Subsidies are usually the subject of purely economic debates. They represent flow of resources from one economic sector to another and can be fundamental to competitive ability of industries in different nations. Subsidies are not restricted to economics, however, and are a pervasive factor in the ecological commerce between ecosystems. In this issue of PNAS, Menge et al. (1) show the influence of subsidies on the pace of ecological interactions in an intertidal community in New Zealand. Subsidies come in the form of nutrients, organic particles, and larvae wafting in from oceanic water masses. Their impact is to dramatically alter the abundances of key animal species and ignite rates of ecological processes such as competition and predation.
All ecosystems receive subsidies. Photons flood every leaf. Estuaries are fueled by nutrient flow from rivers. Amazonian forest soils are enriched by African desert dust that blows relentlessly across the Atlantic Ocean (2, 3). These additional inputs can greatly augment the supply of critical nutrients, such as phosphorus and nitrogen needed for plant growth, and, in so doing, they top up ecosystem fuel supplies. Subsidies like these represent a crucial link among ecosystems because the subsidies that flow into one ecosystem derive ultimately from another.
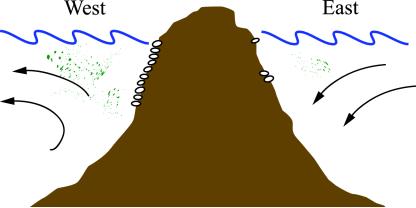
Menge et al. (1) asked whether marine intertidal communities receive different subsidies on opposite coasts of the South Island of New Zealand. The west coast of this large, mountain-backed island is washed by currents that generate an intermittent oceanographic condition called upwelling. Surface currents that flow along coasts and bend out to sea tend to pull deeper waters up behind them. These upwelling waters, with origins in the deep sea, are colder and richer in nutrients than the surface waters they replace. Along the west coast, frequent periods of upwelling generate plumes of nutrient-rich seawater near the western coast of New Zealand (Fig. 1). In contrast, because of current patterns and the rotation of the Earth, the east coast is primarily affected by downwelling, and eastern coasts lack the nutrient subsidies provided by upwelled water masses.
Fig. 1.
Upwelling brings nutrient-rich, deep sea water to the surface on the western coast of the South Island of New Zealand, whereas on the eastern coast, downwelling currents push surface waters toward the bottom. Benthic animals and planktonic particulates tend to be more abundant where upwelling occurs.
Upwelling has long been known to dramatically affect coastal conditions. For example, nearly half of the world's fisheries production currently derives from the <1% of the coasts where upwelling occurs (3). Delivery of marine larvae by coastal currents (4) helped spark strong interest in “supply-side” ecology, a view of ecosystems that emphasized the importance of inputs from outside an area on local ecological processes (5). What is significant about the current work is the penetration of the upwelling effect through a coastal ecosystem, from the basal consumers to the abundance of top carnivores, and the experimental tools deployed to document these patterns.
Menge et al. (1) show a strong correlation between the intermittent upwelling on western coasts and settlement of larvae of intertidal animals such as barnacles and mussels. These larvae feed off ocean plankton as they develop and may be much more likely to survive their juvenile phases where food supplies are high and development times are short. Settling in droves, the larvae metamorphose into rock-dwelling filter-feeders that pull upwelling-enriched planktonic food from the water. Thus subsidized, mussels and barnacles grow much more quickly and cover far more of the intertidal rock surface than in similar habitats on the nonupwelling east coast. These invertebrates are the link between the planktonic ecosystem of the oceans and the rock-bound ecosystems close to shore, so their thriving response to upwelling is expected.
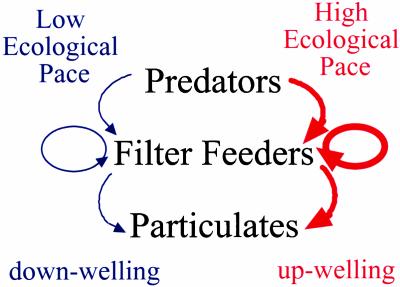
More surprising is that these subsidies at the level of the planktivores also extend to the next level of the food web. Where subsidies are high, coastal predators are also more abundant. Rates of predation are therefore much higher on the west coast than the nutrient-poor east, and overall the entire intertidal ecosystem seems to move at a faster pace on the west coast (Fig. 2).
Fig. 2.
Ecosystem processes run at different rates on opposite shores of New Zealand. Where upwelling prevails on western shores, predators are abundant and predation is strong (top arrow), settlement of filter-feeding invertebrates and their growth rate is high (middle arrow), and feeding on planktonic particulates is high (bottom arrow). Where downwelling currents occur on the eastern shore, all these ecological processes occur at a much lower pace.
Starfish are the top predators in these systems, known to be able to exert strong control over the abundance and diversity of prey communities (6). Recent debates in ecology (7) have centered on discerning the times and places when communities are controlled by the action of predators at the top of the food web (so-called “top down” effects) rather than the availability of primary production at the base of the food web (so-called “bottom-up” effects). The Menge et al. article shows how intimately top-down and bottom-up effects can be connected: the extra subsidies available on upwelling coastlines add to the abundance of filter-feeding producers in the system but also increase levels of predation.
Subsidies impact other food webs as well. Seabirds deposit a wealth of nutrients on offshore islands. This subsidy enriches plant communities and provides the productivity needed for a wide range of animal consumers and predators (8). In many freshwater habitats such as ponds and streams, local primary production is dwarfed by the contribution from surrounding terrestrial ecosystems. For example, Amazonian fish feed in forests almost as much as monkeys do: 50–90% of fish diets derive from forest products (9). Similarly, every hectare of mangrove forest exports 4–8 tons of material into offshore ecosystems annually (3).
Ecological stories such as these can be easily spun by comparisons of communities from place to place, but Menge et al. (1) contribute a research paradigm ideally suited to testing these hypotheses in a rigorous way. They demonstrate the power of a “comparative-experimental” approach to augment the comparative-observational approach typically used. By deploying similar experiments across a range of habitats, ecological processes such as predation, competition, and facilitation can be measured and compared. This can reveal geographic patterns not visible from observational data and provides objective criteria for measuring and comparing the fundamental ecological processes that allow ecosystems to function. This approach also answers a common problem with experimental data. When ecological experiments are done over a small spatial scale, the resulting insights might only apply over that small scale. Experiments placed across the landscape can show when and where ecological processes vary and greatly expand the scope for understanding ecological forces in an environmentally complex world.
The paradigm is not without limitations; the current work compares only one upwelling to one downwelling ecosystem, and differences among them might be ascribed to other factors. The challenge then is to expand the comparative-experimental approach across the grid of global ecosystems to allow the most robust conclusions possible.
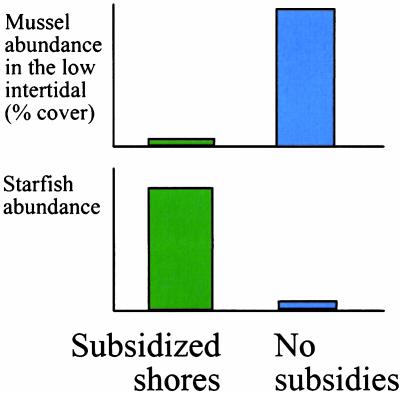
The Menge et al. (1) article also shows clearly that ecological interactions warped by subsidies can have complex affects on ecosystems. Mussels are more abundant on western shores where subsidies are high, but only in the mid- and upper intertidal. In the lower intertidal, mussels are surprisingly rare, eaten by starfish predators made more abundant by rich western subsidies. These subsidies are thus completely consumed on the lower part of western shores. Further up the shore, starfish are hampered by harsh physiological conditions during low tides, and even the subsidized starfish hordes of western shores cannot consume all of the mussels in the upper intertidal. By contrast, on eastern shores, sparse subsidies lower starfish abundance so much that the slow-growing, rarely settling mussels are actually more abundant in the low intertidal than on richer, western shores.
This is a version of the classic “paradox of enrichment,” where rich subsidies sometimes result in poorer diversity or other declines in abundance (Fig. 3). Natural examples are increasingly part of the literature on human impacts on natural ecosystems. For example, nutrient subsidies to the Gulf of Mexico from fertilizer delivered by the Mississippi river are so overwhelming that they generate a oxygen-depleted dead zone. Coral reefs are imperiled by nutrient subsidies from runoff and sewage. The resulting luxuriant algal growth smothers slow growing reef-building corals.
Fig. 3.
The paradox of enrichment. Relative abundance of mussels in the low intertidal show a reverse subsidy effect: they are lower where subsidies are richer. This discrepancy is dictated by the higher relative abundance of predatory starfish on rich western shores, which overeat their subsidies and reduce mussel abundance. Relative abundances are based on figures 2 and 4 of Menge et al. (1).
Globalization of the human economy has focused attention on the ebb and flow of trade around the world. The importance of subsidies from one ecosystem to another demonstrates that these global links are not restricted to human commerce but are critical to the functioning of natural ecosystems. At the same time, there is a continued search for ways to balance the needs of a burgeoning human population with the need to maintain the services provided by functioning ecosystems (10). The subsidies revealed in Menge et al. (1) are a glimpse at the kind of ecological commerce that links all of the Earth's ecosystems into a larger metasystem. Understanding these links demands that we seek enhanced knowledge of ecological communities and their integration into the global system on which human populations depend.
See companion article on page 12229.
References
- 1.Menge, B. A., Lubchenco, J., Bracken, M. E. S., Chan, F., Foley, M. M., Freidenburg, T. L., Gaines, S. D., Hudson, G., Krenz, C., Leslie, H., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 12229–12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrison, V. H., Shinn, E. A., Foreman, W. T., Griffin, D. W., Holmes, C. W., Kellogg, C. A., Majewski, M. S., Richardson, L. L., Ritchie, K. B. & Smith, G. W. (2003) Bioscience 53, 469–480. [Google Scholar]
- 3.Polis, G., Anderson, W. & Holt, R. (1997) Ann. Rev. Ecol. Syst. 28, 289–316. [Google Scholar]
- 4.Roughgarden, J., Gaines, S. & Possingham, H. (1988) Science 241, 1460–1466. [DOI] [PubMed] [Google Scholar]
- 5.Fairweather, P. (1991) Trends Ecol. Evol. 6, 60–63. [DOI] [PubMed] [Google Scholar]
- 6.Paine, R. (1966) Am. Nat. 100, 65–75. [Google Scholar]
- 7.Menge, B. A. (1992) Ecology 73, 755–765. [Google Scholar]
- 8.Polis, G. & Hurd, S. (1996) Am. Nat. 147, 396–423. [Google Scholar]
- 9.Golding, M. (1980) The Fishes and the Forest (Univ. of California Press, Berkeley).
- 10.Daily, G. & Ellison, K. (2003) The New Economy of Nature: The Quest to Make Conservation Profitable (Island Press, Washington, DC).