Abstract
Nuclear and mitochondrial (mt) DNA replication occur within two physically separated compartments and on different time scales. Both require a balanced supply of dNTPs. During S phase, dNTPs for nuclear DNA are synthesized de novo from ribonucleotides and by salvage of thymidine in the cytosol. Mitochondria contain specific kinases for salvage of deoxyribonucleosides that may provide a compartmentalized synthesis of dNTPs. Here we investigate the source of intra-mt thymidine phosphates and their relationship to cytosolic pools by isotope-flow experiments with [3H]thymidine in cultured human and mouse cells by using a rapid method for the clean separation of mt and cytosolic dNTPs. In the absence of the cytosolic thymidine kinase, the cells (i) phosphorylate labeled thymidine exclusively by the intra-mt kinase, (ii) export thymidine phosphates rapidly to the cytosol, and (iii) use the labeled dTTP for nuclear DNA synthesis. The specific radioactivity of dTTP is highly diluted, suggesting that cytosolic de novo synthesis is the major source of mt dTTP. In the presence of cytosolic thymidine kinase dilution is 100-fold less, and mitochondria contain dTTP with high specific radioactivity. The rapid mixing of the cytosolic and mt pools was not expected from earlier data. We propose that in proliferating cells dNTPs for mtDNA come largely from import of cytosolic nucleotides, whereas intra-mt salvage of deoxyribonucleosides provides dNTPs in resting cells. Our results are relevant for an understanding of certain genetic mitochondrial diseases.
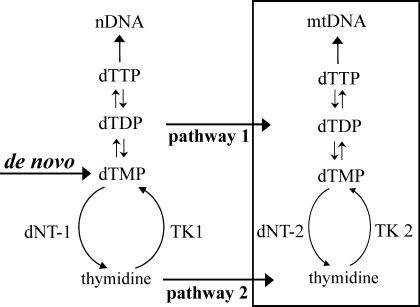
Mitochondria contain two separate potential pathways to provide dTTP for mitochondrial (mt) DNA replication (Fig. 1): (i) deoxynucleotide transporters in the membrane introduce nucleotides from the cytosol (1, 2), and (ii) thymidine kinase 2 (TK2) in the mt matrix phosphorylates thymidine to dTMP (3–6), which is further phosphorylated by nucleotide kinases to dTTP. Preliminary evidence for a third pathway via an intra-mt ribonucleotide reductase (7) has not been followed up, nor could we confirm it. We do not further consider it here.
Fig. 1.
Two pathways for mt dTTP: 1, import from cytosol of nucleotides synthesized in the cytosol; and 2, import of thymidine followed by intra-mt phosphorylation. Substrate cycles between thymidine kinases (TKs) and deoxynucleotidases (dNTs) participate in the regulation of both pathways. nDNA, nuclear DNA.
The first pathway in Fig. 1 relies mainly on de novo synthesis of deoxyribonucleoside diphosphates by ribonucleotide reductase in the cytosol (8) and to a minor extent on the activity of the cytosolic thymidine kinase 1 (TK1) (9). Both enzymes are active only during the S phase of the cell cycle (10, 11). The first pathway is therefore absent from terminally differentiated cells. The second pathway in Fig. 1 uses thymidine imported from the extracellular milieu and is active also outside S phase because TK2 is not cell-cycle regulated. Regulation of this pathway may occur by an intra-mt substrate cycle involving TK2 and 5′(3′)-deoxyribonucleotidase 2 (dNT-2) (12), similar to the cytosolic substrate cycle between TK1 and deoxyribonucleotidase 1 (13). Several genetic diseases affecting mtDNA replication arise from malfunction of enzymes in either mt pathway (14, 15). Also, the genetic loss of the cytosolic thymidine phosphorylase results in mutational damage to mtDNA (16). In this case an increased intra-mt dTTP pool is believed to cause the disease (17). Thus, too little and too much dTTP leads to disease.
Little is known about the interrelations between the two mt pathways and dTTP synthesis in the cytosol. Pioneering work by Clayton and coworkers (3, 4) initially suggested a rather strict spatial and metabolic separation. They demonstrated that cultured cells lacking TK1, but retaining TK2, incorporated labeled thymidine preferentially into mtDNA, suggesting that the labeled dTMP formed by TK2 was largely sequestered in mitochondria.
Our interest in the regulation of the mt dTTP pool started from the discovery of dNT-2 (12), a mt enzyme that specifically dephosphorylates dTMP and dUMP. We proposed that this enzyme serves to prevent an accumulation of dTTP inside mitochondria. To distinguish between thymidine metabolism in mitochondria and in the cytosol, we have now developed a method for the complete separation of the total mt dTTP pool from the cytosolic pool. We used this method to study the kinetics of [3H]thymidine incorporation into cellular thymidine phosphate (dT-P) pools and into DNA. After separation of mt and cytosolic dT-P pools, we determined both the total radioactivity in the different compartments and the specific radioactivity of the two dTTP pools. By comparing the specific radioactivity of the administered thymidine and that of the isolated dTTP we can estimate the amount of de novo synthesis of nonlabeled dTMP. In cells devoid of TK1 the primary phosphorylation of thymidine occurs inside mitochondria. The appearance of labeled dTTP in the cytosol and of labeled DNA in the nucleus then signals transport of dT-P from mitochondria to the cytosol. In cells containing TK1, the phosphorylation of thymidine occurs primarily in the cytosol and most of dTTP recovered in mitochondria originates in the cytosol. From the combined results with TK1– and TK1+ cells we conclude that in both human and mouse cultured cells the cytosolic and mt dTTP pools are in rapid exchange.
Methods
Cell Lines and Cell Growth. The established human tumor lines OSTTK1– and HOSTK1+ and the mouse fibroblast lines 3T3TK1– and 3T3TK1+ were available in this laboratory and grown as described earlier (12, 13) in 10-cm Petri dishes to a final density of ≈3 million (3T3) or 4–8 million (OST and HOS) cells. At this point, between 30% and 40% of the cells were in S phase. We usually used cells from three to five dishes per time point. Two hours before each labeling experiment we replaced the medium with 4 ml of fresh medium containing 20 mM Hepes buffer, pH 7.4, and 10% dialyzed FCS. In chase experiments the labeled medium was replaced with conditioned medium containing nonlabeled thymidine (0.3 μM for TK1+ cells or 1 μM for TK1– cells). We carried out all manipulations of growing cultures in a thermostated room before rapidly transferring them to a37°C incubator containing 5% CO2 and 95% air. All cultures were periodically checked for mycoplasma contamination. Cell numbers were determined in a Coulter Counter (Beckman Coulter).
Isotope Experiments and Separation of mt and Cytosolic dNTPs. We added [3H]thymidine (20,000 cpm/pmol; NEN, Milan) to a final concentration of 1 μM (TK1– cells) or 0.3 μM (TK1+ cells). After the indicated times we transferred the dishes on an ice bath to a cold room, removed the medium, and washed each dish four times with 2 ml of ice-cold PBS with careful draining between washes. We removed the cells from the dish by scraping with a rubber policeman (extraction buffer: 0.21 M mannitol/0.07 M sucrose/10 mM Tris·HCl, pH 7.5/0.2 mM EGTA/0.5 mg/ml BSA; 1.5 ml per three dishes); transferred them to a round-bottomed, 2-ml Eppendorf tube; and homogenized the suspension by aspiration through a 22-gauge 1¼-inch needle into a 2.5-ml syringe followed by rapid expulsion. We found microscopically that a single cycle resulted in complete breakage of cell membranes but incomplete release of mitochondria from nuclei. In several experiments we observed that the yield of mitochondria improved with increasing (up to 15) cycles, but that mt enzymes (citrate synthase, TK2, and dNT-2) started to leak into the cytosol (data not shown). In our standard procedure we therefore settled for one cycle and separated the combined mitochondria and nuclei from the cytosol. We centrifuged the homogenate for 20 min at 19,000 × g to remove the cytosol from the sedimented nuclei and mitochondria, suspended the pellet in 0.5 ml of extraction buffer, and resedimented it. This washing step removed any remaining cytosolic dNTPs from the nuclei and the pellet now contained the mt dNTP pool. To prepare mitochondria in the experiment shown in Fig. 2 A and B, we first sedimented the nuclei from the homogenate by centrifugation at 1,400 × g for 5 min and then sedimented mitochondria by centrifugation at 19,000 × g for 20 min (18). Each pellet was washed with 0.5 ml of washing buffer as described above to give nuclear and mt fractions. In all instances it was imperative to work rapidly and at cold-room temperature to avoid leakage of enzymes and dNTPs.
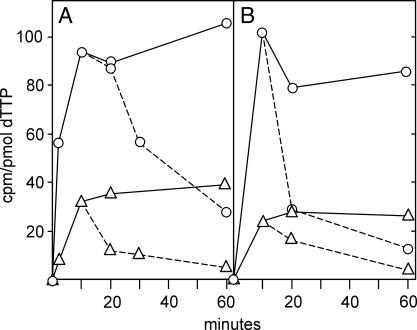
Fig. 2.
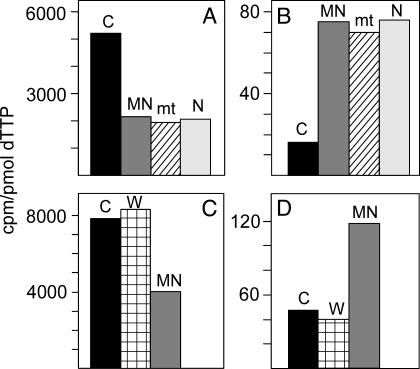
Validation of methodology for the rapid separation of cytosolic and mt dTTP. A and C are results from TK1+ cells; B and D are results from TK1– cells. In A and B, we divided homogenates from cells incubated for 10 min with labeled thymidine into two parts. We used one part for the preparation of the combined mt and nuclear fraction (MN) and for a cytosolic fraction (C) by our method, the other part for the separate preparation of mt and nuclear (N) fractions by a conventional method (18). In C and D we also analyzed the washing (W) from the MN fraction. In all fractions we determined the specific radioactivity of dTTP.
To prepare mt dT-Ps we suspended the pellets in 1 ml of cold 60% methanol and kept them at –20°C for at least 1 h. After centrifugation, we immersed the supernatant solution for 3 min in a boiling water bath to kill any remaining enzyme activity and then evaporated the methanolic solution by centrifugal vacuum evaporation. We dissolved the residue in 0.1 ml of water and used it for further analyses of nucleotides. The pellet remaining after methanol extraction was dissolved in 0.5 ml of 0.3 M NaOH and used for nuclear DNA analysis. We precipitated portions of the dissolved DNA with 0.3 M HClO4, filtered the precipitate onto glass filters, and determined its radioactivity. To prepare cytosolic dT-Ps we precipitated a portion of the cytosolic fraction with 2 vol of 100% methanol and treated the supernatant solution after centrifugation as described above for the supernatant from the pellet.
Determination of Specific Radioactivity of dTTP and Total Radioactivity Incorporated into dT-P. We determined the size and specific radioactivity of the dTTP pools by a DNA polymerase assay (13, 19). Briefly, we used an excess of [α-32P]dATP and between 0.25 and 12 pmol of [3H]dTTP for a standard curve. In the analyzed samples, we calculated the amount of dTTP from 32P cpm and the specific activity from 3H cpm. For the determination of total incorporated radioactivity we did not separate the three thymidine phosphates but determined their total radioactivity after separation from thymidine by stepwise elution from a 2-ml AG 1-X2 acetate column (mesh 100–200; Bio-Rad). We first eluted thymidine with 34 ml of 50 mM acetic acid, followed by elution of thymidine phosphates with 10 ml of 1.0 M HCl and measured the radioactivity of this fraction. Values for the incorporation of isotope are all normalized to one million cells to facilitate comparison between experiments. To determine the distribution of radioactivity between the three dT-Ps and the ratio between the three adenosine phosphates, we separated them by HPLC on a Nucleosil 100 C18 column (Phenomenex, Belmont, CA) by isocratic elution (1 ml/min) with 0.5 M ammonium phosphate, pH 3.5. After 25 min, the eluant was replaced by isocratic elution with 0.5 M ammonium phosphate/10% methanol to remove labeled thymidine from the column. The following retention times were found: dTTP, 8.3 min; dTDP, 9.5 min; dTMP, 18.1 min; ATP, 5.3 min; ADP, 5.7 min; AMP, 10.2 min; and thymidine, 37 min. Thymidine phosphates were quantified from their radioactivity, adenosine phosphates from their peak absorptions.
Enzyme Analyses. We measured citrate synthase (20), TK2 (6), and dNT-2 (12) to check for leakage of enzymes from mitochondria. Cytosolic fractions were analyzed directly; whole cells and mt fractions were extracted (12) before analyses. To distinguish between TK2 and TK1 activity, we used the inhibition of TK2 by deoxycytidine and bromovinyldeoxyuridine (5, 6) and established in this way that only TK2 activity was present in TK1– cells.
Results
Validation of Our Experimental Approach. We used a single centrifugation of the homogenate at 4°C to separate a mixture of nuclei and mitochondria from the cytosol, followed by one washing step of the pellet. Because the mt membrane retains nucleotides, we assumed that mt dTTP remained in the pellet. Nuclei are permeable for nucleotides, and any nuclear dTTP is in rapid equilibrium with the cytosolic pool. The washing step, therefore, was expected to remove from the pellet both the included cytosolic dTTP and dTTP present in nuclei. The nucleotides remaining in the second pellet then represent mt nucleotides.
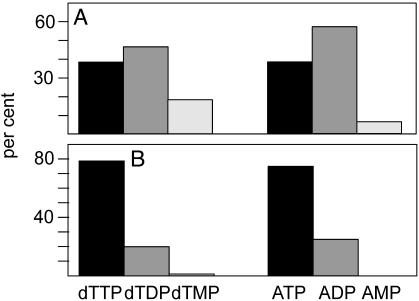
Because all of our results depend on this procedure, it was important to validate its correctness. We did this with three different experiments. First, we compared the specific activity of mt and cytosolic dTTP prepared by our method with the specific activity of dTTP in mitochondria and nuclei separated by a conventional procedure (Fig. 2 A and B). The two methods gave identical values for mt dTTP, which were different from the specific activity of cytosolic dTTP. Note that the nuclear and mt dTTP prepared by the conventional method had identical specific activities, suggesting that the “nuclear” values represent mt contamination. In a second experiment we found that the specific activity of dTTP in the wash solution from the first pellet was that of cytosolic dTTP and differed from mt dTTP (Fig. 2 C and D). A second wash solution contained no dTTP. This finding demonstrates that the washing step removed only cytosolic dTTP from the pellet. In a third experiment (Fig. 3) we determined by HPLC the distribution of isotope among the three dT-Ps and the ATP to ADP to AMP ratio in mitochondria and in the cytosol. In both cellular compartments the distribution of isotope between thymidine phosphates mirrored the molar ratios between adenosine phosphates, with a large preponderance of the triphosphate in the cytosol and a small excess of the diphosphate in mitochondria. Together, the three experiments show that our simplified method functions with minimal cross-contamination of dT-P between mitochondria and the cytosol.
Fig. 3.
Relative amounts of dTMP/dTDP/dTTP and ATP/ADP/AMP in cytosol and mitochondria. By using HPLC, we separated the nucleotide fractions from mitochondria (A) and cytosol (B) from OSTTK1– cells incubated for 10 min with labeled thymidine. We quantified thymidine phosphates from their radioactivity and adenosine phosphates from their peak absorbancies.
Competition Between Salvage and de Novo Synthesis of dTTP. The specific radioactivity of the labeled thymidine provided as a precursor of dTTP was 20,000 cpm/pmol in all of our experiments. From Fig. 2 it appears that this value had decreased to between 2,000 and 10,000 in TK1+ cells and to between 20 and 100 in TK1– cells. When we partially inhibited the de novo synthesis of dTMP in TK1– cells with amethopterin and then labeled the cells with thymidine, the specific activities of both mt and cytosolic dTTP increased 10- to 20-fold. The size of the dTTP pools was much decreased (10-fold in the cytosol and 4-fold in mitochondria), and incorporation of isotope into DNA was inhibited (data not shown), demonstrating the dependence of the cells on de novo synthesis. Our data concerning the mt dTTP pool disagree with an earlier report (21) claiming an increase in the size of this pool during inhibition of dTMP synthesis.
Isotope dilution was evidently due to a competition between salvage of labeled thymidine by thymidine kinase and de novo synthesis of dTMP by thymidylate synthase (see Fig. 1). In the absence of TK1, salvage by TK2 was inefficient, resulting in a very low specific activity of dTTP; however, in the presence of TK1, de novo synthesis still accounted for more than half of the newly synthesized dTTP.
In Vivo Turnover of mt and Cytosolic dT-P Pools. We incubated cell cultures with [3H]thymidine and used isotope flow experiments (13) to determine the time course of isotope incorporation into mt dT-P, cytosolic dT-P, and DNA as well as the specific radioactivities of the two dTTP pools. In chase experiments we substituted nonlabeled thymidine for [3H]thymidine after 10 min and continued incubation for up to 50 min to follow the disappearance of radioactivity from the dTTP pools. Experiments with two different TK1– lines are shown in Figs. 4 and 5. The cytosol of homogenates from both lines contained <2% of the thymidine kinase activity of the cells. All phosphorylation of [3H]thymidine therefore occurred in mitochondria. Fig. 4 shows results of the total isotope incorporation, with 4A giving data for human OST cells and 4B those for mouse 3T3 fibroblasts. Fig. 5 A and B shows the specific activities of the cytosolic and mt dTTP pools from the same experiments.
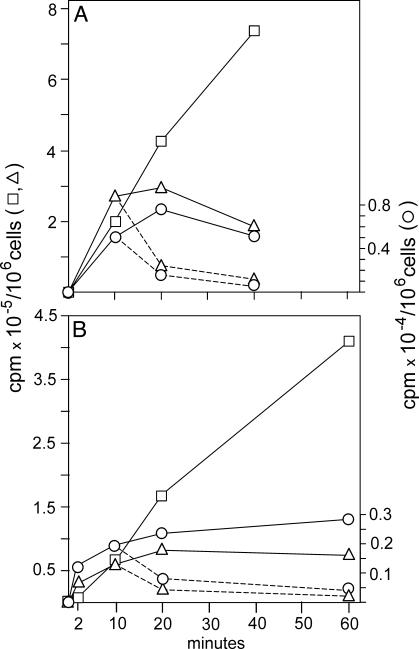
Fig. 4.
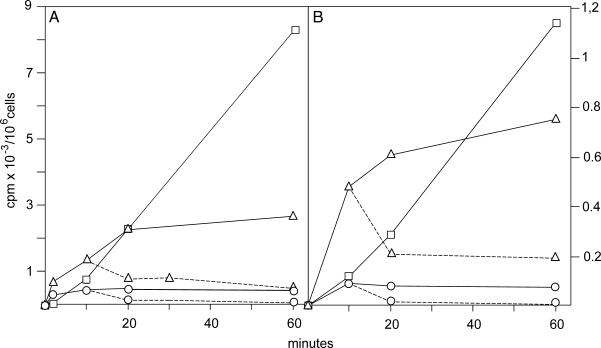
Incorporation of [3H]thymidine into cytosolic dTTP, mt dTTP, and DNA in TK1– cells: OSTTK1– (A) and 3T3TK1– (B). We incubated cells with 1 μM [3H]thymidine for the indicated time periods, isolated cytosolic and mt dTTPs and DNA, and determined incorporation of total radioactivity into each fraction. In a chase experiment (broken lines) we removed the medium from cultures, replaced it with conditioned medium containing 1 μM nonlabeled thymidine, and continued incubation for the additional indicated times. ▵, Cytosolic dTTP; ○, mt dTTP; □, DNA.
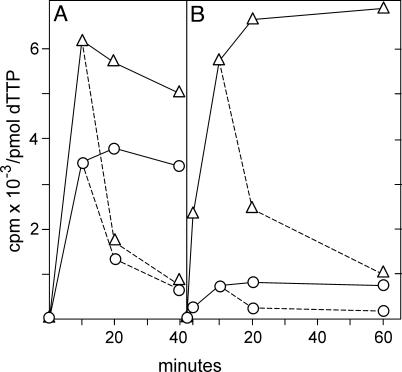
Fig. 5.
Specific radioactivities of cytosolic and mt dTTP in TK1– cells after incubation with [3H]thymidine. This is the same experiment as in Fig. 4, but this figure shows the specific radioactivities of the two dTTP pools instead of total radioactivity. Broken lines indicate chase experiments. ▵, Cytosolic dTTP; ○,mt dTTP.
After a short lag phase, isotope was linearly incorporated into nuclear DNA (Fig. 4) in both cell lines, indicating a continuous synthesis fed from the cytosolic dTTP pool. This pool reached a plateau after 20 min, demonstrating that between 20 and 60 min the loss of dTTP from the pool was replenished by synthesis from thymidine. The rapid turnover of the cytosolic dTTP pool is also apparent from the chase experiment in which the pool rapidly lost radioactivity when nonlabeled thymidine was substituted for labeled thymidine in the medium. Phosphorylation of thymidine by TK2 occurred in mitochondria; therefore, the mt dTTP was the precursor of the cytosolic dTTP. The radioactivity in the mt pool reached a steady state after only 10 min (Fig. 4) and afterward was in a dynamic equilibrium with the cytosolic pool. Again, results from the chase substantiate its rapid turnover. Mouse cells (Fig. 4B) incorporated less isotope than human cells (Fig. 4A), but the general behavior of both lines was identical.
The specific radioactivities from the same experiment are shown in Fig. 5. The values were now almost the same for human (Fig. 5A) and mouse (Fig. 5B) cells. Both mt and cytosolic dTTP pools reached their plateaus after 10 min. The radioactivity was rapidly chased by nonlabeled thymidine. After 2 min the specific radioactivity was 8 times higher in mitochondria than in the cytosol; at later time points the difference decreased to 3-fold, in agreement with a precursor role for mt dTTP. From the plateau values for cytosolic dTTP and the radioactivity incorporated into DNA (Fig. 4) we can calculate the rate of DNA synthesis in the two experiments. In human cells the incorporation into DNA between 20 and 60 min was 150 cpm/min. By division with 35, which is the average specific activity of cytosolic dTTP, we obtain an incorporation of 4.3 pmol of dTMP per min. In a similar calculation the rate of DNA synthesis in mouse 3T3 cells came out as 0.8 pmol of dTMP per min. Thus the incorporation of dTTP into DNA was slower in 3T3 cells than in OST cells. Nevertheless the interrelation between cytosolic and mt dTTP pools remained the same. Moreover, the enormous dilution of isotope indicates that in both types of cells the large majority of dTTP stemmed from the de novo pathway.
We turn now to TK1+ cells. Figs. 6 and 7 show experiments with human HOS TK1+ and mouse 3T3 TK1+ cells patterned on those with TK1– cells described in Figs. 4 and 5. In a separate experiment we found that extracts from TK1+ cells contained 100-fold higher thymidine kinase activity than TK1– cells, and TK1+ cells also incorporated 100 times more radioactivity (Fig. 6). Here, salvage of thymidine competed quite effectively with de novo synthesis. In both human and mouse cells the total radioactivity in the mt and cytosolic dTTP pools reached plateau values at ≈20 min, but at very different levels: The plateau values reached up to 30 times higher in the cytosol. Incorporation into DNA started with a slight lag phase followed by a close to linear phase. The deviation from linearity after 20 min was caused by depletion of the labeled thymidine in the medium, particularly in HOS cells. We had lowered the concentration of thymidine in the medium to 0.3 μM to avoid artifactual expansion of the dTTP pool by the effective salvage of the nucleoside.
Fig. 6.
Incorporation of [3H]thymidine into cytosolic dTTP, mt dTTP, and DNA in TK1+ cells: HOSTK1+ (A) and 3T3TK1+ (B). We incubated cells with 0.3 μM [3H]thymidine for the indicated times and determined incorporation of total radioactivity into dTTP pools and DNA. Broken lines indicate chase experiments conducted with 0.3 μM nonlabeled thymidine as described in Fig. 4. ▵, Cytosolic dTTP; ○, mt dTTP; □, DNA.
Fig. 7.
Specific radioactivities of cytosolic and mt dTTP in TK1+ cells in the experiment described for Fig. 6. Broken lines indicate chase experiments. ▵, Cytosolic dTTP; ○, mt dTTP.
The specific activity of dTTP was much higher in TK1+ cells (Fig. 7) than in TK1– cells, with values of up to 7,000 cpm/pmol in the cytosol compared with 30 (Fig. 4). Now TK1 in the cytosol was responsible for almost all phosphorylation of thymidine. In consequence the cytosolic dTTP pool had a higher specific activity than the mt pool. This is opposite to TK1– cells, where phosphorylation occurred in mitochondria and the specific activity always was highest in mt dTTP. Nevertheless the plateau values for the specific activities of mt dTTP were always higher in TK1+ cells than in TK1– cells (40 times in human cells and 10 times in mouse cells). This finding suggests that most of the radioactive mt dTTP was imported from the cytosol. The contribution of TK2 was probably minimal because of the low activity of the enzyme.
Discussion
This work presents the dynamics of a mt dNTP pool and its connection with the corresponding cytosolic pool. It was made possible by a simple method for the complete separation of mt and cytosolic dNTP pools. The validity of this procedure is supported by the experiments in Fig. 2. We obtained similar results with both human and mouse cells in culture. In addition to the experiments described here, we performed several experiments with HeLaTK1+ and HeLaTK1– cells with similar results (data not shown). We therefore suggest that our results are of general validity for cycling cells in culture.
Our general approach was to determine the flow of tritiated thymidine through cytosolic and mt dTTP pools into DNA by measuring both the time-dependent total incorporation of isotope into the different compartments and the specific radioactivities of mt and cytosolic dTTP. Determinations of specific radioactivities are often neglected but were of crucial importance for our work. Values are independent of losses during preparation of nucleotides and therefore more reliable than total radioactivity. When the specific radioactivity of dTTP was at a plateau after 20 min, the system was in a steady state, and the loss of radioactivity from the pools through incorporation into DNA was balanced by synthesis from thymidine. The attainment of two different plateaus for cytosolic and mt dTTP shows that we were seeing the dynamic behavior of two metabolically distinct pools. This observation by itself does not distinguish between a mechanism involving the independent incorporation of radioactivity into two separate pools or the transfer of radioactivity from one pool to the other. In TK1– cells, however, a distinction can be made, because the only phosphorylation of thymidine occurs by TK2, and any radioactivity in the cytosolic pool must originate from the mt pool. In this case it is clear that the mt pool was a precursor of the cytosolic pool.
Compared with the specific activity of the precursor thymidine, the specific activities of the cellular pools were very low, indicating that a large majority of dTTP was derived from nonlabeled precursors by de novo synthesis. This conclusion is directly apparent from the large increase in specific activity in amethopterin-treated cells, where de novo synthesis is inhibited. We could not determine the nature of the nonlabeled material that contributed to the dilution effect. We found no difference between the specific activities of dTMP, dTDP, and dTTP, indicating a rapid equilibration of isotope between these pools. The size of the intra-mt thymidine pool was too small to be determined. We can therefore not decide whether isotope dilution occurred at the level of thymidine or at the level of a specific thymidine phosphate. We can conclude, however, that also in the presence of thymidine, cycling TK1– cells synthesize the vast majority of their dTTP de novo and that TK1+ cells also strongly depend on de novo synthesis.
A second point emerging from measurements of the specific radioactivities concerns the turnover of both the cytosolic and mt pools. We determined earlier (22) a half-life of 4 min during S phase for the total cellular dTTP pool of 3T3 cells. The present experiments with nonsynchronized cultures were not designed to determine half-lives. Nevertheless one can estimate a similar rapid turnover both from the attainment of isotope equilibrium and from the decay during the chase (Figs. 5 and 7). The important finding is that the mt and cytosolic pools show the same dynamic behavior, suggesting a rapid exchange between them.
The connection between the two pools appears directly from the experiments depicted in Figs. 4, 5, 6, 7. In TK1– cells (Figs. 4 and 5) thymidine first enters the mt dTTP pool, but radioactivity appears in cytosolic dTTP and is incorporated into DNA after only a brief lag period. In TK1+ cells (Figs. 6 and 7) thymidine is phosphorylated almost exclusively by TK1 in the cytosol but then moves rapidly into the mt pool. We did not expect such a rapid exchange between the two compartments. Our experiments show that enzymes in the mt membrane catalyze the rapid movement of thymidine phosphates (and probably other nucleotides) in and out of mitochondria and directly demonstrate the function of the first pathway of Fig. 1. In cycling cells, the function of the second pathway, the salvage of thymidine by TK2, was minimal. We suggest that salvage is important only in resting cells in the absence of de novo synthesis in the cytosol without sufficient dNTPs for import into mitochondria for mtDNA replication and repair. This difference may explain why the symptoms of the genetic absence of the deoxynucleotide transporter appear during embryogenesis (14), whereas the loss of TK2 causes disease only after birth (15).
Acknowledgments
This work was supported by the European Commission (Grant QLRT-CT-2000-01004) and the Italian Association for Cancer Research and Telethon Italia (Grant GP0140Y01 to V.B.).
Abbreviations: mt, mitochondrial; TK1, thymidine kinase 1 (cytosolic); TK2, thymidine kinase 2 (mt); dNT-2, 5′(3′)-deoxyribonucleotidase 2; dT-P, thymidine phosphate.
References
- 1.Bridges, E. G., Jiang, Z. & Cheng, Y. (1999) J. Biol. Chem. 274, 4620–4625. [DOI] [PubMed] [Google Scholar]
- 2.Dolce, V., Fiermonte, G., Runswick, M. J., Palmieri, F. & Walker, J. E. (2001) Proc. Natl. Acad. Sci. USA 98, 2284–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berk, A. J. & Clayton, D. A. (1973) J. Biol. Chem. 248, 2722–2729. [PubMed] [Google Scholar]
- 4.Bogenhagen, D. & Clayton, D. A. (1976) J. Biol. Chem. 251, 2938–2944. [PubMed] [Google Scholar]
- 5.Johansson, M. & Karlsson, A. (1997) J. Biol. Chem. 272, 8454–8458. [DOI] [PubMed] [Google Scholar]
- 6.Wang, L., Munch-Petersen, B., Herrström-Sjöberg, A., Hellman, U., Bergman, T., Jörnvall, H. & Eriksson, S. (1999) FEBS Lett. 443, 170–174. [DOI] [PubMed] [Google Scholar]
- 7.Young, P., Leeds, J. M., Slabough, M. B. & Mathews, C. (1994) Biochem. Biophys. Res. Commun. 203, 9300–9304. [DOI] [PubMed] [Google Scholar]
- 8.Jordan, A. & Reichard, P. (1998) Annu. Rev. Biochem. 67, 71–98. [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw, H. D. J. & Deininger, P. L. (1984) Mol. Cell. Biol. 4, 2316–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffman, M. G. & Kelly, T. J. (1991) Mol. Cell. Biol. 11, 2538–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabes, A. & Thelander, L. (2000) J. Biol. Chem. 275, 17747–17753. [DOI] [PubMed] [Google Scholar]
- 12.Rampazzo, C., Gallinaro, L., Milanesi, E., Frigimelica, E., Reichard, P. & Bianchi, V. (2000) Proc. Natl. Acad. Sci. USA 97, 8239–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazziola, C., Ferraro, P., Moras, M., Reichard, P. & Bianchi, V. (2001) J. Biol. Chem. 276, 6185–6190. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg, M. J., Agarwala, R., Bouffard, G., Davis, J., Fiermonte, G., Hilliard, M. S., Koch, T., Kalikin, L. M., Makalowska, I., Morton, D. H., et al. (2002) Nat. Genet. 32, 175–179. [DOI] [PubMed] [Google Scholar]
- 15.Saada, A., Shaag, A., Mandel, H., Nevo, Y., Eriksson, S. & Elpeleg, O. (2001) Nat. Genet. 29, 342–344. [DOI] [PubMed] [Google Scholar]
- 16.Nishino, I., Spinazzola, A. & Hirano, M. (1999) Science 283, 689–692. [DOI] [PubMed] [Google Scholar]
- 17.Nishigaki, Y., Marti, R., Copeland, W. C. & Hirano, M. (2003) J. Clin. Invest. 111, 1913–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sottocasa, G. L., Kuylenstierna, B., Ernster, L. & Bergstrand, A. (1967) J. Cell Biol. 32, 415–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman, P. A. & Fyfe, J. A. (1989) Anal. Biochem. 180, 222–226. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd, D. & Garland, P. (1969) Methods Enzymol. 13, 11–16. [Google Scholar]
- 21.Bestwick, R. K., Moffet, G. L. & Mathews, C. K. (1982) J. Biol. Chem. 257, 9300–9304. [PubMed] [Google Scholar]
- 22.Spyrou, G. & Reichard, P. (1988) Mutat. Res. 200, 37–43. [DOI] [PubMed] [Google Scholar]