Abstract
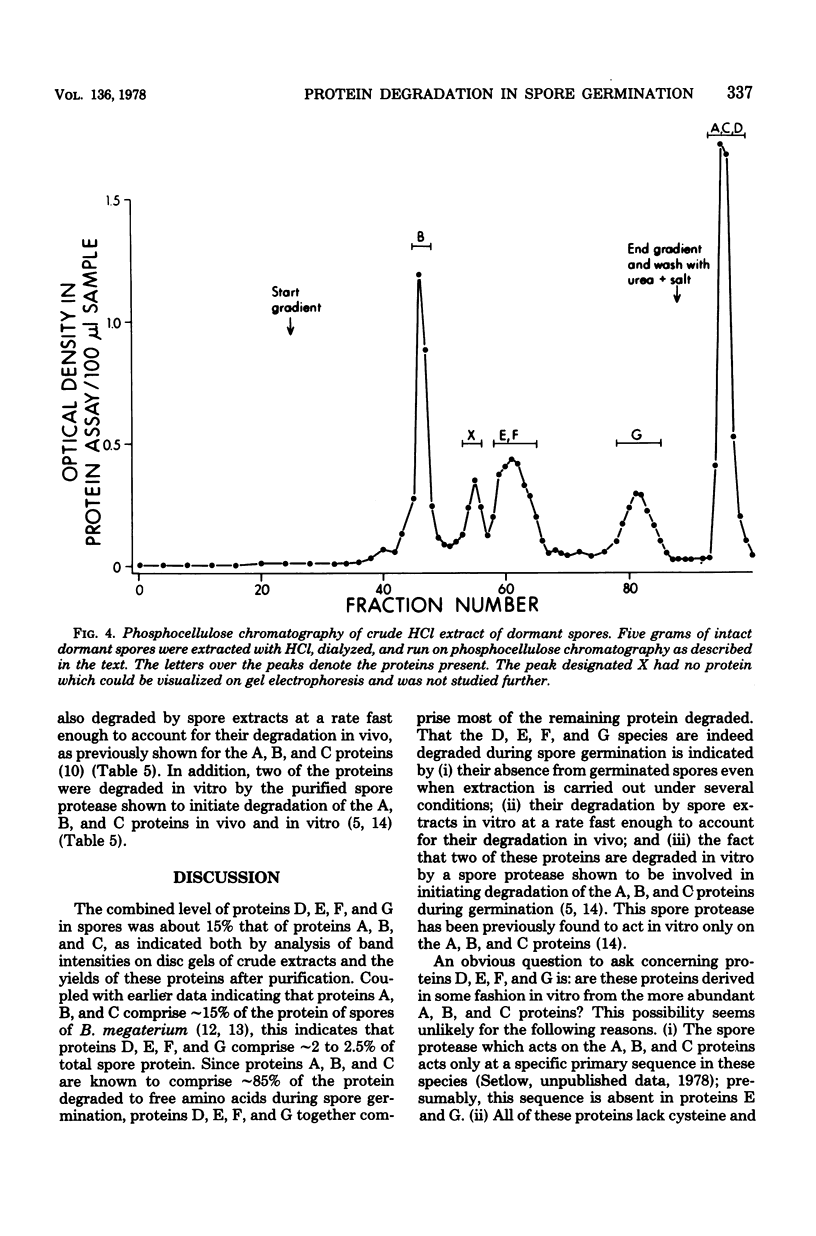
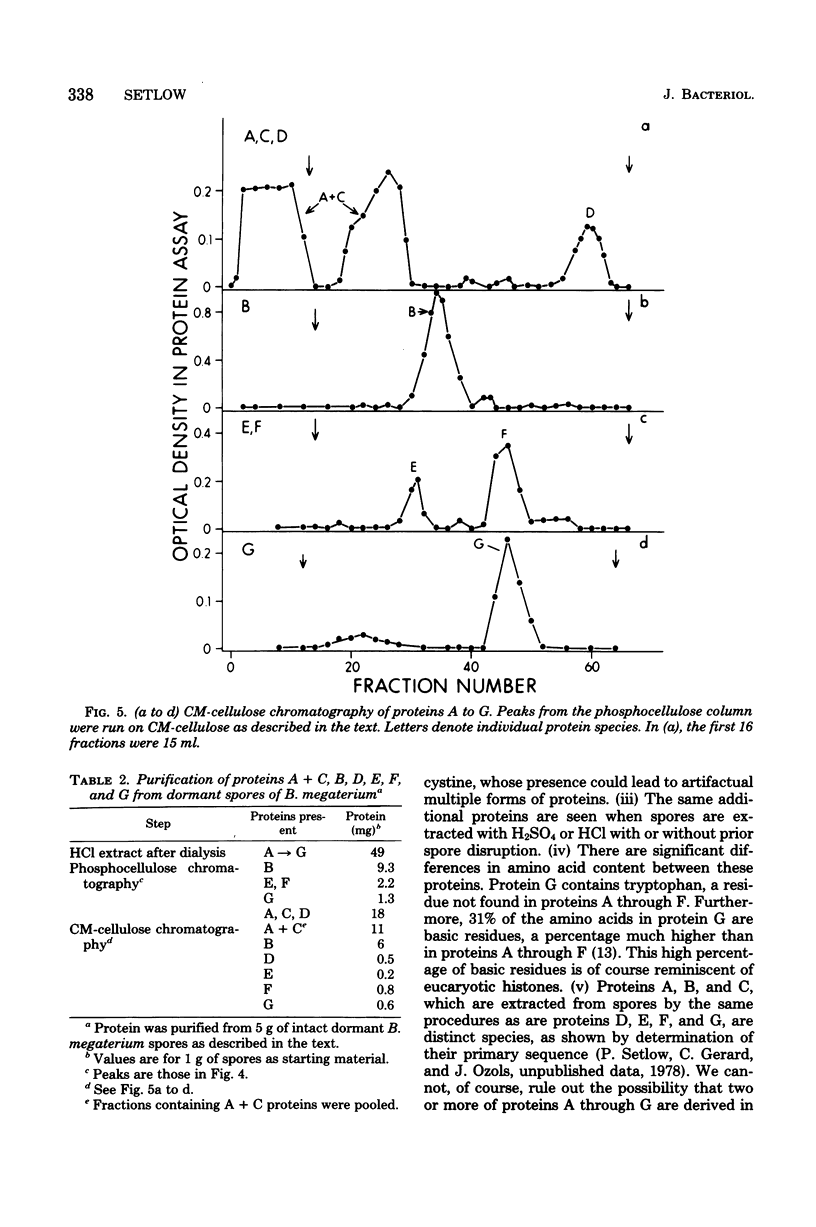
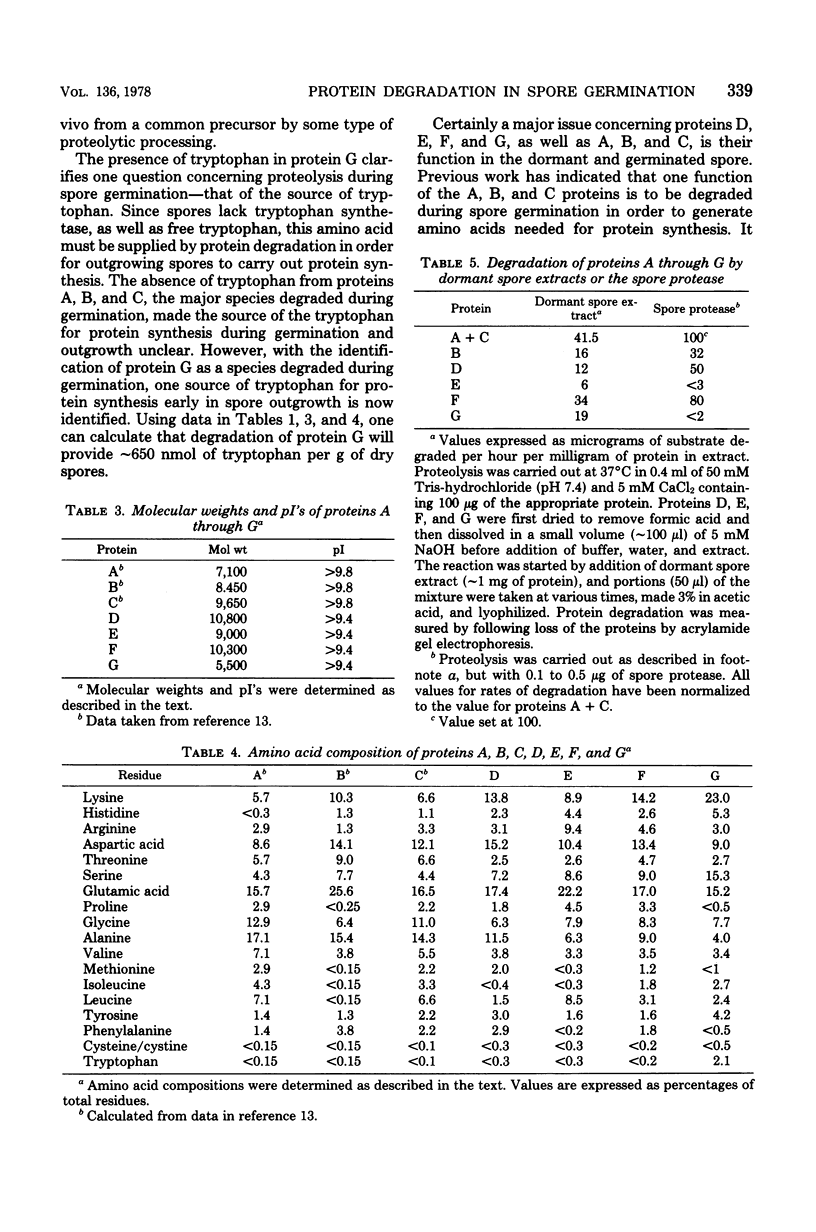
Dormant spores Bacillus megaterium contained a group of low-molecular-weight (5,000 to 11,000) basic (pI greater than 9.4) proteins (termed D, E, F, and G proteins) which could be extracted from disrupted spores with strong acids. These proteins were distinct from the previously described A, B, and C proteins which are degraded during spore germination. However, the D, E, F, and G proteins were also rapidly degraded during spore germination, accounting for 10 to 15% of the protein degraded. Proteins similar to the D, E, F, and G species were also present in spores of other bacterial species. In B. megaterium, the D, E, F, and G proteins were low or absent (less than 15% of the spore level) in vegetative and young sporulating cells and appeared only late in sporulation. The D, E, F, and G proteins were purified to homogeneity, and all contained a high percentage of hydrophilic amino acids; one protein (G) contained 31% basic amino acids and also contained tryptophan. All four proteins were rapidly degraded in vitro by dormant spore extracts. Two proteins (D and F) were degraded in vitro by the previously described spore protease which initiates degradation of the A, B, and C proteins in vivo; the spore enzyme (s) degrading proteins E and G have not been identified.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- Postemsky C. J., Dignam S. S., Setlow P. Isolation and characterization of Bacillus megaterium mutants containing decreased levels of spore protease. J Bacteriol. 1978 Sep;135(3):841–850. doi: 10.1128/jb.135.3.841-850.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- ROBINOW C. F. Spore structure as revealed by thin sections. J Bacteriol. 1953 Sep;66(3):300–311. doi: 10.1128/jb.66.3.300-311.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman Y., Fields M. L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1968 Jan;22(1):168–168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1977 Feb;129(2):857–865. doi: 10.1128/jb.129.2.857-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Identification and localization of the major proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8159–8167. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]
- Setlow P. Protease and peptidase activities in growing and sporulating cells and dormant spores of Bacillus megaterium. J Bacteriol. 1975 May;122(2):642–649. doi: 10.1128/jb.122.2.642-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Protein metabolism during germination of Bacillus megaterium spores. II. Degradation of pre-existing and newly synthesized protein. J Biol Chem. 1975 Jan 25;250(2):631–637. [PubMed] [Google Scholar]
- Setlow P. Purification and properties of a specific proteolytic enzyme present in spores of Bacillus magaterium. J Biol Chem. 1976 Dec 25;251(24):7853–7862. [PubMed] [Google Scholar]
- Setlow P. Purification and properties of some unique low molecular weight basic proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8168–8173. [PubMed] [Google Scholar]
- Setlow P., Waites W. M. Identification of several unique, low-molecular-weight basic proteins in dormant spores of clastridium bifermentans and their degradation during spore germination. J Bacteriol. 1976 Aug;127(2):1015–1017. doi: 10.1128/jb.127.2.1015-1017.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]