Abstract
The SNT309 gene was identified via a mutation that causes lethality of cells in combination with a prp19 mutation. We showed previously that Snt309p is a component of the Prp19p-associated complex and that Snt309p, like Prp19p, is associated with the spliceosome immediately after or concomitantly with dissociation of U4 from the spliceosome. We show here that extracts prepared from the SNT309-deleted strain (ΔSNT309) were defective in splicing but could be complemented by addition of the purified Prp19p-associated complex. Isolation of the Prp19p-associated complex from ΔSNT309 extracts indicated that the complex was destabilized in the absence of Snt309p and dissociated on affinity chromatography, suggesting a role of Snt309p in stabilization of the Prp19p-associated complex. Addition of the affinity-purified Prp19p–Snt309p binary complex to ΔSNT309 extracts could reconstitute the Prp19p-associated complex. Genetic analysis further suggests that Snt309p plays a role in modulating interactions of Prp19p with other associated components to facilitate formation of the Prp19p-associated complex. A model for how Snt309p modulates such interactions is proposed.
Splicing of pre-mRNA takes place on a multicomponent ribonucleoprotein particle called the spliceosome, which consists of five small nuclear RNAs (snRNAs) and a number of protein factors (see refs. 1–8 for reviews). Numerous protein factors involved in the splicing reaction have been identified in mammals and yeast. Some are intrinsic components of the spliceosome (9–11), whereas others associate with the spliceosome only transiently (12–14). Many of the protein factors are components of the sn ribonucleoprotein particles (see refs. 1 and 15–18 for reviews).
Yeast genetics provides a powerful tool for identification of protein factors involved in the splicing reaction, as well as for studying interactions between these components. A large number of PRP (precursor RNA processing) genes that encode protein splicing factors have been identified by screening temperature-sensitive mutants defective in pre-mRNA splicing (19, 20). Other genes were identified through genetic interactions with introns, PRP genes, or snRNA genes (21–27). Over 40 genes that encode protein factors of the splicing machinery have been identified, but the precise functions of these proteins are not well understood.
The yeast PRP19 gene was among the genes identified in a screen for temperature-sensitive mutants defective in splicing (20). Biochemical characterizations indicated that the Prp19p protein is essential for the pre-mRNA splicing reaction in vitro. The protein is not tightly associated with snRNAs, but is associated with the spliceosome during the splicing reaction (11, 28). Prp19p seems to associate with the spliceosome concomitantly with or just after dissociation of U4 from the spliceosome (14, 28), suggesting a possible role for mediating conformational rearrangement at this step of the spliceosome assembly process. Attempts to purify the Prp19p protein for functional studies led to the identification of a protein complex consisting of at least seven proteins associated with Prp19p (29). Among them, at least three can directly interact with Prp19p as analyzed by Far Western blotting (29).
The SNT309 gene, which encodes a component of the Prp19p-associated complex, was identified in a genetic screen for mutations that exacerbate the phenotype of prp19 mutations (27). Cells disrupted in the SNT309 gene, although viable, are temperature-sensitive and accumulated pre-mRNA at the nonpermissive temperature. Snt309p can directly interact with Prp19p but not with any other component in the complex. It was shown further that Snt309p is associated with the spliceosome at the same time as Prp19p (27). In this report, we show that in the absence of Snt309p, the Prp19p-associated complex is destabilized and cannot function properly in the splicing reaction. Snt309p seems to play a role in modulating interactions of Prp19p with other components of the Prp19p-associated complex. A model for how Snt309p modulates such interactions is proposed.
MATERIALS AND METHODS
Strains and Plasmids.
The following strains were used: BJ2168 (MATa prc1 prb1 pep4 leu2 trp1 ura3), YSCC1 (MATa prc1 prb1 pep4 leu2 trp1 ura3 PRP19-HA), YSCC20 (MATa prc1 prb1 pep4 leu2 trp1 ura3 NTC20-HA), YHR13 (MATa prc1 prb1 pep4 leu2 trp1 ura3 PRP19-HA ΔSNT309∷LEU2), and YHR203 (MATa prc1 prb1 pep4 leu2 trp1 ura3 NTC20-HA ΔSNT309∷ LEU2).
The following plasmids were used: pBS.SNT309 (4.0-kb fragment of the SNT309 gene in pBS), pG1.SNT309 [a 824-bp BamHI fragment isolated from pGEM.SNT309 (27) was inserted into the BamHI site of pG1], and pET.SNT309 (a 824-bp BamHI fragment isolated from pGEM.SNT309 was inserted into pET15b).
Construction of the SNT309-Deleted, PRP19 or NTC20 Epitope-Tagged Strain.
The SNT309-deleted, PRP19 or NTC20 epitope-tagged strain was constructed by one-step gene displacement of strains YSCC1 and YSCC20, which are derivatives of strain BJ2168 in that the Prp19p or Ntc20p protein was tagged with the HA-epitope at its carboxyl terminus. The snt309∷LEU2 allele was created by reverse PCR of plasmid pBS.SNT309 with primers flanking the ORF of the SNT309 gene. The PCR product was digested with NruI and ligated with a 2-kb LEU2 DNA fragment. The resulting plasmid was digested with PvuII before transformation into strains YSCC1 and YSCC20. Correct integration was confirmed by Southern blotting. The resulting yeast strains are YHR13 and YHR203.
Preparation of Splicing Extracts and Isolation of the Prp19p-Associated Complex, the Prp19p–Snt309p Binary Complex, and His–Snt309p.
Splicing extracts and the 40% saturated ammonium sulfate fraction of the splicing extract (40P) were prepared according to the method described by Cheng et al. (30). The Prp19p-associated complex was isolated by affinity chromatography of the 40P fraction on an anti-HA antibody column according to the method described by Tarn et al. (14). To isolate the Prp19p–Snt309p binary complex, SNT309 was subcloned into pG1, and PRP19HA was placed under the GPD-promoter control in pRS426. The recombinant plasmids were cotransformed into strain YSCC1. Whole-cell extracts prepared from such a strain were subjected to affinity chromatography on an anti-HA antibody column to isolate the Prp19p–Snt309p binary complex. For each 1 ml of the whole-cell extract, 0.5 ml of the anti-HA antibody column was used. His–Snt309p was isolated from Escherichia coli and purified through a His-Bind column (Novagen) under denaturing conditions according to the manufacturer’s manual.
Splicing Reactions and Complementation Assays.
The splicing reaction was carried out according to the method described by Lin et al. (31). Complementation of the ΔSNT309 extract was performed as in standard splicing reactions with 40% ΔSNT309 extracts and 10–30% complementing protein fractions in different dilutions in buffer D [20 mM Hepes-K+, pH 7.9/0.2 mM EDTA/50 mM NaCl/20% (vol/vol) glycerol].
Far Western Blot Analysis.
The procedure for Far Western blot analysis was modified from that described by Tarn et al. (29), and in vitro phosphorylated Prp19p was used as a probe. The Prp19p protein was fused with an oligo-peptide (RREILSRRPSYRKD) of the protein kinase A site followed by the HA-epitope at its carboxyl terminus. The fusion protein was expressed in yeast under the control of GPD promoter and partially purified by affinity chromatography on an anti-HA antibody-coupled Sepharose column for in vitro phosphorylation. Phosphorylation by cAMP-dependent kinase was completed as described by Ron and Habener (32).
RESULTS
The Prp19p-Associated Complex Was Defective in ΔSNT309 Cells.
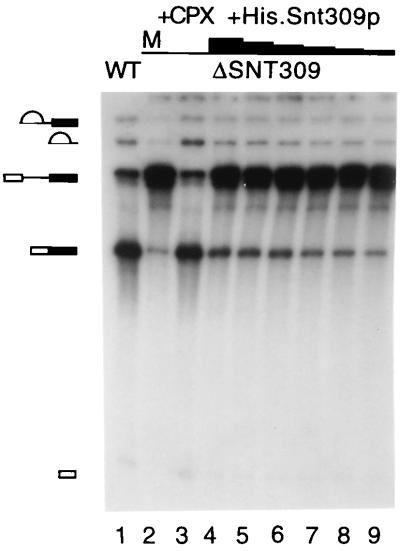
We showed previously that yeast cells disrupted in the SNT309 gene are temperature-sensitive in growth (27). Because Snt309p is a component of the Prp19p-associated complex, it is possible that the growth defect of ΔSNT309 cells reflects malfunction of the Prp19p-associated complex. This possibility was examined by in vitro analysis of the splicing reaction. Splicing extracts were prepared from ΔSNT309 cells grown at 30°C. These extracts consistently gave poor splicing activities (Fig. 1, lane 2, and data not shown) compared with wild-type extracts (Fig. 1, lane 1). However, the splicing activity was quantitatively restored on addition of the affinity-purified Prp19p-associated complex (Fig. 1, lane 3), indicating that ΔSNT309 extracts were defective in the function of the Prp19p-associated complex. This result suggests that the Prp19p-associated complex did not function normally in the absence of the Snt309p protein.
Figure 1.
The splicing activity of the ΔSNT309 extract was complemented by the Prp19p-associated complex and partially complemented by recombinant Snt309p. The splicing reactions were carried out in the wild-type (WT) extract (lane 1) and in ΔSNT309 extracts (lanes 2–9) with the addition of the Prp19p-associated complex (lane 3) or of His.Snt309p (lanes 4–9). The amounts of His.Snt309p added are, for lane 4, 150 ng; for lane 5, 50 ng; for lane 6, 15 ng; for lane 7, 5 ng; for lane 8, 3 ng; and for lane 9, 1.5 ng. M, mock-treated; +CPX, the Prp19p-associated complex added.
When the recombinant Snt309p protein was added to the ΔSNT309 extract, the splicing activity increased only slightly over a wide range of protein concentrations (from 15 μg/ml to 0.15 μg/ml in lanes 4–9). This result indicates that in vitro reconstitution of the Prp19p-associated complex in ΔSNT309 extracts was feasible but inefficient with recombinant Snt309p. A possible explanation for this low efficiency was that the recombinant protein was not folded or modified properly to assume full activity.
The Prp19p-Associated Complex Was Destabilized in the Absence of the Snt309p Protein.
The results described above show that in the absence of Snt309p, the Prp19p-associated complex did not function properly in the splicing reaction. It was therefore important to examine the Prp19p-associated complex lacking Snt309p. To isolate such a complex, the entire ORF of SNT309 was removed from a strain in which the Prp19p protein was tagged with the HA-epitope at its carboxyl terminus (28). The Prp19p-associated complex was isolated from extracts prepared from this strain by affinity chromatography on an anti-HA antibody-coupled Sepharose column (29). Components in the complex were examined by Far Western blotting for proteins that directly interact with Prp19p, and by Western blotting for components whose antibodies were available (27, 37). The Prp19p-associated complex isolated from a control strain with intact SNT309 gene was used for comparison.
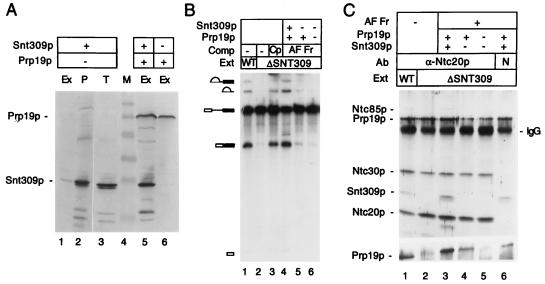
Fig. 2A shows that Snt309p was not detected in the ΔSNT309 complex as indicated on Far Western blots. Surprisingly, except for Prp19p, which directly bound to the antibody column, all the other Prp19p-interacting components in the complex likewise disappeared (Fig. 2A). Neither Ntc85p nor Ntc40p (Ntc represents nineteen complex) was detected in the complex. Western blot analysis with antibodies against Prp19p, Snt309p, and three other components of the Prp19p-associated complex, Ntc85p, Ntc30p and Ntc20p, confirmed that the amounts of these associated proteins were greatly diminished in the complex isolated from ΔSNT309 extracts (Fig. 2B). This result indicates that in the absence of Snt309p, the Prp19p-associated complex was destabilized, and the associated components were dissociated from Prp19p. To see whether all the components of the Prp19p-associated complex became dissociated from each other or whether Prp19p simply was secluded from the complex in the absence of Snt309p, components associated with Ntc20p were examined by immunoprecipitation of Ntc20p. The Ntc20p HA-tagged extracts were used for immunoprecipitation with the anti-HA antibody to distinguish between these possibilities. Fig. 2C shows that, although neither Ntc85p nor Prp19p was associated with Ntc20p in ΔSNT309 extracts, Ntc30p remained tightly associated with Ntc20p, suggesting that Snt309p did not affect the association of Ntc20p and Ntc30p. In the absence of Snt309p, Ntc85p was not associated with either Prp19p or Ntc20p and was separated independently. These results indicate that the Prp19p-associated complex was unstable in the absence of Snt309p and could dissociate into at least three parts, one being or containing Prp19p, one being or containing Ntc85p, and another containing Ntc20p and Ntc30p.
Figure 2.
(A) Far Western blotting of the Prp19p-associated complex isolated from the wild-type (WT) and ΔSNT309 extracts by using 32P-labeled Prp19p as a probe. Prp19p was tagged with the HA-epitope and the complex was isolated by chromatography on an anti-HA antibody-conjugated Sepharose column. (B) Western blotting of the Prp19p-associated complex isolated as in A by using antibodies against Ntc85p, Prp19p, Ntc30p, Snt309p, and Ntc20p. (C) Western blotting of the Prp19p-associated complex immunoprecipitated by the anti-HA antibody in wild-type and ΔSNT309 extracts, in which Ntc20p was tagged with the HA-epitope.
Reconstitution of the Prp19p-Associated Complex.
If Snt309p plays a role in stabilizing the Prp19p-associated complex, addition of the Snt309p protein to ΔSNT309 extracts may reconstitute the stable form of the complex and restore the splicing activity. Because the recombinant Snt309p protein did not complement the ΔSNT309 extract efficiently as shown above in Fig. 1, we tried to overproduce and purify Snt309p from yeast for complementation by placing the SNT309 gene under the control of the GPD promoter. Splicing extracts were prepared from a strain carrying the GPD–SNT309 plasmid. As shown in the Western blot in Fig. 3A, although the Snt309p protein was indeed overproduced as seen in total cell lysates (Fig. 3, lane 3), Snt309p was not present at higher levels in the splicing extract (Fig. 3, lane 1). In fact, the majority of the Snt309p protein was found in the pellet of cell debris during preparation of extracts (lane 2). However, when both SNT309 and PRP19 were overexpressed under GPD-promoter control, Snt309p became soluble and could be isolated in the splicing extract in larger amounts (lane 5). Overproduction of Prp19p alone also yielded a soluble form of Prp19p (lane 6).
Figure 3.
(A) Western blot analysis of splicing extracts prepared from strains overproducing Snt309p alone (lanes 1), both Snt309p and Prp19p (lane 5), or Prp19p alone (lane 6). Lane 2 is the cell pellet from the preparation of the splicing extract of lane 1. Lane 3 represents the total cell lysates from the strain overproducing Snt309p alone. Equivalent amounts of cell extracts were loaded on each lane. Ex, splicing extract; P, cell pellet; T, total lysates; M, molecular mass marker. (B) Complementation of the ΔSNT309 extract (Ext) by Prp19p–Snt309p binary complex. (Lane 1) Wild-type (WT) extract; (lanes 2–6) ΔSNT309 extracts. Fractions used for complementation (Comp): the Prp19p-associated complex (Cp; lane 3); affinity-purified fractions (AF Fr) overproducing Prp19p and Snt309p (lane 4), overproducing Prp19p only (lane 5), and overproducing no protein (lane 6). (C) Reconstitution of the Prp19p-associated complex. ΔSNT309 extracts (lanes 2–6) were added to an anti-HA antibody column-purified fraction from extracts overproducing both Snt309p and Prp19p (lanes 3 and 6), Prp19p only (lane 4), or normal extracts (lane 5), and then precipitated with the anti-Ntc20p antibody (lanes 1–5) or nonspecific (N) antibody (lane 6). Lane 1, wild-type extract.
Because Snt309p interacts strongly with Prp19p (27), it is believed that formation of the Prp19p–Snt309p binary complex prevented Snt309p from precipitating. The Prp19p–Snt309p binary complex then was isolated from an extract overproducing Snt309p and HA-tagged Prp19p by affinity chromatography on the anti-HA antibody column. As shown in Fig. 3B, the purified Prp19p–Snt309p binary complex efficiently restored the splicing activity of the ΔSNT309 extract (lane 4). Identically purified fractions using extracts that did not overproduce Prp19p or Snt309p but contained wild-type levels of HA-tagged Prp19p did not restore the splicing activity of the ΔSNT309 extract (lane 6). This result indicates that complementation by the affinity-purified Prp19p–Snt309p binary complex was not caused by residual amounts of the intact Prp19p-associated complex copurified in these fractions. When Prp19p was overproduced alone, the identically purified fractions did not significantly increase the splicing activity of ΔSNT309 extracts (lane 5), suggesting that complementation by Prp19p–Snt309p binary complex was not caused by an elevating level of Prp19p. These results indicate that the Prp19p–Snt309p binary complex was sufficient to complement splicing deficiency of ΔSNT309 extracts.
To see whether complementation by the Prp19p–Snt309p binary complex indeed reflected reconstitution of the Prp19p-associated complex, components associated with Ntc20p were examined by immunoprecipitation. The Prp19p–Snt309p binary complex was added to the ΔSNT309 extract, and the mixtures were precipitated with the anti-Ntc20p antibody. The anti-HA antibody could not be used in this experiment, because the purified Prp19p-Snt309p binary complex contained the HA-peptide from the purification. It was shown in Fig. 2C that Ntc20p was associated with Prp19p only in the presence of Snt309p. In its absence, Ntc20p was dissociated from Prp19p but remained associated with Ntc30p. Under this condition, Ntc85p was not tightly associated with either Prp19p or Ntc20p. Fig. 3C shows that when the affinity-purified Prp19p–Snt309p binary complex was added to the ΔSNT309 extract, Ntc85p, Snt309p, and Prp19p became associated with Ntc20p and Ntc30p (Fig. 3C, compare lane 2 and lane 3). The situation of Prp19p was not clear in the upper blot from the 12.5% gel because of high background at the position of Prp19p but was clear on that from a 7.5% gel (Fig. 3C, Lower). When the affinity-purified fraction was from the extract overproducing only Prp19p (Fig. 3C, lane 4) or from a normal extract (Fig. 3C, lane 5), no association of Ntc85p was observed. In this experiment, a fraction of Snt309p and Prp19p was found in the pellet when a nonspecific antibody was used for precipitation (lane 6), suggesting that part of Prp19p and Snt309p precipitated by the anti-Ntc20p antibody was caused by nonspecific binding of the binary complex to the resin. Nevertheless, Ntc85p was coprecipitated specifically by the anti-Ntc20p antibody. This result indicates that the Prp19p-associated complex in ΔSNT309 extracts was indeed reconstituted on addition of the Prp19p–Snt309p binary complex.
Growth Dependence of ΔSNT309 Cells on the Amount of Prp19p.
The fact that the Prp19p-associated complex was unstable in the absence of Snt309p may reflect the possibility that formation of the functional complex was unfavorable without Snt309p. Thermodynamically, elevating the level of Prp19p may increase the amount of complex formation under this condition. This increase was not seen in our in vitro complementation assay in which affinity-purified Prp19p did not rescue the splicing activity of ΔSNT309 extracts (Fig. 3C, lane 4). We then tested such dosage compensation in vivo for complementation of the growth defect of ΔSNT309 cells. As shown in Fig. 4, deletion of the entire ORF of SNT309 (ΔSNT309) gave a temperature-sensitive phenotype. ΔSNT309 cells grew well at 25°C, less well at 30°C, and poorly at 33°C or above but grew well at all temperatures tested when carrying SNT309-containing plasmid either on a CEN- or 2μ-based vector (data not shown). When ΔSNT309 cells were transformed with a PRP19-containing plasmid, either on CEN- or 2μ-based vector, transformants grew better at 33°C (Fig. 4A). This result indicates that overexpression of PRP19 could partially rescue the growth defect of ΔSNT309 cells, possibly by facilitating formation of the Prp19p-associated complex.
Figure 4.
Spot assay of ΔSNT309 cells. ΔSNT309 or wild-type cells carrying different plasmids were grown until A600 reached ≈0.5 and then diluted in series by one order. From each diluted culture, 5 μl was spotted on plates. (A) ΔSNT309 cells were temperature-sensitive in growth (−/−) but grew better when carrying a PRP19-containing plasmid either on 2μ- or centromere-based vector (2μ/− and CEN/−). ΔSNT309 cells carrying a 2μ-based SNT309-containing plasmid grew well in the presence of another plasmid carrying PRP19 on CEN or 2μ plasmid, or under the GPD promoter control (CEN/2μ, 2μ/2μ, and GPD/2μ). (B) Wild-type strains carrying PRP19 on CEN or 2μ plasmid, or under the GPD promoter control (CEN, 2μ, and GPD) grew well at all temperatures. (C) ΔSNT309 cells carrying GPD–PRP19 on TRP1 plasmid and the SNT309 gene on URA3 plasmid grew less well by 1–2 orders on 5-fluoroorotic acid plates than when the PRP19 gene was on a 2μ- or CEN-based plasmid. Note that 0, −1, −2, −3, and −4 indicate no, 10−1, 10−2, 10−3, and 10−4 dilutions, respectively.
It was noticed that, on the 2μ plasmid, PRP19 supported growth less well than it did on the CEN plasmid in ΔSNT309 cells (Fig. 4A). This observation suggests that overexpression of PRP19 could partially complement deficiency of ΔSNT309 cells but possibly only in moderate amounts of Prp19p. Overexpression of PRP19 at high levels may deteriorate the growth of ΔSNT309 cells. Indeed, transformation into ΔSNT309 cells of plasmids containing the PRP19 gene under the constitutive strong GPD-promoter control (GPD–PRP19) consistently yielded no transformants unless an SNT309-containing plasmid was cotransformed (data not shown). The same GPD–PRP19 plasmid transformed the wild-type strain normally, and the transformants grew normally (Fig. 4B). Moreover, ΔSNT309 cells containing both GPD–PRP19 and SNT309 on a 2μ plasmid grew normally at all temperatures (Fig. 4A).
To verify that failure to transform ΔSNT309 cells with GPD–PRP19 plasmids was due to lethality caused by overproduction of Prp19p in cells lacking Snt309p, ΔSNT309 cells carrying both the GPD–PRP19 plasmid and SNT309 on a 2μ-based URA3 plasmid (pRS426) were spotted on yeast extract/peptone/dextrose or 5-fluoroorotic acid plates in series dilutions and grown at 25°C. As shown in Fig. 4C, although all cells grew well on yeast extract/peptone/dextrose plates, cells carrying GPD–PRP19 grew less well by 1–2 orders compared with those carrying PRP19 on a CEN- or 2μ-based plasmid on 5-fluoroorotic acid plates, indicating that loss of the SNT309-containing plasmid is unfavorable in ΔSNT309 cells carrying the GPD–PRP19 plasmid. This result confirms that overproduction of Prp19p in large amounts was detrimental to cells lacking Snt309p, which is in contrast to the wild-type strain that is able to overproduce Prp19p ≈500-fold when carrying GPD–PRP19 (data not shown). Thus, the amount of Prp19p is critical for growth of ΔSNT309 cells.
A Model for Modulation of Interaction of Prp19p with Other Components in the Prp19p-Associated Complex by Snt309p.
Our results identify an important function of Snt309p in stabilizing a protein complex essential for the pre-mRNA splicing reaction. Although SNT309 is not required for vegetative yeast growth, deletion of the SNT309 gene impaired growth and pre-mRNA splicing at higher temperatures. The Prp19p-associated complex was destabilized in the absence of Snt309p. Overproduction of Prp19p at low levels partially rescued the growth defect of the SNT309-deleted cells. Overproduction at high levels, on the contrary, was detrimental to SNT309-deleted cells.
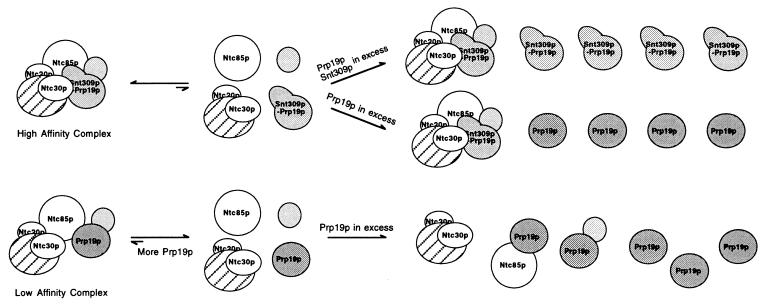
Fig. 5 shows a proposed model for the role of Snt309p in the Prp19p-associated complex. In wild-type cells, the Snt309p protein can interact strongly with Prp19p. This interaction facilitates the interaction of Prp19p with other components of the Prp19p-associated complex to form a stable “high affinity” complex. In the absence of Snt309p, interactions of Prp19p with other components are weakened, and the Prp19p-associated complex formed is less stable as a “low affinity” complex, which is destabilized further at higher temperatures. Increasing the amount of the Prp19p protein may increase the amount of the Prp19p-associated complex, as the equilibrium favors formation of the complex. Further increase of the Prp19p protein may titrate out individual interacting components, because Prp19p can interact with several proteins in the complex individually (29). Such a titration effect was not seen in the presence of Snt309p, because the Prp19p–Snt309p complex interacts with other components either more strongly or in a different fashion to facilitate formation of the intact Prp19p-associated complex. Two lines of evidence argue against enhancement of individual interactions between Prp19p and its associated components by Snt309p. First, neither a two-hybrid assay nor Far Western blotting detected enhancing interaction of Prp19p with other components in the presence of Snt309p (data not shown). Second, cells overproducing high levels of both Prp19p and Snt309p grew normally as shown in Figs. 3B and 4A. If the Prp19p–Snt309p complex interacts with individual components of the Prp19p-associated complex more strongly than Prp19p alone, overproducing both Snt309p and Prp19p should be detrimental to even wild-type cells for the same reason that overproducing Prp19p alone in ΔSNT309 cells because of out-titration of individual interacting components is detrimental. Thus, formation of the stable form of the Prp19p-associated complex may be caused by different modes of interactions of the Prp19p–Snt309p complex with other associated components. The Prp19p–Snt309p complex may interact first with one of the associated components, likely Ntc85p or Ntc40p, which then triggers a conformational change of either Prp19p or its interacting counterpart to facilitate binding of other associated components to form the intact Prp19p-associated complex. This model also suggests that the mode of interaction between Prp19p and its associated components is different in the presence or absence of Snt309p. The Snt309p protein therefore plays an important role in modulating interactions of Prp19p with other components of the Prp19p-associated complex to stabilize this complex, which is essential for the splicing reaction.
Figure 5.
A proposed model for how Snt309p modulates interactions of Prp19p with other components in the Prp19p-associated complex.
DISCUSSION
The SNT309 gene was identified via a mutation that showed a synergistic effect to a prp19 mutation. Biochemical characterizations indicate that Snt309p is Ntc25p of the Prp19p-associated complex and interacts directly with Prp19p. Furthermore, Snt309p associates with the spliceosome in the same manner as Prp19p during spliceosome assembly (27). These results suggest that Snt309p and Prp19p may function in a cooperative fashion during the splicing reaction.
Although the SNT309 gene is not essential for vegetative yeast growth, yeast cells deleted of the SNT309 gene were temperature-sensitive. In vitro analysis indicated that the Prp19p-associated complex was unstable in the absence of Snt309p and could not function properly in the splicing reaction. This result may explain the in vivo temperature-sensitive phenotype of ΔSNT309 cells. In the absence of Snt309p, the Prp19p-associated complex may still maintain its function fully or partially at low temperatures to sustain cellular growth albeit in a less stable form. At higher temperatures, the complex was destabilized further and completely lost its function, resulting in cell lethality.
Many characterized yeast splicing factors, including Prp17p, Prp18p, Mud1p, Mud2p, Mud13p, and Snp1p, also have been shown to be encoded by nonessential genes (21, 25, 33–36). Like SNT309, disruption of PRP17 or PRP18 also yields a temperature-sensitive phenotype. Although both Prp17p and Prp18p are shown to be involved in the second step of the splicing reaction (20) and to interact with U5 snRNA (24, 35), the functional roles of these proteins in the splicing reaction are not known. Our in vitro analysis indicated that Snt309p probably did not function directly in the splicing reaction, as the anti-Snt309p antibody did not inhibit splicing (data not shown). Instead, Snt309p played a role in stabilizing the Prp19p-associated complex, which contains at least two essential splicing factors, including Prp19p and Ntc85p (37), and likely functions as an integral complex. Thus, Snt309p plays an indirect but important role in the splicing reaction by stabilizing a protein complex essential for the reaction. This result provides an example of how auxiliary protein splicing factors may be involved in the splicing reaction. This result also further affirms that maintaining the integrity of the Prp19p-associated complex is important for splicing.
Snt309p seems to stabilize the Prp19p-associated complex through interacting with Prp19p. It is not likely that stabilization is caused by enhancing individual interactions between Prp19p and Ntc85p or Ntc40p in the presence of Snt309, because excessive amounts of the Prp19p–Snt309p binary complex did not out-compete individual interacting components to prevent formation of the integral Prp19p-associated complex. A favorable explanation for stabilization of the Prp19p-associated complex by Snt309p is that, on binding of Snt309p, Prp19p can interact with its associated components in a different fashion to facilitate formation of a stable complex. Under this condition, excessive amounts of Prp19p alone or of both Snt309p and Prp19p do not interfere with formation of the Prp19p-associated complex. This result is in contrast to the situation in ΔSNT309 cells in which overproducing Prp19p is detrimental presumably because individual Prp19p-interacting components are sequestered, preventing formation of the integral Prp19p-associated complex. Therefore, it is more likely that the mode of interaction of Prp19p with other components is regulated by Snt309p in such a way that Prp19p may interact cooperatively with other components to form a stable complex on binding of Snt309p.
When Snt309p was overproduced alone, the majority of the protein was fractionated with cell pellets during preparation of the splicing extract. When Snt309p was overproduced together with Prp19p, the protein became soluble in the extract, presumably as a form of the binary complex. The Prp19p protein, in contrast, was soluble in the cell extract either alone or in complex with Snt309p. The reason why free Snt309p was insoluble in the preparation of cell extract is not clear. One possibility is that Snt309p is prone to self-interaction to form large aggregates when not in complex with Prp19p, although self-interaction of Snt309p was not detected by Far Western blotting or by two-hybrid assays. Alternatively, Snt309p may be characteristic of binding to other cellular components in a nonspecific manner. Indeed, Snt309p was found to bind to protein A–Sepharose and IgG and to give high backgrounds during immunoprecipitation (Fig. 3C). Regardless, high levels of Snt309p were no harm to yeast cells, judging from the fact that they grew normally.
By analyzing interactions between the Prp19p-associated components in ΔSNT309 extracts, a preliminary outline of the architecture of the complex could be viewed. In the absence of Snt309p, the Prp19p-associated complex dissociated easily into three parts among the components identified. Prp19p did not seem to be associated with any other components, because affinity purification of the complex on the anti-HA antibody column using Prp19p HA-tagged extracts isolated essentially only Prp19p. Ntc20p and Ntc30p remained tightly associated with each other, for the anti-Ntc20p antibody precipitated both Ntc20p and Ntc30p but not Prp19p or Ntc85p. Because direct interaction between Ntc20p and Ntc30p was detected neither by Far Western blotting nor by two-hybrid assays (C.-H.C., W.-Y.T., and S.-C.C., unpublished data), it is believed that such interaction is mediated by some other components. Like Prp19p, Ntc85p was not tightly associated with Prp19p or the Ntc20p/Ntc30p complex in the absence of Snt309p. Interestingly, Ntc85p was found to interact directly with Ntc30p and Ntc20p, as determined by two-hybrid assays (C.-H.C., W.-Y.T., and S.-C.C., unpublished data), and with Prp19p, as determined by both Far Western blotting (Fig. 2A) and two-hybrid assays (C.-H.C., W.-Y.T., and S.-C.C., unpublished data). It is thus possible that Ntc85p might play a role in mediating association of Ntc20p/Ntc30p with Prp19p. Identification of other components of the complex is necessary for a complete view of the architecture of this complex.
Acknowledgments
We thank C. Wang and M.-Y. Cheng for critical readings of the manuscript. This work was supported by a grant from the Academia Sinica and by National Science Council (Taiwan) Grant NSC88-2312-B-001-014.
ABBREVIATIONS
- snRNA
small nuclear RNA
- 40P
40% saturated ammonium sulfate fraction of the splicing extract
- CEN
centromere
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Beggs J D. Mol Biol Rep. 1993;18:99–103. doi: 10.1007/BF00986763. [DOI] [PubMed] [Google Scholar]
- 2.Guthrie C. Science. 1991;253:157–163. doi: 10.1126/science.1853200. [DOI] [PubMed] [Google Scholar]
- 3.Maniatis T, Reed R. Nature (London) 1987;325:673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- 4.Moore M J, Query C C, Sharp P A. In: The RNA World. Gesteland R F, Atkins J F, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 303–357. [Google Scholar]
- 5.Rymond B C, Rosbash M. In: The Molecular and Cellular Biology of the Yeast Saccharomyces. Jones E W, Pringle J R, Broach J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. pp. 143–192. [Google Scholar]
- 6.Sharp P A. Cell. 1994;77:805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 7.Staley J P, Guthrie C. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 8.Steitz J A, Black D L, Gerke V, Parker K A, Krämer A, Frendewey D, Keller W. In: Structure and Function of Major and Minor SNURPS. Birnsteil M, editor. New York: Springer; 1988. pp. 115–154. [Google Scholar]
- 9.Abovich N, Legrain P, Rosbash M. Mol Cell Biol. 1990;10:6417–6425. doi: 10.1128/mcb.10.12.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang T-H, Clark M W, Lustig A J, Cusick M E, Abelson J. Mol Cell Biol. 1988;8:2379–2393. doi: 10.1128/mcb.8.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng S-C, Tarn W-Y, Tsao T Y, Abelson J. Mol Cell Biol. 1993;13:1876–1882. doi: 10.1128/mcb.13.3.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin R-J, Lustig A J, Abelson J. Genes Dev. 1987;1:7–18. doi: 10.1101/gad.1.1.7. [DOI] [PubMed] [Google Scholar]
- 13.Schwer B, Guthrie C. Nature (London) 1991;349:494–499. doi: 10.1038/349494a0. [DOI] [PubMed] [Google Scholar]
- 14.Tarn W-Y, Lee K-R, Cheng S-C. Proc Natl Acad Sci USA. 1993;90:10821–10825. doi: 10.1073/pnas.90.22.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beggs J D. In: Pre-mRNA Processing. Lamond A I, editor. Austin, TX: Landes; 1995. pp. 79–95. [Google Scholar]
- 16.Will C L, Lührmann R. Curr Opin Biol. 1997;9:320–328. doi: 10.1016/s0955-0674(97)80003-8. [DOI] [PubMed] [Google Scholar]
- 17.Will C L, Lührmann R. In: Eukaryotic mRNA Processing. Krainer A R, editor. Oxford: IRL; 1997. pp. 130–173. [Google Scholar]
- 18.Krämer A. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- 19.Maddock J R, Roy J, Woolford J L., Jr Nucleic Acids Res. 1996;24:1037–1044. doi: 10.1093/nar/24.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayraghavan U, Company M, Abelson J. Genes Dev. 1989;3:1206–1216. doi: 10.1101/gad.3.8.1206. [DOI] [PubMed] [Google Scholar]
- 21.Abovich N, Liao X C, Rosbash M. Genes Dev. 1994;8:843–854. doi: 10.1101/gad.8.7.843. [DOI] [PubMed] [Google Scholar]
- 22.Chapon C, Legrain P. EMBO J. 1992;11:3279–3288. doi: 10.1002/j.1460-2075.1992.tb05406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couto G R, Tamm J, Parker R, Guthrie C. Genes Dev. 1987;1:445–455. doi: 10.1101/gad.1.5.445. [DOI] [PubMed] [Google Scholar]
- 24.Frank D, Patterson B, Guthrie C. Mol Cell Biol. 1992;12:5197–5205. doi: 10.1128/mcb.12.11.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao X C, Tang J, Rosbash M. Genes Dev. 1993;7:419–428. doi: 10.1101/gad.7.3.419. [DOI] [PubMed] [Google Scholar]
- 26.Wells S E, Neville M, Haynes M, Wang J, Igel H, Ares M., Jr Genes Dev. 1996;10:220–232. doi: 10.1101/gad.10.2.220. [DOI] [PubMed] [Google Scholar]
- 27.Chen H-R, Jan S-P, Tsao T Y, Sheu Y-J, Banroques J, Cheng S-C. Mol Cell Biol. 1998;18:2196–2204. doi: 10.1128/mcb.18.4.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarn W-Y, Lee K-R, Cheng S-C. Mol Cell Biol. 1993;13:1883–1891. doi: 10.1128/mcb.13.3.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarn W-Y, Hsu C-H, Huang K-T, Chen H-R, Kao H-Y, Lee K-R, Cheng S-C. EMBO J. 1994;13:2421–2431. doi: 10.1002/j.1460-2075.1994.tb06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng S-C, Newman A, Lin R-J, McFarland G D, Abelson J N. Methods Enzymol. 1990;181:89–96. doi: 10.1016/0076-6879(90)81114-a. [DOI] [PubMed] [Google Scholar]
- 31.Lin R-J, Newman A J, Cheng S-C, Abelson J. J Biol Chem. 1985;260:14780–14792. [PubMed] [Google Scholar]
- 32.Ron D, Habener J F. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 33.Seshadri V, Vaidya V C, Vijayraghavan U. Genetics. 1996;143:45–55. doi: 10.1093/genetics/143.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilleren P J, Kao H-Y, Siliciano P G. Mol Cell Biol. 1995;15:6341–6350. doi: 10.1128/mcb.15.11.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horowitz D S, Abelson J. Mol Cell Biol. 1993;13:2959–2970. doi: 10.1128/mcb.13.5.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colot H V, Stutz F, Rosbash M. Genes Dev. 1996;10:1699–1708. doi: 10.1101/gad.10.13.1699. [DOI] [PubMed] [Google Scholar]
- 37.Tsai W-Y, Chow Y-T, Chen H-R, Huang K-T, Hong R-I, Jan S-P, Kuo N-Y, Tsao T Y, Chen C-H, Cheng S-C. J Biol Chem. 1999;274:9455–9462. doi: 10.1074/jbc.274.14.9455. [DOI] [PubMed] [Google Scholar]