman J, Faraone SV, Keenan K, et al. Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1990;29:526-33. [DOI] [PubMed]
- 15.Mick E, Biederman J, Faraone SV, et al. Case-control study of attention-deficit/hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry 2002;41:378-85. [DOI] [PubMed]
- 16.Ben Amor L, Grizenko N, Schwartz G, et al. Perinatal complications in children with attention-deficit hyperactivity disorder and their unaffected siblings. J Psychiatry Neurosci 2005;30:120-6. [PMC free article] [PubMed]
- 17.Wadhwa P. Prenatal stress and life-span development. In: Friedman HS, editor. Encyclopedia of mental health. San Diego: Academic Press; 1998. p. 265-280.
- 18.Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res 1992;574:131-7. [DOI] [PubMed]
- 19.Henry C, Kabbaj M, Simon H, et al. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol 1994;6:341-5. [DOI] [PubMed]
- 20.Clarke AS, Wittwer DJ, Abbott DH. Long-term effects of prenatal stress on HPA axis activity in juvenile Rhesus monkeys. Dev Psychobiol 1994;27:257-69. [DOI] [PubMed]
- 21.Teixeira JMA, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318:153-7. [DOI] [PMC free article] [PubMed]
- 22.Seckl JR. Glucocorticoids, feto-placental 11β-hydroxysteroid dehydrogenase type 2, and early life origins of adult disease. Steroids 1997;62:89-94. [DOI] [PubMed]
- 23.Pliszka SR, Crismon ML, Hughes CW, et al. The Texas Children's Medication Algorithm Project: Revision of the Algorithm for Pharmacotherapy of Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry 2006;45:642-57. [DOI] [PubMed]
- 24.Barkley RA. A Review of stimulant drug research with hyperactive children. J Child Psychol Psychiatry 1977;18:137-65. [DOI] [PubMed]
- 25.Spencer TJ, Biederman J, Wilens TE, et al. Novel treatments for attention-deficit/hyperactivity disorder in children. J Clin Psychiatry 2002;63:16-22. [PubMed]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. rev. Washington: The Association; 2000.
- 27.Shaffer D, Ficher P, Lucas CP, et al. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 2000;39:28-38. [DOI] [PubMed]
- 28.Achenbach TM. The Child Behavior Checklist/4-18. Burlington (VT): University of Vermont; 1991.
- 29.Conners CK. Conners' Global Index-Parents. North Noranda (NY): Multihealth Systems; 2003.
- 30.Conners CK, Sitarenios G, Parker JD, et al. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:279-91. [DOI] [PubMed]
- 31.Wechsler D. Wechsler Intelligence Scale for Children — Third Edition: manual. San Antonio (TX): Psychological Corporation; 1991.
- 32.McNeil TF, Cantor-Graae E, Sjöstrom K. Obstetric complications as antecedents of schizophrenia: empirical effects of using different obstetric complication scales. J Psychiatr Res 1994;28:519-30. [DOI] [PubMed]
- 33.McNeil TF, Sjöstrom K. McNeil-Sjöstrom Scale for Obstetric Complications. Malmo (Sweden): Department of Psychiatry, Lund University; 1995.
- 34.Joober R, Grizenko N, Sengupta S, et al. Dopamine transporter 3 — UTR VNTR genotype and ADHD: a pharmaco-behavioral genetic study with methylphenidate. Neuropsychopharmacology 2007;32:1370-6. [DOI] [PubMed]
- 35.Barkley RA. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: Guilford Press; 1990.
- 36.Conners CK. Conners Continuous Performance Test computer program. Toronto: Multihealth Systems; 1995.
- 37.Rickwood DJ, Braithwaite VA. Social-psychological factors affecting help-seeking for emotional problems. Soc Sci Med 1994; 39:563-72. [DOI] [PubMed]
- 38.Garland AF, Zigler EF. Psychological correlates of help-seeking attitudes among children and adolescents. Am J Orthopsychiatry 1994;64:586-93. [DOI] [PubMed]
- 39.Deane FP, Chamberlain K. Treatment fearfulness and distress as predictors of professional psychological help-seeking. British Journal of Guidance & Counselling 1994;22:207-17.
- 40.Van Hemert AM, Bakker CH, Vandenbrouke JP, et al. Psychological distress as a long term predictor of medical utilisation. Int J Psychiatry Med 1993;23:295-305. [DOI] [PubMed]
- 41.Webster RG. The buffering effects of social support on psychological states and obstetrical outcomes in pregnancy. The Sciences & Engineering 1996;57(3-B):2170.
- 42.Da Costa DM. A prospective study on the influence of stress, social support and coping on birth outcomes and depressive symptomatology during pregnancy and the postpartum. The Sciences & Engineering 2000;60(8-B):4213.
- 43.Lemaire V, Lemarque S, Lemoal M, et al. Post natal stimulation of the pups counteracts prenatal stress induced deficits in hippocampal neurogenesis. Biol Psychiatry 2006;59:786-92. [DOI] [PubMed]
- 44.Weaver ICG, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci 2004;7:847-54. [DOI] [PubMed]
- 45.Meijer A. Child psychiatric sequelae of maternal war stress. Acta Psychiatr Scand 1985;72:505-11. [DOI] [PubMed]
- 46.Huttunen MO, Niskanen P. Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry 1978;35:429-32. [DOI] [PubMed]
- 47.Grizenko N, Kovacina B, Ben Amor L, et al. Relationship between response to methylphenidate treatment in children with ADHD and psychopathology in their family. J Am Acad Child Adolesc Psychiatry 2006;45:47-53. [DOI] [PubMed]


