Abstract
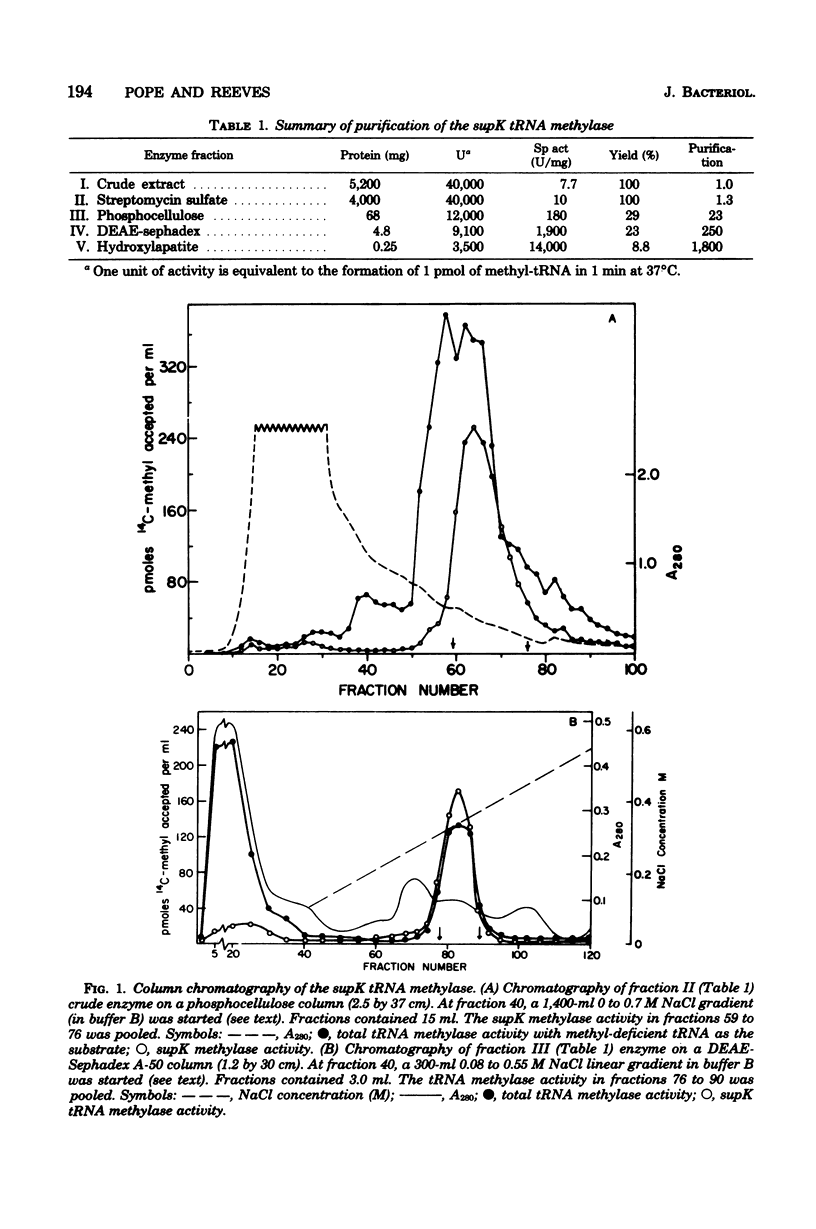
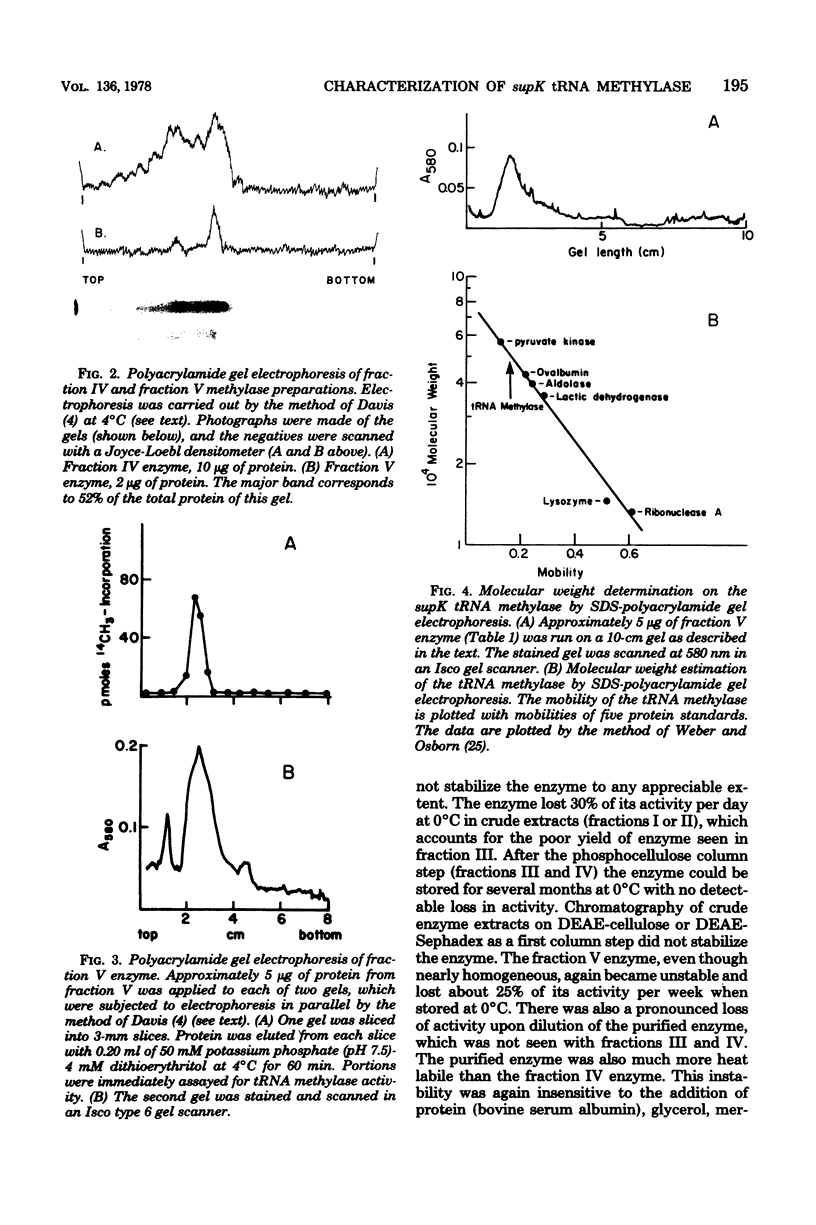
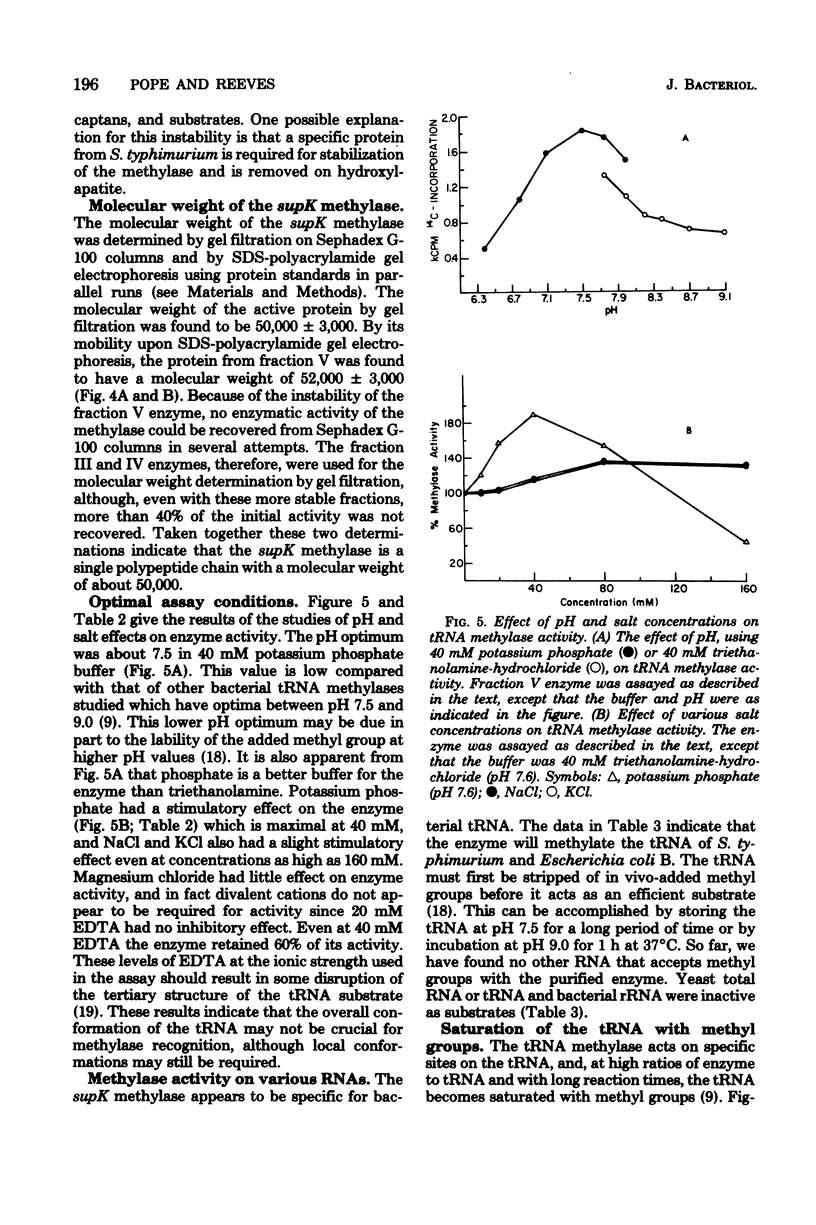
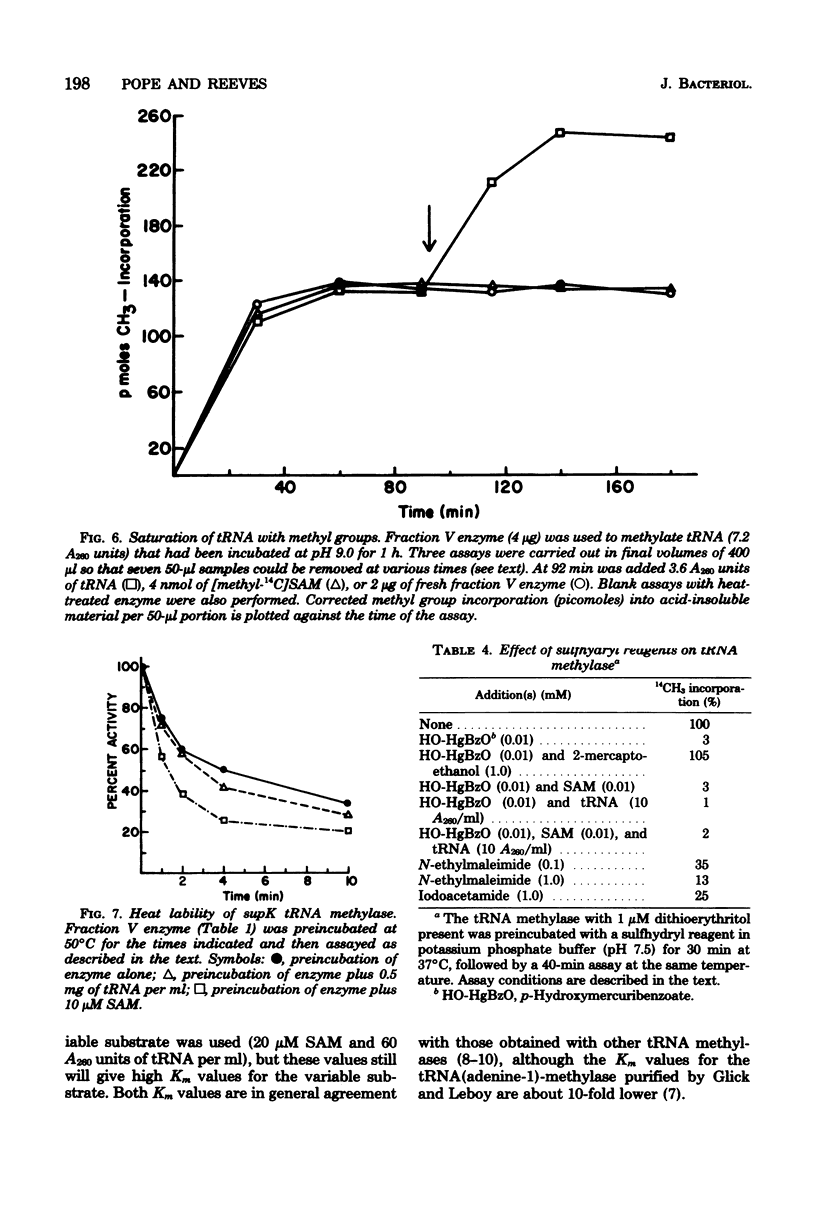
A tRNA methylase, in which supK strains of Salmonella typhimurium are deficient, was purified from strain LT2 and characterized. Column chromatography of protein extracts from wild-type cells on phosphocellulose, diethylaminoethyl-Sephadex A-50, and hydroxlapatite resulted in an enzyme that was estimated to be about 50% pure. tRNA from S. typhimurium which had been incubated at pH 9.0 served as a substrate for this methylase. The enzyme has a molecular weight of about 50,000 as estimated by gel chromatography and by electrophoresis on sodium dodecyl sulfate-polyacrylamide gels. The optimal assay conditions, as well as the kinetics and stability of the enzyme, were studied. As with other tRNA-methylating enzymes, S-adenosylhomocysteine is a potent inhibitor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschhoff H. J., Elten H., Arnold H. H., Mahal G., Kersten W., Kersten H. 7-Methylguanine specific tRNA-methyltransferase from Escherichia coli. Nucleic Acids Res. 1976 Nov;3(11):3109–3122. doi: 10.1093/nar/3.11.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Ryce S. UGA and non-triplet suppressor reading of the genetic code. Nature. 1974 Jun 7;249(457):527–530. doi: 10.1038/249527a0. [DOI] [PubMed] [Google Scholar]
- Calendar R., Berg P. Purification and physical characterization of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1681–1690. doi: 10.1021/bi00869a033. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Lorenzo F., Ames B. N. Histidine regulation in Salmonella typhimurium. VII. Purification and general properties of the histidyl transfer ribonucleic acid synthetase. J Biol Chem. 1970 Apr 10;245(7):1710–1716. [PubMed] [Google Scholar]
- Fangman W. L., Nass G., Neidhardt F. C. Immunological and chemical studies of phenylalanyl sRNA synthetase from Escherichia coli. J Mol Biol. 1965 Aug;13(1):202–219. doi: 10.1016/s0022-2836(65)80090-0. [DOI] [PubMed] [Google Scholar]
- Glick J. M., Leboy P. S. Purification and properties of tRNA(adenine-1)-methyltransferase from rat liver. J Biol Chem. 1977 Jul 25;252(14):4790–4795. [PubMed] [Google Scholar]
- Glick J. M., Ross S., Leboy P. S. S-adenosylhomocysteine inhibition of three purified tRNA methyltransferases from rat liver. Nucleic Acids Res. 1975 Oct;2(10):1639–1651. doi: 10.1093/nar/2.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURWITZ J., GOLD M., ANDERS M. THE ENZYMATIC METHYLATION OF RIBONUCLEIC ACID AND DEOXYRIBONUCLEIC ACID. IV. THE PROPERTIES OF THE SOLUBLE RIBONUCLEIC ACID-METHYLATING ENZYMES. J Biol Chem. 1964 Oct;239:3474–3482. [PubMed] [Google Scholar]
- Izzo P., Gantt R. Partial purification and characterization of an N2-guanine RNA methyltransferase from chicken embryos. Biochemistry. 1977 Aug 9;16(16):3576–3581. doi: 10.1021/bi00635a012. [DOI] [PubMed] [Google Scholar]
- Joseph D. R., Muench K. H. Tryptophanyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification of the enzyme and of tryptrophan transfer ribonucleic acid. J Biol Chem. 1971 Dec 25;246(24):7602–7609. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapointe J., Söll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. 3. Influence of the 46K protein on the affinity of the 56K glutamyl transfer ribonucleic acid synthetase for its substrates. J Biol Chem. 1972 Aug 25;247(16):4982–4985. [PubMed] [Google Scholar]
- Murao K., Saneyoshi M., Harada F., Nishimura S. Uridin-5-oxy acetic acid: a new minor constituent from E. coli valine transfer RNA I. Biochem Biophys Res Commun. 1970 Feb 20;38(4):657–662. doi: 10.1016/0006-291x(70)90631-5. [DOI] [PubMed] [Google Scholar]
- Pope W. T., Brown A., Reeves R. H. The identification of the tRNA substrates for the supK tRNA methylase. Nucleic Acids Res. 1978 Mar;5(3):1041–1057. doi: 10.1093/nar/5.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. H., Cantor C. R., Chambers R. W. Effect of magnesium ions on the conformation of two highly purified yeast alanine transfer ribonucleic acids. Biochemistry. 1970 Sep 29;9(20):3993–4002. doi: 10.1021/bi00822a019. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Roth J. R. A recessive UGA suppressor. J Mol Biol. 1971 Mar 28;56(3):523–533. doi: 10.1016/0022-2836(71)90399-8. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Roth J. R. Transfer ribonucleic acid methylase deficiency found in UGA supressor strains. J Bacteriol. 1975 Oct;124(1):332–340. doi: 10.1128/jb.124.1.332-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle D. L., Roth J. R. Frameshift suppressors. 3. Effects of suppressor mutations on transfer RNA. J Mol Biol. 1972 May 28;66(3):495–506. doi: 10.1016/0022-2836(72)90429-9. [DOI] [PubMed] [Google Scholar]
- Taya Y., Nishimura S. Biosynthesis of 5-methylaminomethyl-2-thiouridylate. I. Isolation of a new tRNA-methylase specific for 5-methylaminomethyl-2-thiouridylate. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1062–1068. doi: 10.1016/0006-291x(73)90035-1. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]




