Abstract
Expression of amino acid biosynthesis genes in bacteria is often repressed when abundant supplies of the cognate amino acid are available. Repression of the Bacillus subtilis lysC gene by lysine was previously shown to occur at the level of premature termination of transcription. In this study we show that lysine directly promotes transcription termination during in vitro transcription with B. subtilis RNA polymerase and causes a structural shift in the lysC leader RNA. We find that B. subtilis lysC is a member of a large family of bacterial lysine biosynthesis genes that contain similar leader RNA elements. By analogy with related regulatory systems, we designate this leader RNA pattern the “L box.” Genes in the L box family from Gram-negative bacteria appear to be regulated at the level of translation initiation rather than transcription termination. Mutations of B. subtilis lysC that disrupt conserved leader features result in loss of lysine repression in vivo and loss of lysine-dependent transcription termination in vitro. The identification of the L box pattern also provides an explanation for previously described mutations in both B. subtilis and Escherichia coli lysC that result in lysC overexpression and resistance to the lysine analog aminoethylcysteine. The L box regulatory system represents an example of gene regulation using an RNA element that directly senses the intracellular concentration of a small molecule.
The lysine biosynthesis pathway is required in bacteria for production of lysine for protein synthesis and cell wall biosynthesis and for generation of diaminopimelate (DAP), a key cell wall component in certain bacteria (Fig. 1A). This pathway also generates dipicolinate, a major component of bacterial endospores. No consistent pattern for regulation of the lysine pathway in bacteria has emerged, although repression of enzyme synthesis and enzyme activity has been observed in multiple systems (1–3). In Escherichia coli, the lysA gene encoding meso-DAP decarboxylase, which carries out the final step in lysine biosynthesis, is regulated at the level of transcription initiation by the LysR transcriptional activator (4). Repression of Bacillus subtilis lysA by lysine was reported, but the mechanism is unknown (5). Expression of the B. subtilis lysC gene, encoding a lysine-responsive aspartokinase (designated AKII), was previously shown to be regulated at the level of premature termination of transcription (6). Termination at an intrinsic terminator 270 bp downstream of the transcription start site was observed during growth in the presence of lysine, whereas synthesis of the full-length transcript increased during lysine deprivation. A potential competing antiterminator structure was identified in the leader, but the mechanism for response to lysine availability was not understood. Sequence similarity was noted in the upstream regions of lysC genes of B. subtilis and a thermophilic Bacillus (7), with more limited similarity to the E. coli lysC leader (8); however, there was no evidence for a leader region transcriptional terminator in the E. coli gene, suggesting that the regulatory mechanism was not conserved. Mutations conferring resistance to the lysine analog aminoethylcysteine (AEC, also known as thialysine) mapped to upstream regions of the lysC genes of both B. subtilis and E. coli (8–10), but no basis for the resulting lysC overexpression was uncovered.
Fig. 1.
L box lysine biosynthesis genes. (A) Lysine biosynthesis pathway in B. subtilis. Genes found to contain L box leaders in any organism are labeled (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org), and the corresponding enzyme product is shown in parentheses. Three genes encoding aspartokinase have been identified in B. subtilis; lysC encodes aspartokinase II, the lysine responsive form of the enzyme. Alternative roles of compounds in this pathway are shown with dashed arrows. (B) Structures of lysine and related compounds.
Several recent studies have revealed a crucial role for conserved leader region motifs in regulation of gene expression at the level of premature termination of transcription. The T box system in Gram-positive organisms uses direct interaction of uncharged tRNA with the leader RNA to promote transcription antitermination of aminoacyl-tRNA synthetase, amino acid biosynthesis, and transporter genes of a variety of amino acid classes, with each transcriptional unit responding individually to its cognate tRNA (11–13); no lysine genes have been found to be regulated by the T box mechanism. The S box regulatory system also involves transcription termination, but in this case the regulated genes respond in concert to a single effector, S-adenosylmethionine (SAM), which binds to the leader RNA and promotes a structural rearrangement that stabilizes the terminator helix (14–16). Similarly, thiamine pyrophosphate, flavin mononucleotide, and guanine regulate thiamine, riboflavin, and purine biosynthesis genes, respectively, via direct interaction with the leader RNA (17–20). There are also many systems in which modulation of the leader RNA structure is determined by binding of a regulatory protein that responds to the availability of the effector molecule. Systems of this type include the B. subtilis trp operon, the E. coli bgl and B. subtilis sac operons, and the B. subtilis pyr operon (see ref. 21 for review). In each of these regulatory systems, comparative sequence analysis was useful in identification of key leader RNA features important for regulation.
In this study, we use phylogenetic analyses of lysine biosynthesis genes to reveal a complex leader RNA structural array that is conserved in a variety of organisms, including members of the low G+C Gram-positive bacteria and the γ-proteobacteria. These RNAs exhibit a highly similar pattern of secondary structural features, despite very little primary sequence conservation. Putative transcriptional terminators and competing antiterminators were found in the lysine genes from Gram-positive organisms, and termination of the B. subtilis lysC gene was shown to occur in direct response to lysine. In contrast, the genes from Gram-negative organisms appear to be regulated at the level of translation initiation.
Materials and Methods
Bacterial Strains and Growth Conditions. B. subtilis strain BR151 (lys-3 metB10 trpC2) was used as the source of chromosomal DNA for amplification by PCR and for measurement of lysC-lacZ expression. A 450-bp lysC DNA fragment containing sequences 83 bp upstream of the transcription start site to a position 95 bp downstream of the leader region terminator (10) was generated by PCR, inserted into the lacZ transcriptional fusion vector pFG328 (22), and integrated in single copy into the chromosome of strain BR151 by recombination into a bacteriophage SPβ prophage. Oligonucleotide primers were purchased from Integrated DNA Technologies (Coralville, IA). Leader region mutations (Fig. 2) were generated by PCR using oligonucleotides containing the desired sequence alterations. All constructs were verified by DNA sequencing. Cells containing lacZ fusions were grown in Spizizen minimal medium (23) containing lysine, methionine, and tryptophan at 50 μg/ml until midexponential growth phase and were then divided into two cultures containing methionine and tryptophan at 50 μg/ml and lysine at 50 or 5 μg/ml. Growth was continued for 4 h, and cells were harvested at 1-h intervals and assayed for β-galactosidase activity, expressed in Miller units (24). All growth experiments were carried out in triplicate, and variation was <10%.
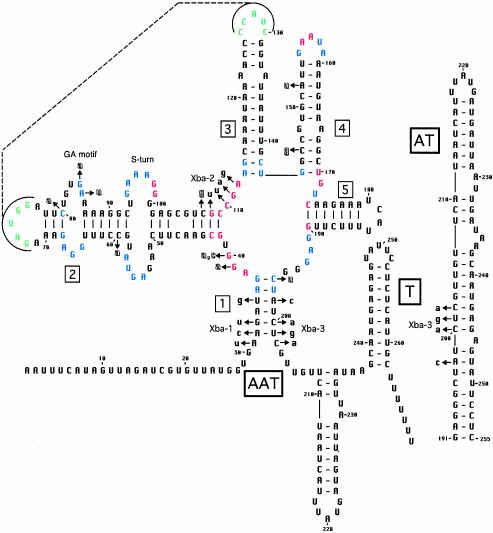
Fig. 2.
Structural model of the B. subtilis lysC leader. Numbering is relative to the transcription start point (10). Helices 1–5 are labeled with boxed numbers. T, terminator; AT, antiterminator; AAT, anti-antiterminator. The antiterminator is formed by pairing of residues from positions 191–217 with residues from positions 223–255. Red residues are found in 100% of the sequences, and blue residues are found in >80% of the sequences (Fig. 5); green residues and dashed lines show a possible tertiary interaction between the loops of helices 2 and 3. Hollow lowercase letters indicate mutations previously shown to cause resistance to AEC and/or constitutive lysC expression (refs. 9 and 10 and H. Paulus, personal communication). Mutations constructed in this study (Xba-1, Xba-2, Xba-3) are labeled with solid lowercase letters. Sequence alterations in the mutants are: Xba-1, A31U/A32C/G33U/U35G; Xba-2, G108U/C110U/G111A/A112G; Xba-3, A198C/C200A/U201G/C202A. Putative S-turn and GA motif elements are labeled.
In Vitro Transcription Assays. Templates for in vitro transcription were generated by PCR using oligonucleotide primers that contained the B. subtilis glyQS promoter region (12) and hybridized within the leader region of the B. subtilis lysC gene. The resulting PCR product contained the glyQS promoter fused to the lysC leader region so that transcription initiated 1 nt upstream of C17 of the lysC transcript (10). This location was selected to allow initiation with the dinucleotide ApC and a halt at position G42 (Fig. 2) by initiation in the absence of CTP. The promoter-distal end of the PCR fragment was 95 bp downstream of the leader region termination site, to allow resolution of terminated and read-through products (253 and 348 nt, respectively). PCR products were purified with a Qiagen PCR cleanup kit (Qiagen, Chatsworth, CA). Templates for leader region mutants were generated by PCR with template DNAs containing the appropriate alleles. The ykrW template has been described (15).
Single-round transcription reactions were carried out as described (12) with template DNA (10 nM) and His-tagged B. subtilis RNA polymerase (RNAP) (6 nM) purified as described by Qi and Hulett (25). The initiation reactions, containing 1× transcription buffer (26), ApC (150 μM; Sigma), GTP (2.5 μM), ATP (2.5 μM), UTP (0.75 μM), and [α-32P]UTP (0.25 μM, 800 Ci/mmol; 1 Ci = 37 GBq), were incubated at 37°C for 15 min. Heparin (20 μg/ml; Sigma) was added to block reinitiation, and elongation was triggered by the addition of NTPs to 10 μMinthe presence of other reagents as indicated. Elongation reactions were incubated at 37°C for an additional 15 min and terminated by extraction with phenol. Transcription products were resolved by denaturing PAGE and visualized by PhosphorImager (Molecular Dynamics) analysis.
Structural Mapping by RNase H Cleavage. RNase H cleavage experiments were carried out with two lysC transcripts, both of which were uniformly labeled by in vitro transcription with B. subtilis RNAP in the presence of α-32P]UTP. Template DNAs were generated by PCR of WT or mutant lysC DNAs described above. The shorter transcripts (including lysC sequences from +17 to +228) contained the 5′ side of the antiterminator but lacked the 3′ region, so that formation of the anti-antiterminator could be monitored without competition from the alternate antiterminator (Fig. 2). The longer transcripts (including lysC sequences from +17 to +253) contained the entire antiterminator, but lacked the 3′ side of the terminator helix. Transcription reactions were carried out as described above in the presence or absence of l-lysine (3 mM). Antisense oligonucleotides were added at 100-fold molar excess to the transcription complexes, and the reactions were incubated at 35°C (oligo a) or 30°C (oligo b) for 5 min before addition of RNase H (Ambion, 10 units) and further incubation for 10 min. Oligonucleotide a (5′-ACGAGATAGCCC-3′) is complementary to positions 193–204 relative to the lysC transcription start point; oligonucleotide b (5′-ATACTCTCATTGCTTA-3′) is complementary to positions 235–250. Products were resolved by denaturing PAGE and visualized by PhosphorImager analysis.
Results
Phylogenetic Analysis of Lysine Gene Regulatory Elements. Comparative sequence analysis was crucial to recognition of the T box and S box regulatory systems (11, 14). In an attempt to elucidate the mechanism for regulation of B. subtilis lysC in response to lysine, and the effect on lysC expression of mutations conferring resistance to AEC (6, 9, 10), we identified additional lysC homologs from Bacillus sp. We examined these sequences for conserved features, including small primary sequence elements, helical domains, and RNA structural elements known to be important in the T box and S box regulatory RNAs. The lysC sequences were found to be capable of folding into a consistent pattern (Fig. 2), despite little primary sequence conservation. We then searched for this pattern upstream of additional lysine biosynthesis genes in these and other microbial genomes. A total of 21 variants were identified upstream of lysC, lysA, and dapA (dihydropicolinate synthase, Fig. 1 A) genes in Bacillus, Clostridium, Staphylococcus, Desulfitobacterium, Thermoanaerobacter, Carboxydothermus, and Oceanobacillus (Gram-positive bacteria), as well as upstream of lysC genes in E. coli, Erwinia, and Klebsiella (γ-proteobacteria; see Table 2). Most of the searched genomes contain a single gene with this sequence pattern, whereas in Bacillus anthracis it was found upstream of both lysC and lysA, and in Bacillus halodurans the pattern was found upstream of lysC, lysA, and dapA. A structure-based alignment of these leader sequences is shown in Fig. 5, which is published as supporting information on the PNAS web site. Because all of the genes with these features are involved in lysine biosynthesis, we designate this leader RNA pattern the “L box.”
Structural Arrangement of the L Box Motif. The L box leader region pattern consists of five helical domains (helices 1–5) upstream of the leader region intrinsic terminator (Fig. 2); in the genes from Gram-negative organisms (all of which are lysC), the terminator helix is replaced by a helical structure that includes the lysC Shine-Dalgarno sequence, suggesting that it functions to block access of the ribosome to the mRNA. In each leader, an alternate structure can be formed that pairs sequences on the 3′ side of helix 1 with sequences on the 5′ side of the terminator (or translational repressor helix), so that the two structures are mutually exclusive. This alternate element is proposed to serve as an antiterminator (for the genes from Gram-positive organisms) or as an antirepressor of translation (for the genes from Gram-negative organisms).
The five helical domains of the L box radiate from a central core (Fig. 2). The junction region between helices 1 and 2 contains a conserved AGG sequence immediately adjacent to helix 1. Helix 2 contains two consistently placed internal loops. The loop proximal to the central core region contains conserved 5′-AGUA-3′ and 5′-GAAA-3′ elements on opposing sides, an arrangement consistent with the S-turn (or loop E) RNA structural motif, which imparts a turn to the backbone of the helix (27). The distal internal loop of helix 2 is asymmetric, with opposing GA residues; this arrangement matches the GA motif found in both T box and S box leader RNAs (28) and corresponds to the kink-turn motif that forms a kink in the RNA backbone (29). The GA motif is absent in the Staphylococcus L box genes, whereas the S-turn motif is less prominent in the leaders of Gram-negative origin; however, all helix 2 domains contain two internal loops, at least one of which fits the S-turn or GA motif pattern.
Helices 3 and 4 appear to be a simple elements that usually include weak pairs (G:U or U:G wobble pairs, sheared G:A pairs, or mismatches). The loop of helix 4 exhibits significant primary sequence conservation, and helices 3 and 4 are flanked by very highly conserved residues (Fig. 2). Helix 5 is relatively short in the genes of Gram-positive origin and is extended in the genes of Gram-negative origin; the Klebsiella pneumoniae lysC leader contains two extra helical elements within helix 5. The junction between helices 5 and 1 contains conserved GAG residues, usually immediately 3′ to helix 5. Residues forming base pairs at the junction regions of each helix are also conserved at the primary sequence level, suggesting that the structural arrangement in the core region is tightly constrained.
To gain further insight into the possible tertiary structure of the L box motif, we examined all unpaired regions for complementarity. The loops of helices 2 and 3 exhibited complementary residues, usually with 5 nt capable of pairing; the two loop sequences exhibit extensive covariation. The internal loops in helix 2 and the weak pairs in helix 3 may contribute to the ability of these loop regions to interact. The conserved residues in the core region at the junction of helices 1–5 have the potential to form additional pairings, but the very high conservation prevents corroboration by covariation.
Effect of lysC Leader Region Mutations. In each leader RNA, the terminator (or translational repressor) helix is predicted (based on helix length and number of G-C pairs) to be less stable than the competing antiterminator (or antirepressor) helix, which is formed by pairing sequences on the 5′ side of the terminator with sequences on the 3′ side of helix 1, often including sequences in the region between helix 5 and helix 1. This arrangement suggested a model in which helix 1 serves as an anti-antiterminator, which prevents formation of the antiterminator and therefore promotes termination when lysine is abundant.
To test this model, we constructed mutations (Fig. 2) that disrupt the 5′ side of helix 1 (Xba-1), the 3′ side of helix 1 (Xba-3), or conserved residues in the helix 2/3 junction (Xba-2) and examined the effect of these mutations on expression of a B. subtilis lysC-lacZ transcriptional fusion (Table 1). The WT fusion exhibited low expression during growth in the presence of lysine, with 40-fold induction in response to starvation for lysine. The Xba-1 mutation, which is predicted to block formation of the anti-antiterminator element, resulted in high expression in the presence or absence of lysine, whereas the Xba-3 mutation, which alters residues involved in formation of both helix 1 and the antiterminator, resulted in loss of repression by lysine, but reduced expression relative to that of the Xba-1 mutant. The residual unregulated expression exhibited by the Xba-3 mutant may be because this allele is not predicted to completely disrupt the antiterminator structure. The Xba-2 mutation resulted in high, constitutive expression, consistent with the prediction that highly conserved residues are important for regulation. These results support the model that formation of helix 1 is required for termination, formation of the antiterminator is required for efficient read-through, and conserved elements in the helix 1–5 junction region are required for repression during growth in lysine.
Table 1. Expression of lysC-lacZ transcriptional fusions.
| β-galactosidase activity*
|
|||
|---|---|---|---|
| Fusion | Low lysine | High lysine | Induction ratio† |
| WT | 180 | 4.3 | 42 |
| Xba-1 | 410 | 360 | 1.1 |
| Xba-2 | 310 | 180 | 1.7 |
| Xba-3 | 49 | 74 | 0.66 |
Fusions were integrated in single copy in strain BR151 (lys-3 metB10 trpC2). Cells were grown in Spizizen minimal medium (23) in the presence of lysine, then were split and grown in the presence of high lysine (50 μg/ml) or low lysine (5 μg/ml). Cells were harvested and assayed for β-galactosidase activity, expressed in Miller units (24). Maximal induction was observed 3 h after incubation in medium with low lysine.
Induction ratio is the ratio of activity under inducing conditions (low lysine) to the activity under repressing conditions (high lysine).
Lysine Promotes Transcription Termination During in Vitro Transcription by B. subtilis RNAP. DNA templates containing the B. subtilis glyQS promoter fused to position C17 of the B. subtilis lysC leader were generated to allow examination of the regulatory properties of the leader sequence independent of the lysC promoter. Read-through of the leader region terminator was efficient in the absence of lysine, and termination was stimulated by the addition of l-lysine at 3 mM (Fig. 3A, lanes 1 and 2). The response to lysine was specific, because DAP and SAM had no effect (Fig. 3A, lanes 3 and 5); AEC exhibited weaker termination activity (Fig. 3A, lane 4), and a 10-fold increase in AEC concentration was required to give a response comparable to that observed with lysine (data not shown). Lysine had no effect on termination of the ykrW S box leader (Fig. 3A, lane 7), which responds instead to SAM (Fig. 3A, lane 8, and ref. 15). These results indicate that lysine specifically promotes termination of the lysC leader and does not generally cause transcription termination by B. subtilis RNAP. Similar results were obtained with E. coli RNAP (data not shown), indicating that the effect is independent of the source of RNAP.
Fig. 3.
In vitro transcription of B. subtilis lysC. Arrows show positions of the read-through (RT) and terminated (T) transcripts. Percent termination (%T) is shown at the bottom of each lane. DNA templates (lysC or ykrW) were transcribed with B. subtilis RNAP. (A) Lysine-dependent transcription termination. Lysine (lys), DAP, AEC, and SAM were added at 3 mM. (B) In vitro transcription of mutant lysC templates. Lysine was added at 3 mM (+). WT, WT lysC; Xba-1, Xba-1 mutation; Xba-2, Xba-2 mutation; Xba-3, Xba-3 mutation; Xba-1/3, Xba-1 and Xba-3 double mutant. Mutations are shown in Fig. 2.
The effect of leader mutations on lysine-directed termination in vitro was also tested (Fig. 3B). The Xba-1 and Xba-2 mutations resulted in loss of termination, consistent with the in vivo results. The Xba-3 mutation, which affects residues on the 3′ side of helix 1 (Fig. 2), resulted in an increase in termination independent of the presence of lysine, indicating that disruption of helix 1 blocks the response to lysine; the failure of this mutation to confer complete loss of read-through is consistent with the in vivo results and may be caused by the fact that this allele does not completely disrupt the antiterminator element. Combination of the Xba-1 and Xba-3 alleles was predicted to restore pairing in helix 1, with an increase in helix stability. The double mutant exhibited increased termination relative to the Xba-3 mutant, consistent with the prediction that formation of helix 1 promotes termination. The absence of a response to lysine could be caused by lysine-independent stabilization of the anti-antiterminator and destabilization of the antiterminator and/or by effects of the sequence alterations on lysine binding. Overall these results provide support for the model and for the hypothesis that read-through is the default state of the system.
Lysine Causes a Structural Transition in the B. subtilis lysC Leader. The model for the L box leader predicts that the antiterminator is paired during growth in the absence of lysine and unpaired in its presence. Oligonucleotides complementary to the 5′ and 3′ regions of the antiterminator of B. subtilis lysC (Fig. 4A) were used to probe this structural transition; annealing of the oligonucleotides was detected by sensitivity to RNase H, which specifically cleaves RNA–DNA hybrids. Transcripts were generated by transcription with B. subtilis RNAP and included lysC sequences from +17 to +228 or +17 to +253; the shorter transcript (211 nt) contains only the 5′ side of the antiterminator, whereas the longer transcript (236 nt) contains the entire antiterminator but lacks the 3′ side of the terminator. Oligo a, which is complementary to sequences predicted to form the 3′ side of helix 1 in the presence of lysine, triggered cleavage of the transcript ending at +228 only when incubated with the RNA in the absence of lysine (Fig. 4B, lane 3), consistent with the prediction that the targeted region is available only in the absence of lysine when the 3′ side of the antiterminator is missing. The Xba-1 mutation, which alters sequences on the 5′ side of helix 1, resulted in efficient cleavage in the presence or absence of lysine (Fig. 4B, lanes 7 and 8), consistent with the prediction that pairing of helix 1 is responsible for loss of cleavage of the WT sequence in the presence of lysine. The Xba-2 mutation also resulted in lysine-independent cleavage (Fig. 4B, lanes 11 and 12), consistent with loss of repression by lysine in vivo and in vitro. Oligo b, which is complementary to sequences that form the 3′ side of the antiterminator or the 5′ side of the terminator, targets sequences predicted to be paired in the absence of lysine in the RNA ending at +253, because the 3′ side of the terminator region is absent (Fig. 4A). For the WT transcript, oligo b-directed cleavage was strongly enhanced by the addition of lysine, indicating that lysine inhibits formation of the antiterminator structure (Fig. 4C). In contrast, cleavage of RNAs containing the Xba-1 or Xba-2 mutations was inefficient in the presence or absence of lysine, as expected if the ability to form helix 1 is impaired. These results indicate that lysine promotes a structural rearrangement of the antiterminator region of the lysC leader RNA, consistent with the model.
Fig. 4.
Lysine-dependent structural transition in the lysC RNA. (A) Structural model of the B. subtilis lysC leader in the read-through (-lysine) and termination (+lysine) conformations. Positions of pairing of antisense oligonucleotides a and b, positions of Xba-1 and Xba-2 mutations (Fig. 2), and endpoints of transcription products are shown. (B) Antisense oligonucleotide a-directed RNase H cleavage of transcripts containing lysC sequences from +17 to +228 (211-nt transcript, containing the 5′ side of the antiterminator, but not the 3′ side). Transcription was in the presence (+) or absence (-) of lysine (3 mM). Arrows indicate the RNase H cleavage products, which were observed only when the oligonucleotide was added. (C) Antisense oligonucleotide b-directed RNase H cleavage of transcripts containing lysC sequences from +17 to +253 (236-nt transcript, containing the entire antiterminator, but lacking the 3′ side of the terminator). Arrows indicate the RNase H cleavage products, which were observed only when the oligonucleotide was added.
Discussion
The recently discovered ability of nascent transcripts to directly sense effector molecules and respond by modulation of the RNA structure, thereby affecting the fate of the transcription complex, represents a novel mechanism for gene regulation in bacteria. Binding of uncharged tRNA and small molecules such as thiamine pyrophosphate, flavin mononucleotide, SAM, and guanine has been shown to control premature transcription termination (12, 15–20); it has been suggested that riboflavin and thiamine biosynthesis genes in Gram-negative bacteria are instead regulated at the level of translation initiation (17–19). In this study, we find that the B. subtilis lysC gene is regulated by direct interaction of lysine, the final product of the lysine biosynthesis pathway, with the lysC leader RNA. Conservation of leader sequence patterns suggests that multiple lysine biosynthesis genes in Gram-positive species are regulated by a similar mechanism, whereas lysC genes from Gram-negative organisms with these leader features appear to be translationally regulated. However, translational control has not yet been experimentally demonstrated for any of these systems. Regulation of the B. subtilis trp genes by TRAP trp RNA binding attenuation protein) also uses analogous leader RNA structural transitions to mediate regulation at either the transcriptional or translational level (30).
The L box leader pattern was uncovered by using phylogenetic comparisons based on conserved positioning of primary sequence elements and helical domains identified by covariation. The RNAs in this group exhibit very little primary sequence conservation; the largest conserved element is only 5 nt in length, and there is variable spacing between conserved sequence elements. The S box leaders, which also recognize a small molecule (SAM), exhibit a structural arrangement similar to that of the L box leaders, with four helices emanating from a core region (14, 15). A similar terminator/antiterminator/anti-antiterminator arrangement is found in both systems, and mutations in the conserved elements cause similar effects. The arrangement of the GA motif in helix 2 is also very similar in the two classes of RNAs. In contrast to the L box pattern, the S box leaders exhibit high primary sequence conservation within the helix 1–4 region, corresponding to the helix 1–5 region of L box leaders. One important difference between these systems is that the S box pattern is often found in many copies per genome (e.g., 11 in B. subtilis), whereas the L box pattern is found less frequently, with only one copy in most organisms. The mechanism of regulation of lysine biosynthesis genes that do not contain L box leaders is unknown. The overall structural similarity of the S box and L box sensory RNAs is intriguing, perhaps reflecting their common role in regulation of amino acid biosynthesis. Other metabolite binding RNAs exhibit varying levels of complexity, with the guanine binding determinant apparently the simplest (20). In both the S box and L box RNAs, unpaired residues in the helix junction region are highly conserved; it is likely that these residues play a crucial role in effector recognition and binding.
The B. subtilis lysC leader was shown to respond specifically to lysine, and not to DAP or SAM; AEC was 10-fold less effective than lysine in promoting transcription termination. Discrimination against DAP, which differs from lysine by an extra carboxyl group (Fig. 1B), is important in appropriate regulation of the lysA gene, the product of which converts DAP to lysine. It appears that the other L box genes, which encode products responsible for earlier steps in the pathway, also monitor lysine. In organisms that use DAP or lysine as a cell wall component, it is essential that this pathway be sufficiently active to provide adequate levels for both cell wall biosynthesis and protein synthesis. The intracellular pool of free lysine in B. subtilis has been reported at 2 mM during growth in the absence of exogenous lysine, conditions under which lysine biosynthesis genes are expressed (31). The requirement for 3 mM lysine for efficient transcription termination in vitro is therefore within a physiologically relevant range. The S box genes respond to much lower concentrations of SAM (≈10 μM for efficient termination in vitro), consistent with the lower intracellular pools of SAM (15). The differential sensitivity of these regulatory RNAs suggests that each system is calibrated to sense the in vivo concentration of the effector molecule.
In contrast to DAP, AEC is not a normal cellular component. AEC differs from lysine by substitution of a single carbon with sulfur (Fig. 1B). Resistance to AEC in B. subtilis and E. coli can result from derepression of lysC expression; the identification of the L box pattern provides an explanation for the effect of previously characterized AECR mutations (refs. 8–10 and H. Paulus, personal communication). The increase in aspartokinase II activity observed in the mutants is apparently sufficient for accumulation of higher lysine pools, which reduce misincorporation of AEC. An alternate possibility is that AEC acts as a mimic of lysine, repressing L box gene expression, so that resistance occurs by loss of repression by AEC. However, the requirement for 10-fold higher concentrations of AEC than lysine for efficient lysC transcription termination in vitro suggests that AEC is unlikely to cause lysC repression in vivo.
It is remarkable that in each regulatory system that uses leader RNAs to directly monitor an effector molecule there is very high specificity for the cognate effector. For example, The T box leader RNAs selectively recognize only the cognate tRNA and further discriminate between uncharged and charged tRNA. The S box RNAs respond to SAM, but not S-adenosylhomocysteine. The L box RNAs are specific for lysine, and not DAP. This specificity is an inherent requirement for biological function of these sensory RNAs in monitoring physiological signals within the complex environment of the cell. It will be of great interest to compare the features of each group of RNAs required for recognition of the cognate effector and discrimination against related molecules.
Supplementary Material
Acknowledgments
We thank H. Paulus for providing unpublished data on AECR mutations and lysC expression and F. M. Hulett for providing the strain for production of B. subtilis RNAP. This work was supported by National Institutes of Health Grant GM63615.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RNAP, RNA polymerase; DAP, diaminopimelate; AEC, aminoethylcysteine; SAM, S-adenosylmethionine.
References
- 1.Belitsky, B. R. (2002) in Bacillus subtilis and Its Closest Relatives: From Genes to Cells, eds. Sonenshein, A. L., Hoch, J. A. & Losick, R. (Am. Soc. Microbiol., Washington, DC), pp. 203–231.
- 2.Paulus, H. (1993) in Bacillus subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology, and Molecular Genetics, eds. Sonenshein, A. L., Hoch, J. A. & Losick, R. (Am. Soc. Microbiol., Washington, DC), pp. 237–267.
- 3.Patte, J.-C. (1996) in Escherichia coli and Salmonella: Cellular and Molecular Biology, eds. Neidhardt, F. C., Curtis, R., III, Ingraham, J. L., Lin, E. C. C., Low, K. B., Magasanik, B., Reznikoff, W. S., Riley, M., Schaechter, M. & Umbarger, H. E. (Am. Soc. Microbiol., Washington, DC), pp. 528–541.
- 4.Stragier, P., Richaud, F., Borne, F. & Patte, J.-C. (1983) J. Mol. Biol. 168, 307–320. [DOI] [PubMed] [Google Scholar]
- 5.Rosner, A. (1975) J. Bacteriol. 121, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochhar, S. & Paulus, H. (1996) Microbiology 142, 1635–1639. [DOI] [PubMed] [Google Scholar]
- 7.Schendel, F. J. & Flickinger, M. C. (1992) Appl. Environ. Microbiol. 58, 2806–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patte, J.-C., Akrim, M. & Mejean, V. (1998) FEMS Microbiol. Lett. 169, 165–170. [DOI] [PubMed] [Google Scholar]
- 9.Lu, Y., Chen, N. Y. & Paulus, H. (1991) J. Gen. Microbiol. 137, 1135–1143. [DOI] [PubMed] [Google Scholar]
- 10.Lu, Y., Shevtchenko, T. N. & Paulus, H. (1992) FEMS Microbiol. Lett. 92, 23–28. [DOI] [PubMed] [Google Scholar]
- 11.Grundy, F. J. & Henkin, T. M. (1993) Cell 74, 475–482. [DOI] [PubMed] [Google Scholar]
- 12.Grundy, F. J., Winkler, W. C. & Henkin, T. M. (2002) Proc. Natl. Acad. Sci. USA 99, 11121–11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy, F. J. & Henkin, T. M. (2003) Front. Biosci. 8, d20–d31. [DOI] [PubMed] [Google Scholar]
- 14.Grundy, F. J. & Henkin, T. M. (1998) Mol. Microbiol. 30, 737–749. [DOI] [PubMed] [Google Scholar]
- 15.McDaniel, B. A. M., Grundy, F. J., Artsimovitch, I. & Henkin, T. M. (2003) Proc. Natl. Acad. Sci. USA 100, 3083–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epshtein, V., Mironov, A. S. & Nudler, E. (2003) Proc. Natl. Acad. Sci. USA 100, 5052–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler, W., Nahvi, W. & Breaker, R. R. (2002) Nature 419, 952–956. [DOI] [PubMed] [Google Scholar]
- 18.Winkler, W. C., Cohen-Chalamish, S. & Breaker, R. R. (2002) Proc. Natl. Acad. Sci. USA 99, 15908–15913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mironov, A. S., Gusarov, I., Rafikov, R., Lopez, L. E., Shatalin, K., Kreneva, R. A., Perumov, D. A. & Nudler, E. (2002) Cell 111, 747–756. [DOI] [PubMed] [Google Scholar]
- 20.Mandal, M., Boese, B., Barrick, J. E., Winkler, W. C. & Breaker, R. R. (2003) Cell 113, 577–586. [DOI] [PubMed] [Google Scholar]
- 21.Henkin, T. M. & Yanofsky, C. (2002) BioEssays 24, 700–707. [DOI] [PubMed] [Google Scholar]
- 22.Grundy, F. J., Waters, D. A., Allen, S. H. G. & Henkin, T. M. (1993) J. Bacteriol. 175, 7348–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anagnostopoulos, C. & Spizizen, J. (1961) J. Bacteriol. 81, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. H. (1972) Experiments in Molecular Genetics (Cold Spring Harbor Lab. Press, Plainview, NY).
- 25.Qi, Y. & Hulett, F. M. (1998) Mol. Microbiol. 28, 1187–1197. [DOI] [PubMed] [Google Scholar]
- 26.Grundy, F. J., Moir, T. R., Haldeman, M. T. & Henkin, T. M. (2002) Nucleic Acids Res. 30, 1646–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leontis, N. B. & Westhof, E. (1998) J. Mol. Biol. 283, 571–583. [DOI] [PubMed] [Google Scholar]
- 28.Winkler, W. C., Grundy, F. J., Murphy, B. A. & Henkin, T. M. (2001) RNA 7, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein, D. J., Schmeing, T. M., Moore, P. B. & Steitz, T. A. (2001) EMBO 20, 4214–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du, H., Tarpey, R. & Babitzke, P. (1997) J. Bacteriol. 179, 2582–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tempest, D. W., Meers, J. L. & Brown, C. M. (1970) J. Gen. Microbiol. 64, 171–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.