Abstract
Exosite 1 of thrombin consists of a cluster of basic residues (Arg-35, Lys-36, Arg-67, Lys-70, Arg-73, Arg-75, and Arg-77 in chymotrypsinogen numbering) that play key roles in the function of thrombin. Structural data suggest that the side chain of Arg-35 projects toward the active site pocket of thrombin, but all other residues are poised to interact with thrombomodulin (TM). To study the role of these residues in TM-mediated protein C (PC) activation by thrombin, a charge reversal mutagenesis approach was used to replace these residues with a Glu in separate constructs. The catalytic properties of the mutants toward PC were analyzed in both the absence and presence of TM and Ca2+. It was discovered that, with the exception of the Arg-67 and Lys-70 mutants, all other mutants activated PC with similar maximum rate constants in the presence of a saturating concentration of TM and Ca2+, although their affinity for interaction with TM was markedly impaired. The catalytic properties of the Arg-35 mutant were changed so that PC activation by the mutant no longer required Ca2+ in the presence of TM, but, instead, it was accelerated by EDTA. Moreover, the activity of this mutant toward PC was improved ≈25-fold independent of TM. These results suggest that Arg-35 is responsible for the Ca2+ dependence of PC activation by the thrombin–TM complex and that a function for TM in the activation complex is the allosteric alleviation of the inhibitory interaction of Arg-35 with the substrate.
Protein C (PC) is a multidomain vitamin K-dependent plasma serine protease zymogen that, on activation by thrombin in complex with thrombomodulin (TM), down-regulates the coagulation cascade by inactivating factors Va and VIIIa by limited proteolysis (1–3). Similar to the structures of other vitamin K-dependent coagulation proteases, the structure of PC consists of an amino-terminal γ-carboxyglutamic residue (Gla) domain followed by two epidermal growth factor (EGF)-like domains, and a C-terminal catalytic domain with a trypsin-like primary specificity pocket (4, 5). The Gla domain contains several cooperative low-affinity Ca2+-binding sites, which mediate the metal ion-dependent interaction of PC with endothelial protein C receptor and the thrombin–TM complex on membrane surfaces (1, 6). In addition to the Gla domain, the amino-terminal EGF domain and the catalytic domain of PC each have a high-affinity Ca2+-binding site with important roles for the structure and function of the protein (4, 7). The Ca2+-binding site on the protease domain of PC is located on the 70–80 loop (7). With the exception of prothrombin, the same loop is also known to bind Ca2+ in other vitamin K-dependent coagulation proteases (8, 9) and in trypsin (10). The binding of Ca2+ to this loop plays a complex role in PC activation by thrombin: it is inhibitory for activation by thrombin alone but stimulatory for activation by the thrombin–TM complex (11). The Ca2+-binding site in the amino-terminal domain does not appear to play a role in PC activation by thrombin in solution, because the deletion of either the Gla domain or both the Gla and the amino-terminal EGF domains does not influence the rate or the Ca2+ dependence of PC activation by thrombin either alone or in complex with the detergent-solubilized TM or the TM fragment containing EGF-like domains 4, 5, and 6 (TM456) (12, 13). TM improves the catalytic efficiency of thrombin toward PC in the presence of Ca2+ by ≈3 orders of magnitude by improving both the Km and the kcat of the PC activation reaction (1). Recent structural, molecular modeling, and mutagenesis data have indicated that TM, by binding to both exosite 1 of thrombin and a basic exosite of PC, presents the substrate to the activation complex for efficient activation (14, 15). However, neither structural nor mutagenesis data have provided any insight into the mechanism by which Ca2+ exerts its cofactor function in PC activation by the thrombin–TM complex.
The basic residues of exosite 1 of thrombin play a pivotal role in the catalytic function of thrombin (16–20). In a previous study, we replaced all basic residues of this site, including Arg-35, Lys-36, Arg-67, Lys-70, Arg-73, Arg-75, and Arg-77 [the chymotrypsinogen numbering system has been used throughout the study (21)] with Glu, and showed that these residues are also critical recognition sites on the substrate for prothrombinase (22). By contrast to all other residues, Arg-35 is not known to interact with the exosite-1-specific ligands of thrombin. In this study, we evaluated the kinetic properties of these mutants toward PC in both the absence and the presence of TM and Ca2+. It was found that reversing the charge of Arg-35 leads to a diminished requirement for both TM and Ca2+ in the rapid activation of PC by the thrombin–TM complex. The results suggest that TM modulates the conformation of Arg-35, and a cofactor function of TM is to alleviate the inhibitory interaction of Arg-35 with PC in the activation complex.
Methods
Construction and Expression of Mutant Proteins. The expression of wild-type prethrombin 1 by the pNUT-PL2 expression/purification vector system in baby hamster kidney (BHK) cells has been described (23). Prethrombin 1 mutants in the chymotrypsinogen numbering system, Arg-35 → Glu or Ala (R35E, R35A), Lys-36 → Glu (K36E), Arg-67 → Glu (R67E), Lys-70 → Glu (K70E), Arg-73 → Glu (R73E), Arg-75 → Glu (R75E), and Arg-77 → Glu (R77E), were prepared by PCR mutagenesis methods as described (23). All mutant constructs were expressed in BHK cells and purified to homogeneity as described (13, 23). Prethrombin 1 derivatives were activated to thrombin and purified on a Mono S column, and their concentrations were determined based on their absorbance at 280 nm and by titrations with a known concentration of antithrombin (AT) as described (23). The EGF-like domains 4, 5, and 6 of TM (TM456) (13), recombinant human PC (24), Gla-domainless protein C (GD-PC) (13), and recombinant human AT (25) were prepared as described. The chromogenic substrate Spectrozyme (SPCa) was purchased from American Diagnostica (Greenwich, CT), and S2238 was purchased from Kabi Pharmacia/Chromogenix (Franklin, OH). Heparin and dextran sulfate with an average molecular weight of 8,000 were purchased from Sigma. Normal pooled plasma was purchased from George King Bio-Medical (Overland Park, KS).
Amidolytic Activity and Reactivity with AT. The steady-state kinetics of hydrolysis of S2238 by thrombin derivatives were as described (22). The rate of inactivation of thrombin derivatives by human AT was measured under pseudo-first-order rate conditions by a discontinuous assay as described (23).
PC Activation. The initial rate of PC or GD-PC activation by thrombin derivatives was measured in both the absence and the presence of Ca2+, EDTA, and TM456 as described (13). In the absence of TM456, the time course of PC or GD-PC (1 μM) activation by each thrombin mutant (1–50 nM) was studied in 0.1 M NaCl/0.02 M Tris·HCl, pH 7.5, containing 0.1 mg/ml BSA and 0.1% polyethylene glycol 8000 (TBS) in the presence of either 2.5 mM CaCl2 (referred to as TBS/Ca2+) or 1 mM EDTA in 96-well assay plates. At different intervals, thrombin activity was quenched by 500 nM AT in complex with 1 μM heparin, and the rate of PC activation was measured from the cleavage rate of SPCa (200 μM) reading absorbance at 405 nm by a Vmax Kinetic Microplate Reader (Molecular Devices) as described (13). The experimental conditions in the presence of TM456 were the same, except that PC activation by thrombin (0.5–1.0 nM) was carried out in the presence of a saturating concentration of TM456. The concentration of activated PC (APC) in reaction mixtures was determined by reference to a standard curve that was prepared by total activation of PC with excess thrombin at the time of the experiments as described (13). The apparent Kd [Kd(app)] for the interaction of thrombin mutants with TM456 was determined from the cofactor-dependent (1 nM to 14 μM) increase in the rate of GD-PC (0.5 μM) activation by similar procedures. The Kd(app) and the maximum rate of activation were determined from the saturable dependence of the TM456mediated APC generation according to a hyperbolic equation. The TM456 concentration in all activation reactions was in excess of 5 × Kd(app) values. When a detailed kinetic analysis was done to determine Km and kcat values, the initial rates of activation were measured as a function of the GD-PC concentration by using 1–10 nM thrombin in complex with a saturating concentration of either TM456 or dextran sulfate (25 μg/ml) as described above. All reactions were carried out at room temperature, and it was ensured that <10% substrate was used in all activation reactions.
Clotting Activity. The clotting activity of wild-type and selected thrombin mutants was compared by using normal pooled plasma or purified fibrinogen (Hyphen BioMed, Andresy, France) and an ST4 Biocoagulometer (Diagnostica/Stago, Asnieres, France) as described (26). Plasma clotting was initiated by addition of 100 μl of thrombin in TBS/Ca2+ to 100 μl of citrated human plasma at 37°C to give final concentrations of 0.25–1.0 units/ml thrombin (≈2.8–11 nM). The same conditions were used for fibrinogen clotting except that 100 μl of 6 mg/ml human fibrinogen in 0.15 M NaCl/0.02 M Tris·HCl, pH 7.5, was used instead of human plasma. The clotting activities of mutants were calculated from standard curves prepared by using different dilutions of wild-type thrombin (0.25–1.0 units/ml). The clotting activities of mutants were calculated from at least four dilutions (0.25–4 units/ml) that yielded clotting times in the linear range of the standard curves (17–61 sec).
Results
Amidolytic Activity and Reactivity with AT. With the exception of the K70E mutant, which exhibited a higher Km value, all mutants cleaved S2238 with kinetic constants that were similar to those observed for wild-type thrombin (Table 1) (22). Previously, we showed that the conformation of the P3–P1 binding pocket of the K70E mutant has been altered (22). With the exception of K70E, all mutants also exhibited normal or improved reactivity with AT (Table 1), suggesting that the mutagenesis did not adversely affect the folding or the reactivity of the active site pocket of mutant proteins. This result is consistent with previous exosite 1 mutagenesis studies (20, 27, 28).
Table 1. Kinetic constants for the hydrolysis of S2238, μM-1·s-1 reaction with AT, interaction with TM456, and activation of PC by thrombin derivatives.
| Thrombin | kcat/Km (S2238) | k2, 103·M-1·s-1 (AT) | Kd(app), nM (TM456) | Maximum rate, nM·min-1·nM-1 (PC) |
|---|---|---|---|---|
| WT | 16 ± 2 | 9.2 ± 0.4 | 6.2 ± 0.7 | 0.17 ± 0.01 |
| R35E | 22 ± 4 | 28.0 ± 5.0 | 51.8 ± 7.2 | 0.18 ± 0.01 |
| R35A | 25 ± 7 | 6.2 ± 0.7 | 12.5 ± 3.8 | 0.14 ± 0.02 |
| K36E | 19 ± 2 | 8.1 ± 0.5 | 170.2 ± 16.3 | 0.12 ± 0.01 |
| R67E | 23 ± 4 | 9.3 ± 0.2 | 4,235 ± 850 | 0.003 ± 0.0002 |
| K70E | 0.45 ± 0.04 | 0.01 ± 0.0002 | ND | ND |
| R73E | 19 ± 5 | 6.6 ± 0.5 | 4,100 ± 300 | 0.06 ± 0.003 |
| R75E | 20 ± 3 | 9.8 ± 0.9 | 211.4 ± 11.1 | 0.17 ± 0.01 |
| R77E | 20 ± 4 | 10.1 ± 0.5 | 1,480 ± 70 | 0.19 ± 0.01 |
The kcat/Km values for the cleavage of the chromogenic substrate S2238 are derived from ref. 22. The other kinetic values were determined as described in Methods. ND, not determinable. All values are the average of at least two measurements ± standard errors.
TM Dependence of PC Activation. The cofactor dependence of the initial rate of GD-PC activation was evaluated as a function of increasing concentrations of TM456. The Kd(app) for the interaction of mutants with TM456 was impaired to various degrees (Table 1). Although no value for K70E could be determined, the increase in Kd(app) values for other mutants ranged from a minimum of approximately 2- to 8-fold for R35A and R35E to a maximum of ≈3 orders of magnitude for both R67E and R73E mutants. With the exception of the latter two mutants, no decrease in the rate of substrate activation was observed for all other mutants if a saturating concentration of TM456 was used in the reaction. In agreement with previous results (20), these findings suggest a key role for Arg-67, Lys-70, and, to a lesser extent, Arg-73 in the TM-mediated catalytic function of thrombin.
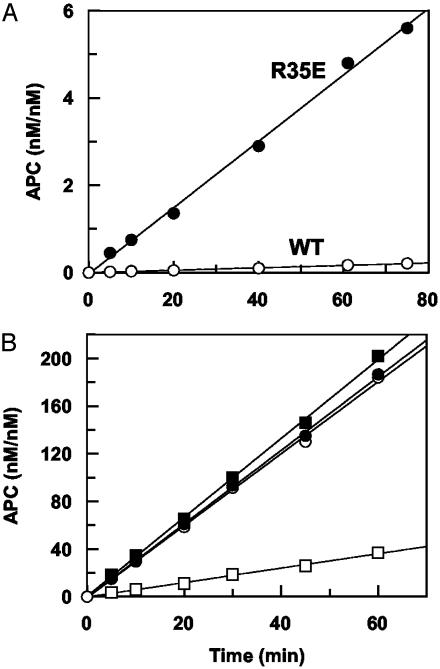
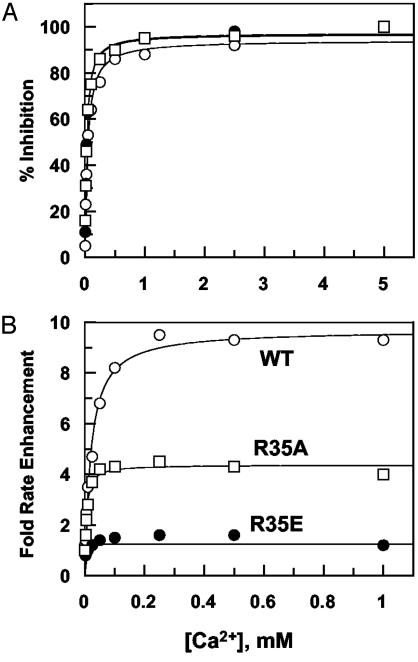
With the exception of Arg-35 mutants, the initial rates of PC activation by all other mutants were decreased in the absence of TM. The extent of impairment was the greatest for R67E and K70E thrombins (>10-fold) and ranged from 2- to 4-fold for other mutants (data not shown). On the other hand, the activity of R35E toward both PC and GD-PC was improved ≈25-fold (Fig. 1A, shown for GD-PC only). The rate of GD-PC activation by R35A was improved ≈5-fold (data not shown). Wild-type and mutant thrombins, in complex with a saturating concentration of TM456, activated GD-PC with similar rates in TBS/Ca2+ (Fig. 1B, shown for R35E only). Unlike activation of wild-type and R35A thrombins, the TM-mediated PC activation by R35E did not require Ca2+, because an identical rate of GD-PC activation was observed in either 2.5 mM Ca2+ or 1 mM EDTA. It is known that Ca2+ inhibits the rate of PC and GD-PC activation by thrombin in the absence of TM (1). Similar to activation by wild type, GD-PC activation by all mutants was inhibited by Ca2+ with half-maximal values of 30–60 μM in the absence of TM456 (Fig. 2A, shown for wild-type, R35A, and R35E thrombins). In the presence of TM456, all mutants, with the exception of R35E, exhibited a similar Ca2+ dependence of PC activation with half-maximal values of 15–30 μM at room temperature (data not shown). However, GD-PC activation by R35E did not require Ca2+ (Fig. 2B). Under the experimental conditions described in the legend of Fig. 2, Ca2+ in the presence of TM456 enhanced the rate of activation of 1 μM GD-PC by wild-type and R35A thrombins 10- and 5-fold, respectively. Under the same conditions, the metal ion exhibited ≈1.5-fold stimulatory effect with R35E thrombin (indistinguishable rates in the presence of 2.5 mM Ca2+ and 1 mM EDTA). These results suggest that reversing the charge of Arg-35 eliminates the requirement for Ca2+ in GD-PC activation by the thrombin–TM complex.
Fig. 1.
Initial rate of GD-PC activation by wild-type and R35E thrombins. (A)In the absence of TM, GD-PC (1 μM) was incubated with 50 nM wild-type (○) or 5 nM R35E thrombin (•) in TBS/Ca2+. At the indicated intervals, the activity of thrombin was inhibited by AT and the rate of APC generation was determined as described in Methods. The activation rates were 0.00276 nM/min and 0.076 nM/min for wild-type (WT) and R35E thrombin, respectively. (B)In the presence of TM456 (250 nM), the time course of GD-PC activation by 0.5 nM thrombin (○, Ca2+; □, EDTA) or R35E thrombin (•, Ca2+; ▪, EDTA) was carried out as in A. The activation rates were 3.0 nM/min (Ca2+) and 0.6 nM/min (EDTA) and 3.1 nM/min (Ca2+) and 3.3 nM/min (EDTA) for wild-type and R35E thrombin, respectively.
Fig. 2.
Ca2+-concentration dependence of GD-PC activation. (A) In the absence of TM, GD-PC (1 μM) activation was monitored at room temperature by 10 nM wild-type (WT, ○), 1 nM R35E (•), or 5 nM R35A thrombin (□)inthe presence of increasing concentrations of Ca2+. Data were normalized to 100% inhibition in the presence of 5 mM Ca2+.(B) The same as in A, except that the Ca2+-mediated enhancement in the activation rates in the presence of TM456 was measured and plotted vs. concentration of Ca2+. With the exception of R35E, all data fitted well to a hyperbolic binding isotherm yielding Kd(app) values of ≈30 μM and 15 μMCa2+ in the absence (A) and presence (B)ofTM.
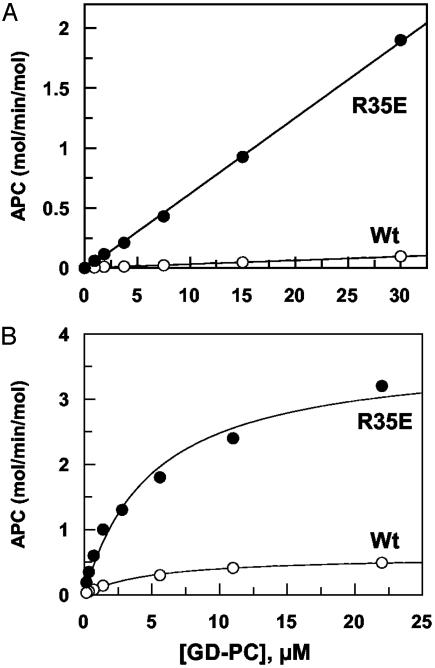
Concentration Dependence of PC Activation. To determine whether the mutagenesis of Arg-35 improves the Km or kcat of activation in the absence of TM, the concentration dependence of GD-PC activation by wild-type and R35E thrombins was studied. The initial rate of GD-PC activation remained linear for up to 30 μM substrate, suggesting a Km(app) of much greater than 30 μM GD-PC for both thrombins in the presence of Ca2+ (Fig. 3A). Bovine thrombin has a Km of ≈60 μM for bovine PC in TBS/Ca2+ (11). Assuming this is also true for the human proteins, a 25-fold improvement in the activation rate with the mutant thrombin cannot be accounted for by only a decrease in the Km value. It follows, therefore, that the Arg-35 → Glu substitution may have improved the kcat of PC activation by thrombin. To confirm this hypothesis, we conducted the GD-PC activation studies in the presence of dextran sulfate or heparin, both of which dramatically reduce the Km of PC activation by thrombin without influencing the kcat of the reaction (29). Both enzymes activated GD-PC with a similar Km(app) of ≈5 μMinthe presence of dextran sulfate; however, the kcat of activation by R35E thrombin was improved from 0.6 mol of APC per min per mol for the wild-type thrombin to 3.7 mol of APC per min per mol for the mutant (Fig. 3B and Table 2). In contrast to a 25-fold improvement in the catalytic activity of R35E toward GD-PC, the improvement in the catalytic activity of R35E in the presence of dextran sulfate was 6-fold. The reason for this discrepancy is not known. Nevertheless, the results support the conclusion that the improvement in the catalytic function of R35E toward PC is primarily in the kcat of the reaction.
Fig. 3.
The concentration dependence of the GD-PC activation by wild-type (Wt) and R35E thrombin. (A) Twenty nanomolar wild-type (○) or 5 nM R35E thrombin (•) was incubated with increasing concentrations of GD-PC (0.5–30 μM GD-PC) in TBS/Ca2+ at room temperature for 50 min. (B) The same as in A, except that the initial rate of activations by 10 nM thrombin (○) or 2 nM R35E thrombin (•) was determined in the presence of 25 μg/ml dextran sulfate. The solid lines are best fit of data to the Michaelis–Menten equation.
Table 2. Kinetic parameters for activation of GD-PC by thrombin in the presence of cofactors.
| kcat, mol·min-1·mol-1 | Km, μM | |
|---|---|---|
| Thrombin | ||
| GD-PC, TM456, Ca2+ | 13.8 ± 1.2 | 6.8 ± 0.3 |
| GD-PC, TM456, EDTA | ND | ND |
| GD-PC, dextran sulfate, Ca2+ | 0.59 ± 0.02 | 4.9 ± 0.5 |
| R35E thrombin | ||
| GD-PC, TM456, Ca2+ | 14.0 ± 0.7 | 4.5 ± 0.9 |
| GD-PC, TM456, EDTA | 55.5 ± 2.3 | 13.0 ± 1.1 |
| GD-PC, EDTA | 61.4 ± 2.2 | 15.8 ± 1.0 |
| GD-PC, dextran sulfate, Ca2+ | 3.7 ± 0.3 | 5.0 ± 0.9 |
| R35A thrombin | ||
| GD-PC, TM456, Ca2+ | 14.2 ± 0.2 | 4.8 ± 0.2 |
| GD-PC, TM456, EDTA | ND | ND |
The concentration dependence of the initial rate of GD-PC (0.2-22 μM) activation by wild-type, R35E, and R35A thrombins (1 nM each) in the presence of TM456 (500 nM) or dextran sulfate (25 μg/ml) was determined as described in Methods. ND, not determinable.
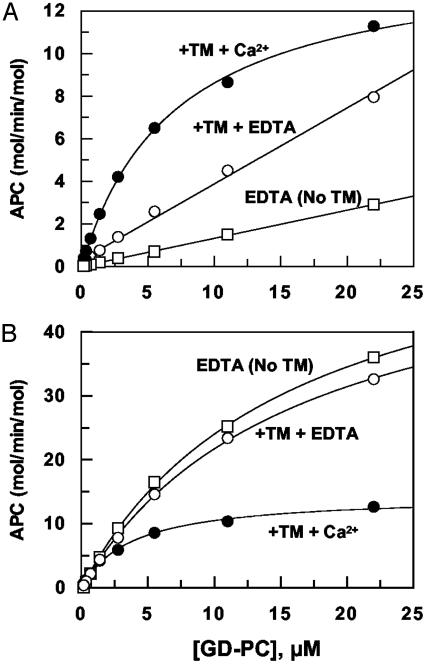
In the presence of TM456 and Ca2+, wild-type thrombin activated GD-PC with Km(app) and kcat values of 7 μM and 14 mol of APC per min per mol at room temperature (Fig. 4A and Table 2). As expected, no values for activation by the thrombin–TM456 complex could be obtained in EDTA, because the Km value was much greater than 22 μM, the highest concentration of substrate used in the activation reaction. TM456 enhanced the initial rate of GD-PC activation by thrombin 3- to 4-fold in the presence of EDTA. On the other hand, the R35E thrombin–TM456 complex activated GD-PC in the presence of Ca2+ with kinetic constants similar to those of the wild-type thrombin. However, EDTA was stimulatory in the reaction, and thus both Km and kcat parameters could be measured (Fig. 4B and Table 2). GD-PC activation by R35E in EDTA was entirely independent of TM (Fig. 4B). Thus, a Glu at position 35 fully replaced the cofactor function of TM in the absence of Ca2+. Relative to activation in the presence of Ca2+, the kcat by the R35E thrombin–TM456 complex was improved ≈4-fold in EDTA (Fig. 4B and Table 2). Unlike R35E thrombin, GD-PC activation by the R35A thrombin–TM456 complex exhibited Ca2+ dependence of activation similar to the wild-type thrombin (data not shown, but see Table 2). Taken together, these results indicate that, by reversing the charge of Arg-35, we have reversed the Ca2+-dependent PC activation property of the thrombin–TM complex.
Fig. 4.
The concentration dependence of the GD-PC activation by wild-type and R35E thrombins in the absence and presence of TM456 and Ca2+.(A) Initial rate of GD-PC activation by 1 nM thrombin alone in 1 mM EDTA (□) or in complex with 500 nM TM456 was determined in the presence of either 1 mM Ca2+ (•) or EDTA (○). (B) The same as in A except that R35E thrombin was used in the reaction.
Clotting Activity. One unit per milliliter wild-type thrombin (≈11 nM) yielded a clotting time of ≈17 s. This time was prolonged to ≈80 s with the same concentration of the mutant. Thus, 3-fold more R35E than wild type was required to obtain an equivalent clotting time. On the other hand, the clotting activity of R35A was minimally affected (75% of wild type). The poor plasmaclotting activity of R35E may be related to its enhanced reactivity with AT (Table 1). To confirm this possibility, the fibrinogen-clotting activities were evaluated in a purified system. Although wild type and R35A exhibited identical activity, yielding a clotting time of ≈17 s with 1 unit/ml thrombin, this time was prolonged to ≈30 s for R35E. Approximately 2-fold more R35E than wild-type thrombin was required to obtain an equivalent clotting time with the purified fibrinogen. Thus, the ability of this mutant to interact with fibrinogen has been impaired ≈2-fold.
Discussion
PC activation by thrombin is a cofactor-dependent reaction requiring both TM and Ca2+. Kinetic data have indicated that TM and Ca2+ together enhance the catalytic efficiency of thrombin toward PC by 3 orders of magnitude (1). Structural and mutagenesis data have indicated that the interaction of TM456 with basic residues of both exosite 1 on thrombin and a homologous site on PC facilitates the formation of a productive ternary complex that results in efficient activation of the substrate (14, 15). The Ca2+-binding site, critical for the activation of PC by the thrombin–TM complex, is located on the 70–80 loop of the substrate (7). It is not known how Ca2+ binding to this loop of PC stimulates the activation reaction. The complexity of the role of Ca2+ in the PC activation process may be underscored by the observation that the metal ion is also inhibitory for the substrate activation by thrombin in the absence of TM, with half-maximal stimulatory and inhibitory effects occurring at similar concentrations of Ca2+ (≈50–100 μMat37°C) (1). On the basis on such results, Esmon (1) hypothesized that the binding of Ca2+ to PC is associated with a conformational change in the activation peptide of the substrate and that the altered conformation is not complementary for docking into the active site pocket of thrombin. According to this hypothesis, the active site of thrombin also undergoes a conformational change on interaction with TM, thus optimizing its complementarity with the Ca2+-stabilized conformation of PC. Some of the spectroscopic, kinetic, and mutagenesis results that have supported this hypothesis include the following: (i) changes in the intrinsic Trp fluorescence properties of PC, but not APC, on binding to Ca2+ (30), (ii) alteration of the spectroscopic properties of the active-sitelabeled thrombin on binding to either TM56 or TM456 (31, 32), (iii) alteration of the chromogenic substrate specificity of thrombin on interaction with exosite-1-specific ligands (18), and (iv) enhancement in the affinity of thrombin for binding to the Kunitz inhibitor, bovine pancreatic trypsin inhibitor, in the presence of TM456 (33).
Recently, however, this hypothesis has been discounted because no significant structural change in the active site pocket of thrombin has been observed in the x-ray crystal structure of the thrombin–TM456 complex (14). We believe that the unique catalytic properties of R35E thrombin toward PC in both the absence and presence of TM and Ca2+ support the initial PC activation model proposed by Esmon (1). Our results are consistent with the following model for PC activation by the thrombin–TM complex: the interaction of Ca2+ with PC alters the conformation of a basic site of PC, thereby impeding the docking of the substrate into the active site pocket of thrombin because of its repulsive interactions with Arg-35 of the protease. On interaction with TM, the side chain of Arg-35 of thrombin is relocated, facilitating its productive interaction with a negatively charged region of PC. Thus, TM not only overcomes the inhibitory interaction of Arg-35 with PC, but may also facilitate its interaction with a complementary site of the substrate in the presence of Ca2+. According to the results presented in Figs. 3B and 4B, such an interaction can improve the kcat of the activation reaction. The observation that the activity of R35E toward PC in the presence of Ca2+ is improved is consistent with this hypothesis, suggesting that Glu-35 in the mutant thrombin makes ion-pair interactions with a basic site of the substrate. By the same token, the modulation of the side chain of Glu-35 by TM would be inhibitory, rather than stimulatory, for activation by the mutant in the presence of Ca2+. The observation that EDTA enhanced the kcat of the activation (Fig. 4 and Table 2) is also consistent with this model. The interesting observation in the presence of EDTA was that, unlike wild-type and R35A thrombins, PC activation by R35E was also entirely independent of TM. These results suggest that a cofactor function of TM in PC activation by thrombin is to alleviate the repulsive charge–charge interaction of Arg-35 with the substrate. Thus, the side chain of Arg-35, in the vicinity of the active site pocket of thrombin, is subject to modulation by TM, and reversing the charge of this residue leads to a diminished requirement for TM and almost a complete loss of the Ca2+ requirement for the rapid activation of PC by the thrombin–TM complex.
The relative orientation of the side chain of Arg-35 and the orientations of other basic residues of exosite 1 in thrombin are shown in Fig. 5 (21). The side chains of Arg-35 and Lys-36 are oriented in opposite directions in the structure of thrombin. The ammonium group of Lys-36 points away from the active site pocket, extending toward exosite 1 to interact with TM; however, the guanidinium group of Arg-35 points toward the active site pocket of thrombin. Thus, the positive charge of this residue near the substrate entrance pocket of thrombin can regulate the access of this pocket by a positively charged substrate such as PC. The likely candidate residues in PC that are inhibitory for interaction with the Arg-35 of thrombin in the presence of Ca2+ may involve Lys-174, Arg-177, or Arg-178, which are located at the P5′, P8′, and P9′ sites of the activation peptide of the substrate. In support of this hypothesis, previous replacement of these residues with three Glu residues has resulted in a 12-fold enhancement in the rate of PC activation by thrombin in the presence, but not in the absence of Ca2+ (34). Consistent with the PC activation model described above, TM did not enhance the rate of activation of this mutant by thrombin, possibly suggesting that either one or all of these residues are required for the productive interaction of PC with the TM-altered conformation of thrombin. Further mutagenesis study is needed to test the validity of this hypothesis.
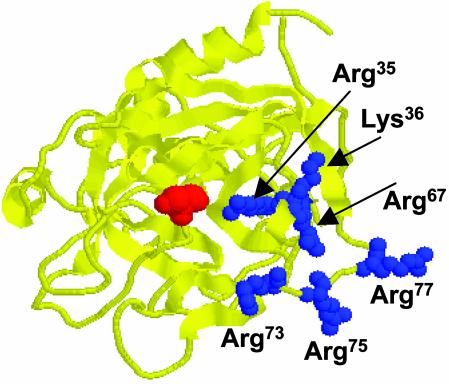
Fig. 5.
Crystal structure of human thrombin. The Arg-35 and basic residues of exosite 1 (Lys-36, Arg-67, Arg-73, Arg-75, and Arg-77) are shown in blue. The catalytic residue Ser-195 is shown in red. The coordinates of Protein Data Bank code 1PPB (21) were used to prepare the figure.
Another interesting feature of the activation peptide of PC is that it contains two Asp residues at the P3 and P3′ sites, both of which are known to be inhibitory for PC activation by thrombin in the absence, but not in the presence of TM (13, 35, 36). It has been hypothesized that PC activation peptide cannot optimally dock into the active site pocket of thrombin. This inability is thought to be caused by repulsive interactions of these residues with two acidic residues, Glu-192 and Glu-39, which have been demonstrated to be critical for determining the P3- and P3′-binding specificity of thrombin, respectively (1, 35, 36). Studies with PC mutants and synthetic peptides spanning the P7–P5′ residues of the PC activation peptide, containing Gly instead of Asp at either the P3 or P3′ positions, have confirmed this hypothesis (13, 36). Moreover, the replacement of Glu-192 with a Gln or Glu-39 with a Lys in thrombin has eliminated some of the requirement for the TM cofactor function during PC activation (35, 36). Consequently, it has been hypothesized that TM modulates the conformation of these residues by an allosteric mechanism. Our results in this study lend support to this hypothesis because Glu-39 is hydrogen-bonded to Arg-35 (21, 36), and thus, the allosteric modulation of Arg-35 by TM may involve the entire 35–39 loop of thrombin. Thus, it is also possible that the loss of ionic interactions between Arg-35 and Glu-39 may reposition the side chain of Glu-39, thus mimicking the TM effect by overcoming the inhibitory interaction of this residue with the P3′ Asp of the substrate (36). Similarly, Glu-192 has a flexible side chain at the base of the active site pocket of thrombin and is spatially adjacent to this loop; thus, it is also likely to be subject to a direct or an indirect modulation by TM. It appears that the primary role of all three residues (Arg-35, Glu-39, and Glu-192) is to improve the kcat of PC activation by thrombin (35, 36). Noting the complex role of Ca2+ in PC activation by thrombin and the importance of electrostatic interactions of these residues with PC in the ternary thrombin-TM–PC complex, it is not surprising that the crystal structure of the binary thrombin–TM456 complex, which has been resolved at a very acidic pH, did not provide any evidence for the TM modulation of the thrombin structure by an allosteric mechanism. Thus, the initial commentaries that immediately followed the publication of the thrombin–TM456 structure, claiming that the allosteric modulation of thrombin activity by TM “has been put to rest,” were hasty and premature (37).
Acknowledgments
We thank Audrey Rezaie for proofreading the manuscript. This research was supported by National Heart, Lung, and Blood Institute, National Institutes of Health Grant HL 68571 (to A.R.R.).
Abbreviations: PC, protein C; APC, activated PC; TM, thrombomodulin; EGF, epidermal growth factor; TM456, TM fragment containing the EGF-like domains 4, 5, and 6; Gla, γ-carboxyglutamic residue; GD-PC, Gla-domainless PC from which the amino-terminal residues 1–45 have been removed by recombinant DNA methods; AT, antithrombin.
References
- 1.Esmon, C. T. (1993) Thromb. Haemostasis 70, 1–5. [PubMed] [Google Scholar]
- 2.Walker, F. J. & Fay, P. J. (1992) FASEB J. 6, 2561–2567. [DOI] [PubMed] [Google Scholar]
- 3.Davie, E. W., Fujikawa, K. & Kisiel, W. (1991) Biochemistry 30, 10363–10370. [DOI] [PubMed] [Google Scholar]
- 4.Stenflo, J. (1991) Blood 78, 1637–1651. [PubMed] [Google Scholar]
- 5.Mather, T., Oganessyan, V., Hof, P., Huber, R., Foundling, S., Esmon, C. & Bode, W. (1996) EMBO J. 15, 6822–6831. [PMC free article] [PubMed] [Google Scholar]
- 6.Oganesyan, V., Oganesyan, N., Terzyan, S., Qu, D., Dauter, Z., Esmon, N. L. & Esmon, C. T. (2002) J. Biol. Chem. 277, 24851–24854. [DOI] [PubMed] [Google Scholar]
- 7.Rezaie, A. R., Mather, T., Sussman, F. & Esmon, C. T. (1994) J. Biol. Chem. 269, 3151–3154. [PubMed] [Google Scholar]
- 8.Wildgoose, P., Foster, D., Schiodt, J., Wiberg, F. C., Birktoft, J. J. & Petersen, L. C. (1993) Biochemistry 32, 114–119. [DOI] [PubMed] [Google Scholar]
- 9.Rezaie, A. R. & Esmon, C. T. (1994) J. Biol. Chem. 269, 21495–21499. [PubMed] [Google Scholar]
- 10.Bode, W. & Schwager, P. (1975) J. Mol. Biol. 98, 693–717. [DOI] [PubMed] [Google Scholar]
- 11.Esmon, N. L., DeBault, L. E. & Esmon, C. T. (1983) J. Biol. Chem. 258, 5548–5553. [PubMed] [Google Scholar]
- 12.Rezaie, A. R., Esmon, N. L. & Esmon, C. T. (1992) J. Biol. Chem. 267, 11701–11704. [PubMed] [Google Scholar]
- 13.Rezaie, A. R. & Esmon, C. T. (1992) J. Biol. Chem. 267, 26104–26109. [PubMed] [Google Scholar]
- 14.Fuentes-Prior, P., Iwanaga, Y., Huber, R., Pagila, R., Rumennik, G., Seto, M., Morser, J., Light, D. R. & Bode, W. (2000) Nature 404, 518–525. [DOI] [PubMed] [Google Scholar]
- 15.Yang, L. & Rezaie, A. R. (2003) J. Biol. Chem. 278, 10484–10490. [DOI] [PubMed] [Google Scholar]
- 16.Rydel, T. J., Ravichandran, K. G., Tulinsky, A., Bode, W., Huber, R., Roitsch, C. & Fenton, J. W., II (1990) Science 249, 277–280. [DOI] [PubMed] [Google Scholar]
- 17.Fenton, J. W., II (1995) Thromb. Haemostasis 74, 493–498. [PubMed] [Google Scholar]
- 18.Liu, L., Vu, T. H., Esmon, C. T. & Coughlin, S. R. (1991) J. Biol. Chem. 266, 16977–16980. [PubMed] [Google Scholar]
- 19.Tsiang, M., Lentz, S. & Sadler, J. E. (1992) J. Biol. Chem. 267, 6164–6170. [PubMed] [Google Scholar]
- 20.Pineda, A. O., Cantwell, A. M., Bush, L. A., Rose, T. & Di Cera, E. (2002) J. Biol. Chem. 277, 32015–32019. [DOI] [PubMed] [Google Scholar]
- 21.Bode, W., Mayr, I., Baumann, U., Huber, R., Stone, S. R. & Hofsteenge, J. (1989) EMBO J. 8, 3467–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, C., Yang, L. & Rezaie, A. R. (2003) J. Biol. Chem. 278, 27564–27569. [DOI] [PubMed] [Google Scholar]
- 23.Rezaie, A. R. (1998) Protein Sci. 7, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang, L., Manithody, C. & Rezaie, A. R. (2002) Biochemistry 41, 6149–6157. [DOI] [PubMed] [Google Scholar]
- 25.Rezaie, A. R. & Yang, L. (2001) Biochim. Biophys. Acta 1528, 167–176. [DOI] [PubMed] [Google Scholar]
- 26.Rezaie, A. R. (1997) Biochemistry 36, 7437–7446. [DOI] [PubMed] [Google Scholar]
- 27.Sadler, J. E., Lentz, S. R., Sheehan, J. P., Tsiang, M. & Wu, Q. (1993) Haemostasis 23, 183–193. [DOI] [PubMed] [Google Scholar]
- 28.Tsiang, M., Jain, A. K., Dunn, K. E., Rojas, M. E., Leung, L. L. K. & Gibbs, C. S. (1995) J. Biol. Chem. 270, 16854–16863. [DOI] [PubMed] [Google Scholar]
- 29.Rezaie, A. R. (1998) Blood 91, 4572–4580. [PubMed] [Google Scholar]
- 30.Rezaie, A. R. & Esmon, C. T. (1995) Biochemistry 34, 12221–12226. [DOI] [PubMed] [Google Scholar]
- 31.Ye, J., Esmon, N. L., Esmon, C. T. & Johnson, A. E. (1991) J. Biol. Chem. 266, 23016–23021. [PubMed] [Google Scholar]
- 32.Ye, J., Liu, L., Esmon, C. T. & Johnson, A. E. (1992) J. Biol. Chem. 267, 11023–11028. [PubMed] [Google Scholar]
- 33.Rezaie, A. R., He, X. & Esmon, C. T. (1995) Biochemistry 37, 693–699. [DOI] [PubMed] [Google Scholar]
- 34.Grinnell, B. W., Gerlitz, B. & Berg, D. T. (1994) Biochem. J. 303, 929–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bonniec, B. F. & Esmon, C. T. (1991) Proc. Natl. Acad. Aci. USA 88, 7371–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bonniec, B. F., MacGillivray, R. T. A. & Esmon, C. T. (1991) J. Biol. Chem. 266, 13796–13803. [PubMed] [Google Scholar]
- 37.Overduin, M. & de Beer, T. (2000) Nat. Struct. Biol. 7, 267–269. [DOI] [PubMed] [Google Scholar]