Abstract
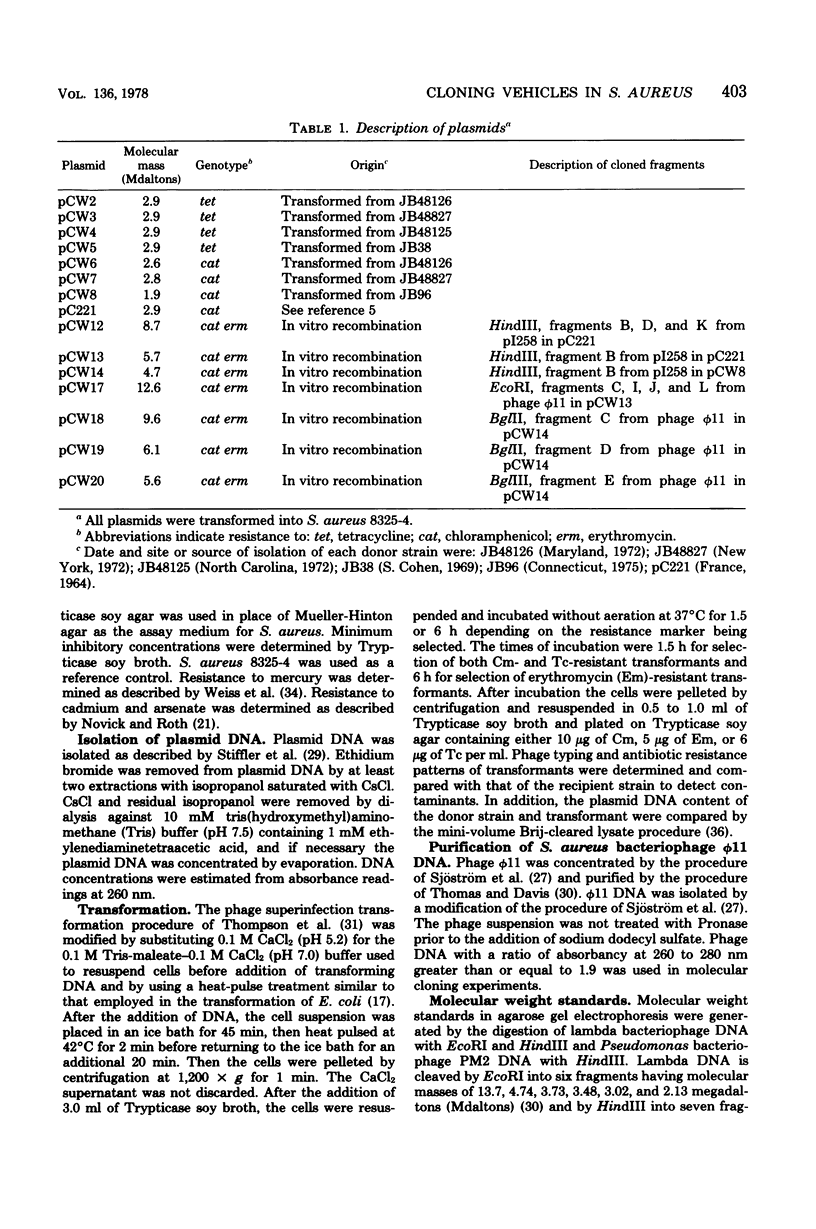
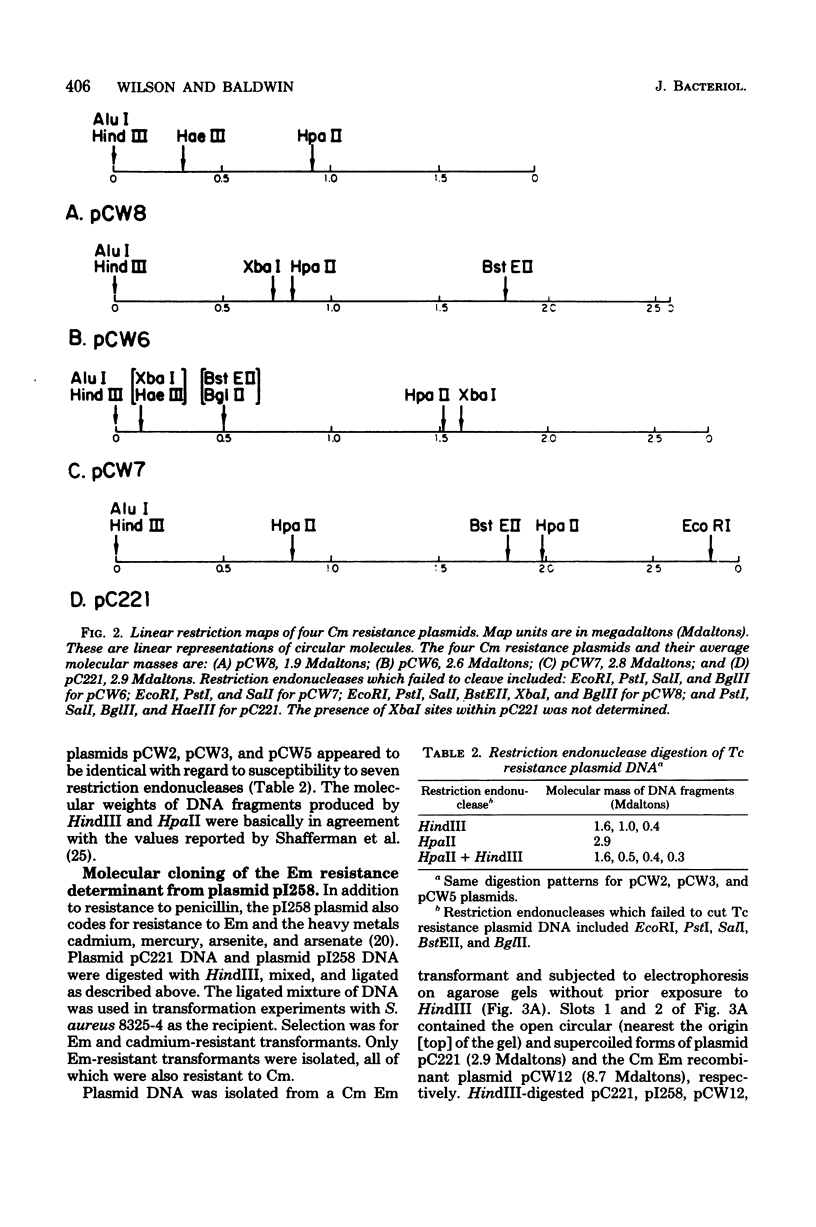
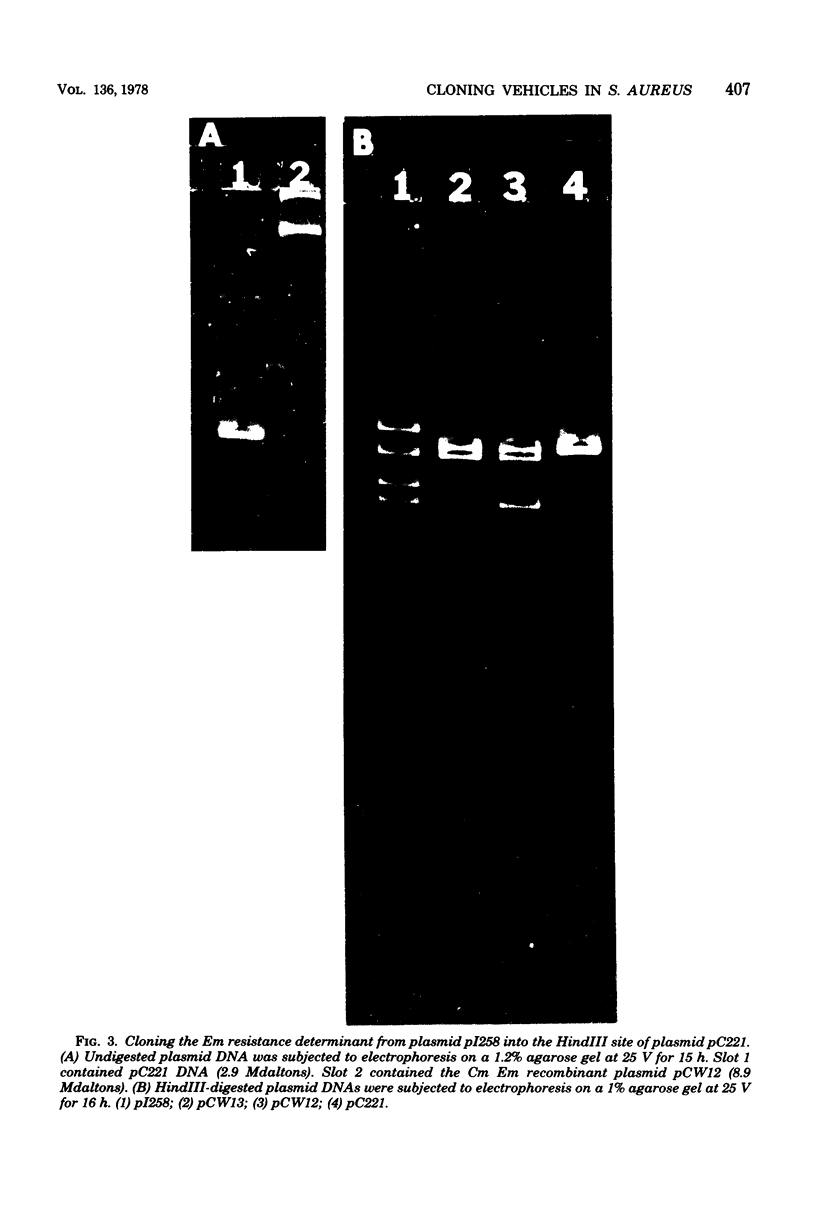
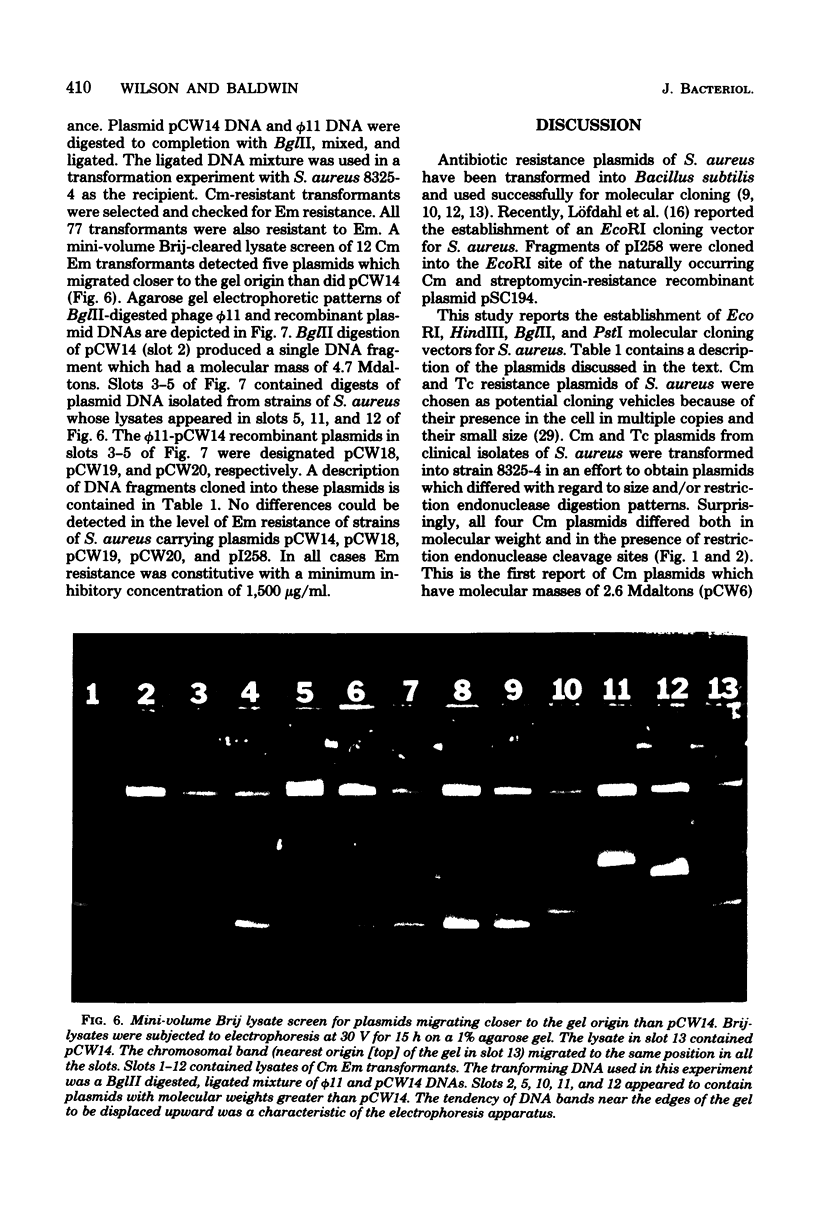
Four chloramphenicol resistance (Cm) and four tetracycline resistance (Tc) plasmids from Staphylococcus aureus were characterized by restriction endonuclease mapping. All four Tc plasmids had molecular masses of 2.9 megadaltons (Mdaltons) and indistinguishable responses to seven different restriction endonucleases. The four Cm plasmids (pCW6, pCW7, pCW8, and pC221) had molecular masses of 2.6, 2.8, 1.9, and 2.9 Mdaltons, respectively. The four Cm plasmids also differed both in the level of resistance to Cm and in susceptibility to retriction endonucleases. Single restriction endonuclease sites contained within each plasmid included the following: in pCW6 for HindIII, XbaI, HpaII, and BstEII; in pCW7 for HindIII, BstEII, BglII, HaeIII, and HpaII; in pCW8 for HindIII, HaeIII, and HpaII; in pC221 for HindIII, BstEII, and EcoRI. The molecular cloning capabilities of pCW8 and pC221 were determined. Cm and erythromycin resistance (Em) recombinant plasmids pCW12, PCW13, and pCW14 were constructed and used to transform S. aureus 8325-4. A 2.8-Mdalton HindIII fragment from plasmid pI258 was found to encode Em resistance and contain single sites for the retriction endonucleases BglII, PstI, HaeIII, and HpaII. The largest EcoRI fragment (8 Mdaltons) from pI258 contained the HindIII fragment encoding Em resistance intact. Cloning of DNA into the BglII site of pCW14 did not alter Em resistance. Cloning of DNA into the HindIII site of pCW8 and the HindIII and EcoRI sites of pC221 did not disrupt either plasmid replication of Cm resistance.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Berg P. E., Gayda R., Avni H., Zehnbauer B., Markovitz A. Cloning of Escherichia coli DNA that controls cell division and capsular polysaccharide synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):697–701. doi: 10.1073/pnas.73.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABBERT Y. A., BAUDENS J. G., GERBAUD G. R. VARIATIONS SOUS L'INFLUENCE DE L'ACRIFLAVINE ET TRANSDUCTION DE LA R'ESISTANCE A LA KANAMYCINE ET AU CHLORAMPH'ENICOL CHEZ LES STAPHYLOCOQUES. Ann Inst Pasteur (Paris) 1964 Nov;107:678–690. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Biochemical construction and selection of hybrid plasmids containing specific segments of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4361–4365. doi: 10.1073/pnas.72.11.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. DNA cloning in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1433–1436. doi: 10.1073/pnas.75.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Dubnau D. Construction and properties of chimeric plasmids in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1428–1432. doi: 10.1073/pnas.75.3.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov J. I., Kalinina N. A., Gening L. V., Rebentish B. A., Strongin A. Y., Bogush V. G., Debabov V. G. A suitable method for construction and cloning hybrid plasmids containing EcoRI-fragments of E. coli genome. Mol Gen Genet. 1977 Jan 18;150(2):211–219. doi: 10.1007/BF00695401. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. A vector for recombinant DNA in Staphylococcus aureus. Gene. 1978 Apr;3(2):161–172. doi: 10.1016/0378-1119(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. Characterization of small plasmids from Staphylococcus aureus. Gene. 1978 Apr;3(2):145–159. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Larson T. J., Dowhan W. Gene cloning for the isolation of enzymes of membrane lipid synthesis: phosphatidylserine synthase overproduction in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1412–1416. doi: 10.1073/pnas.74.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafferman A., Shalita Z., Hertman I. Cleavage maps of a tetracycline plasmid from Staphylococcus aureus. J Bacteriol. 1978 Apr;134(1):345–348. doi: 10.1128/jb.134.1.345-348.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Co-transduction of plasmids mediating resistance to tetracycline and chloramphenicol in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):934–934. doi: 10.1128/jb.120.2.934-944.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Thompson N. E., Pattee P. A. Transformation in Staphylococcus aureus: role of bacteriophage and incidence of competence among strains. J Bacteriol. 1977 Feb;129(2):778–788. doi: 10.1128/jb.129.2.778-788.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Alton N. K., Bassett C. L., Kushner S. R. Amplification in Escherichia coli of enzymes involved in genetic recombination: construction of hybrid ColE1 plasmids carrying the structural gene for exonuclease I. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3492–3496. doi: 10.1073/pnas.73.10.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Wilson C. R., Totten P. A., Baldwin J. N. Rapid procedure for the detection of plasmids in Staphylococcus epidermidis. Appl Environ Microbiol. 1978 Aug;36(2):368–374. doi: 10.1128/aem.36.2.368-374.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]