Abstract
The enzyme 5-lipoxygenase (5-LO) initiates the synthesis of leukotrienes from arachidonic acid. In resting cells, 5-LO can accumulate in either the cytoplasm or the nucleoplasm and, upon cell stimulation, translocates to membranes to initiate leukotriene synthesis. Here, we used mutants of 5-LO with altered subcellular localization to assess the role that nuclear positioning plays in determining leukotriene B4 (LTB4) synthesis. Mutation of either a nuclear localization sequence or a phosphorylation site reduced LTB4 synthesis by 60%, in parallel with reduced nuclear localization of 5-LO. Mutation of both sites together or mutation of all three nuclear localization sequences on 5-LO inhibited LTB4 synthesis by 90% and abolished nuclear localization. Reduced LTB4 generation in mutants could not be attributed to differences in 5-LO amount, enzymatic activity, or membrane association. Instead, 5-LO within the nucleus acts at a different site, the nuclear envelope, than does cytosolic 5-LO, which acts at cytoplasmic and perinuclear membranes. The significance of this difference was suggested by evidence that exogenously derived arachidonic acid colocalized with activated nuclear 5-LO. These results unequivocally demonstrate that the positioning of 5-LO within the nucleus of resting cells is a powerful determinant of the capacity to generate LTB4 upon subsequent activation.
Leukotriene B4 (LTB4) is a lipid mediator that is important for host defense, as it stimulates leukocyte chemotaxis, degranulation, phagocytosis, and superoxide generation (reviewed in ref. 1). The underproduction of leukotrienes (LTs) results in susceptibility to infection (2, 3), whereas the overproduction of LTB4 contributes to inflammatory disease (4, 5). The enzyme 5-lipoxygenase (5-LO) initiates the synthesis of LTB4 from arachidonic acid (AA). Thus, the action of 5-LO is a key point of regulation in LTB4 synthesis.
In resting leukocytes, 5-LO is a soluble enzyme. Elevated intracellular calcium, as occurs upon cell stimulation, causes translocation of 5-LO to nuclear and perinuclear membranes (6, 7). Membrane association places the enzyme close to its substrate and initiates LT synthesis. LT synthesis can also be stimulated by activation of p38 mitogen-activated protein kinase (MAPK) (8), which is thought to be due to phosphorylation of 5-LO on Ser-271 by MAPK-activated protein (MAPKAP) kinase 2 (9) and phosphorylation-dependent nuclear association of 5-LO (10).
Entirely distinct from its ability to move to membranes upon cell stimulation, 5-LO can redistribute in unstimulated cells; 5-LO can be imported into the nucleus (11, 12), and it can be exported from the nucleus (13). The import of soluble 5-LO protein, through nuclear pores on the nuclear envelope, is mediated by three distinct nuclear localization signals (NLSs) on 5-LO (14, 15). The import process can be triggered by adherence (11, 12) or by cytokines (16, 17). Movement of 5-LO from the cytoplasm into the interior of the nucleus also occurs during leukocyte recruitment into sites of inflammation (11, 18). Nuclear import in many cell types is regulated by a phosphorylation-dependent mechanism (19), and it is possible that phosphorylation of 5-LO on Ser-271 also affects its nuclear import.
The effect of the subcellular localization of 5-LO in resting cells on its capacity to synthesize LTs upon subsequent cell stimulation is unclear. Nuclear import of 5-LO induced by adherence increases LT synthesis in neutrophils (11) but inhibits LT synthesis in eosinophils (12). And, although LT production by eosinophils is reduced when import is induced by adherence, LT generation in the same cell type is enhanced when import is promoted by cytokines (16, 17). Certainly, factors like adherence or cytokines have additional effects that confound our understanding of the relationship between 5-LO localization and LT synthesis.
In this study, we used site-directed mutagenesis to alter residues of 5-LO that are necessary for nuclear localization but not for catalytic activity. This molecular approach allowed us to directly assess the effect of 5-LO localization on LTB4 synthesis.
Experimental Procedures
Plasmids and Mutagenesis. Plasmids expressing WT, mutNLS1 (where NLS1 is at Arg-518 on human 5-LO), and mutNLS1,2,3 (mutation of NLSs at Arg-518, Arg-112, and Lys-158) 5-LO/GFP have been described (14, 15, 20). All plasmids use the enhanced GFP gene, which has reduced absorption at 450 nm as compared with GFP. To construct p(S271A)5-LO/GFP and p(mutNLS1+S271A)5-LO/GFP, Ser-271 was substituted with Ala in p(WT)5-LO/GFP and p(mutNLS1)5-LO/GFP, respectively, using the QuikChange site-directed mutagenesis kit (Stratagene) following the manufacturer's directions. Mutations were verified by DNA sequence analysis (DNA Sequencing Core, University of Michigan). Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA). Plasmid and oligonucleotide sequences are available on request.
Cell Culture and Transfection. NIH 3T3 cells were obtained from American Type Culture Collection and grown under 5% CO2 in DMEM (Invitrogen) with 10% calf serum and 100 units/ml each penicillin and streptomycin. Cells were transfected with 4 μg of plasmid DNA by using Polyfect (Qiagen, Valencia, CA) following the manufacturer's directions. Transient transfectants were evaluated microscopically with a Nikon E600 fluorescent microscope or Leica DMIRB fluorescent inverted microscope, live or after fixation with 2% paraformaldehyde, 16 h after transfection.
Cell Stimulation, LTB4 Analysis, and Immunoblotting. Cells (3.2 × 106 per construct) were transfected and, 16 h later, washed, pooled, and divided equally into four aliquots. To stimulate LTB4 production, the cells were incubated in 0.5 ml of serum-free DMEM containing 1.6 mM CaCl2, 10 μM calcium ionophore A23187, and 10 μM AA at 37°C for 15 min. Immunoreactive LTB4 in conditioned media was quantitated by ELISA (Cayman Chemical, Ann Arbor, MI) following the supplier's instructions. The detection limit for LTB4 was 4 pg/ml; cross-reactivity for AA, 5-hydroxyeicosatetraenoic acid, LTC4, LTD4, and LTE4 was <0.01%. To standardize LTB4 synthesis for an amount of expressed 5-LO protein, immunoblot analysis on whole-cell lysates from all transfected, activated samples was performed. Cells were lysed in SDS/PAGE sample buffer and boiled, and 20-μl samples were separated by SDS/PAGE and transferred to nitrocellulose. Membranes were probed with rabbit polyclonal antibodies raised against purified human leukocyte 5-LO (a generous gift from J. Evans, Merck Frosst Centre for Therapeutic Research, Pointe Claire Dorval, QB, Canada; titer 1:3,000) (21) or GFP (Santa Cruz Biotechnology; titer 1:500) followed by peroxidase-conjugated secondary antibody and enhanced chemiluminescence detection (Amersham Biosciences).
Cell-Free 5-LO Enzymatic Activity. 3T3 cells (5 × 106) transfected with 5 μg of plasmid were harvested 20 h after transfection, washed with PBS, and sonicated for 90 s in 10-s bursts on ice in 50 mM Tris·HCl (pH 7.5), 0.1 mM EDTA, 1 mM DTT, and protease inhibitor mixture (Complete TM EDTA-free, Roche Molecular Biochemicals). Lysates were centrifuged (6,000 × g for 10 min at 4°C) to remove cell debris, and level of protein expression for each construct was confirmed first by immunoblot analysis by using anti-GFP based on 10 μg of total protein per sample. The 5-LO activity of cell lysates (200 μg of total protein) was determined in 0.25-ml reaction mixtures containing 50 mM Tris·HCl (pH 7.5), 0.6 mM CaCl2, 0.1 mM EDTA, 0.1 mM ATP, 12 μg/ml phosphatidylcholine (Avanti Polar Lipids), 20 μM AA (Cayman Chemicals), including ≈100,000 dpm [3H]AA (Du-Pont/NEN) and 10 μM 13(s)-hydroperoxy-9-cis-11-transoctadecadienoic acid (Cayman Chemicals). After 30 min incubation at room temperature, reactions were stopped by adding 1 ml of ether/methanol/1 M citric acid (30:4:1, vol/vol/vol) and mixed thoroughly. The mixture was centrifuged at 5,000 rpm, 5 min, and the upper phase was collected, evaporated under nitrogen, and stored at –70°C. Lipid residues were dissolved in 250 μl of 50% acetonitrile/trifluoroacetic acid (1,000:1, vol/vol) and 50% water/trifluoroacetic acid (1,000:1, vol/vol), analyzed by reverse-phase HPLC as described (14). There were no LTB4/LTB4 isomers detected in the cell lysates; 5-hydroperoxyeicosatetraenoic acid (5-HPETE) and 5-hydroxyeicosatetraenoic acid (5-HETE) coeluted as a single peak, clearly separated from unmetabolized AA. The 5-LO specific activity was calculated as the percent conversion of radiolabeled AA to 5-HPETE/5-HETE.
Ca2+-Dependent Membrane Translocation. To assess 5-LO membrane translocation in cell-free conditions, WT and various mutant 5-LO constructs were transfected into 3T3 cells and cells were homogenized and analyzed 20 h after transfection as described (22) with minor modification. Briefly, cells were sonicated on ice three times, 20 s each, in KPB-1 buffer (100 mM NaCl/2 mM EGTA/1 mM DTT/50 mM potassium phosphate buffer, pH 7.1, and protease inhibitor mixture). Cell lysates were centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were then divided equally into two parts, one to which CaCl2 was added (4 mM final concentration), and the other to which vehicle (water) was added. After 5 min at room temperature, supernatants were further centrifuged at 100,000 × g for 70 min at 4°C. The resulting supernatant was designated the soluble fraction, and the pellet, washed and resuspended in KPB-1 buffer, was designated the microsomal fraction. To assess 5-LO membrane translocation in intact cells, transfected cells were stimulated with vehicle (DMSO) or 10 μM A23187 in media for 20 min at 37°C. After stimulation, cells were washed twice and sonicated in KPB-1 buffer, and soluble and microsomal fractions were prepared as described above. Protein concentrations of fractions were determined by a modified Coomassie dye binding assay (Pierce), and 10 μg protein per sample were separated by SDS/PAGE and evaluated by immunoblot as described above by using GFP antibody.
Fluorescent AA Preparation and Microscopic Imaging. Fluorescent diazoalkanes react spontaneously with carboxylic acids of fatty acids (23) and have been used to fluorescently label AA for HPLC analysis (24). In this study, AA was labeled with 1-pyrenyldiazomethane (PDAM, Molecular Probes) before addition to 3T3 cells. Equimolar concentrations of AA and PDAM were incubated at 37°C for 3 h to ensure conjugation of AA and PDAM. Cells on chamber slides were mounted on a temperature-regulated (37°C) stage on a Leica DMIRB fluorescent inverted microscope, washed, and incubated with PBS and imaged by phase contrast and fluorescence microscopy before AA addition. Cells were subsequently imaged in 3-min intervals after the addition of AA/PDAM (10 μM final concentration). For 5-LO activation, cells were stimulated with 10 μM A23187 in calcium-containing medium before washing and AA/PDAM addition.
Statistical Analysis. Statistical significance was evaluated by one-way ANOVA, using P < 0.05 as indicative of statistical significance. Pairs of group means were analyzed by using the Tukey–Kramer post hoc test.
Results and Discussion
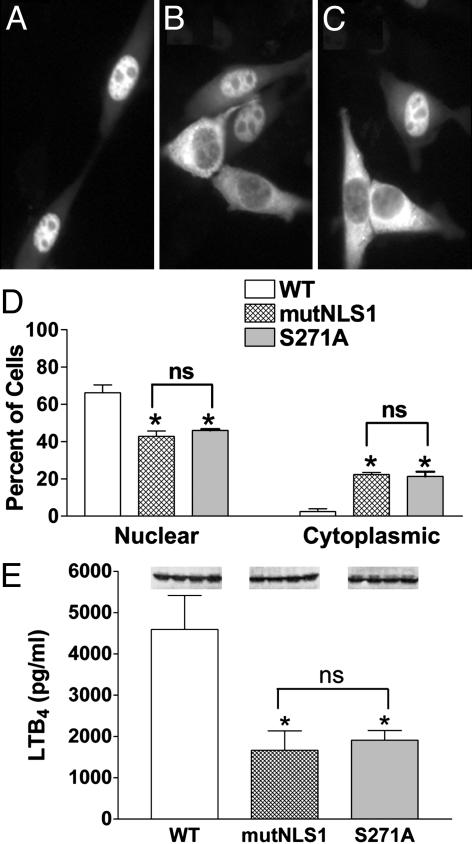
Reduced 5-LO Nuclear Localization: Decreased LTB4 Synthesis. As shown (15), the WT 5-LO/GFP protein accumulates in the nucleus of transfected 3T3 cells (Fig. 1A). Mutation of NLS1 on 5-LO/GFP produced a mixed population, with some cells with nuclear accumulation and others showing a failure to import (Fig. 1B). Quantitation indicated that both the decrease in cells with nuclear fluorescence and the increase in cells with cytoplasmic fluorescence induced by mutation of NLS1 were statistically significant (Fig. 1D); some cells had a balanced distribution, and their numbers were not affected by mutation of NLS1 (ref. 15 and data not shown). Because nuclear import of proteins can be regulated by phosphorylation (19) and 5-LO can be phosphorylated on Ser-271 (9), we asked whether Ser-271 was important in determining 5-LO localization. Site-directed replacement of Ser-271 with Ala resulted in a protein distribution that visually (Fig. 1C) and quantitatively (Fig. 1D) resembled mutation of NLS1. Thus, two distinctly different mutations, one at Arg-518–Lys-530 that alters an import sequence and another at Ser-271 that eliminates a phosphorylation site, produced essentially identical changes in the subcellular distribution of 5-LO.
Fig. 1.
Different mutations that reduce nuclear localization of 5-LO also reduce LTB4 synthesis. 3T3 cells were transfected with WT, mutNLS1, or S271A 5-LO/GFP and, after 16 h, evaluated for 5-LO localization and LTB4 synthesis. Representative fields show WT 5-LO/GFP (A), mutNLS1 5-LO/GFP (B), or S271A 5-LO/GFP (C) from one experiment and are representative of 10 experiments. (D) Quantitative analysis of transfected populations of cells expressing WT, mutNLS1, or S271A 5-LO/GFP, with localization determined as described in Experimental Procedures. Data are means ± SE of n = 4 independent transfections per construct (*, P < 0.05 vs. WT; ns, not statistically different). (E) LTB4 synthesis. Transfected cells were stimulated with 10 μM A23187 and 10 μMAA at 37°C for 15 min, and LTB4 in conditioned media was measured by ELISA. Data are means ± SE (n = 4) (*, P < 0.05 vs. WT; ns, not statistically different). (Inset) Protein expression of the examined constructs assessed by immunoblotting. Results are from one experiment and are representative of three.
To evaluate the effect of nuclear localization on the ability of 5-LO to produce LTB4, cells overexpressing WT, mutNLS1, and S271A 5-LO/GFP were stimulated with the calcium ionophore A23187 with exogenous AA. Preliminary results indicated that 3T3 cells lacked the 5-LO activating protein and secreted negligible LTB4 when transfected with 5-LO (with or without GFP) and activated with ionophore alone (data not shown). Also, cells that were mock-transfected did not secrete detectable LTB4 when stimulated (data not shown). However, cells overexpressing WT 5-LO/GFP protein produced significant LTB4 (4,570 ± 807 pg/ml) when stimulated with both ionophore and AA (Fig. 1E). In contrast, cells overexpressing either mutNLS1 or S271A 5-LO/GFP produced significantly less LTB4 (1,666 ± 469 and 1,912 ± 232 pg/ml, respectively). Immunoblot (Fig. 1E Inset) and densitometric (data not shown) analyses of protein expression of cell lysates after cell stimulation showed that there was no difference in protein expression between the different constructs. Thus, two distinct mutants of 5-LO that share comparable reductions in nuclear import have similarly reduced capacities to convert exogenous AA to LTB4 upon cell stimulation.
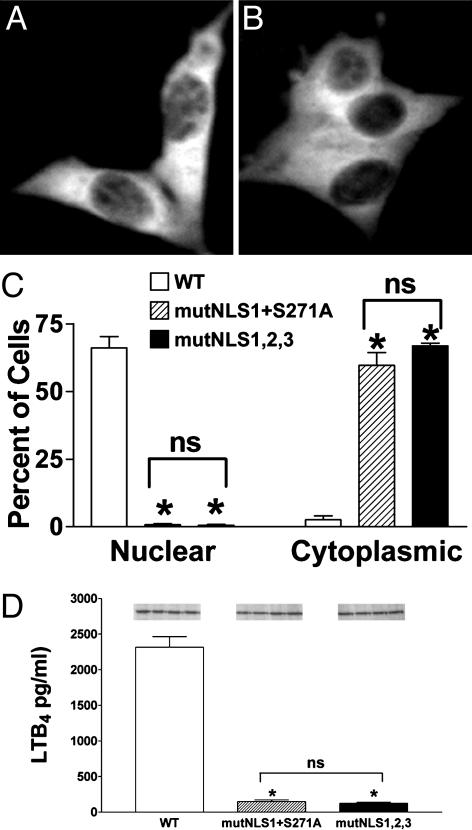
Combined Mutations Eliminate 5-LO Nuclear Accumulation and Minimize LTB4 Synthetic Capacity. The similarity of the effects of the two distinct mutations suggested that the two affected sites overlap in function and that phosphorylation at Ser-271 regulated activation of NLS1. If so, then mutation of both sites together should be similar to either mutation alone. However, when both NLS1 and Ser-271 were mutated in 5-LO/GFP, the resulting protein was distributed almost exclusively in the cytoplasm (mutNLS1+S271A; Fig. 2 A and C). This finding indicated that phosphorylation at Ser-271 did not regulate localization of 5-LO solely through its effects on NLS1. It seems likely, then, that phosphorylation at Ser-271 regulates a different NLS, modulates nuclear export, or affects 5-LO localization through some other mechanism altogether.
Fig. 2.
Different combinations of mutations that eliminate nuclear localization of 5-LO also strongly reduce LTB4 synthesis. 3T3 cells were transfected with WT, mutNLS1+S271A, or mutNLS1,2,3 5-LO/GFP and, after 16 h, evaluated for 5-LO localization and LTB4 synthesis. Representative fields show mutNLS1+S271A 5-LO/GFP (A) or mutNLS1,2,3 5-LO/GFP (B) from one experiment and are representative of eight experiments. (C) Quantitative analysis of transfected populations of cells expressing WT, mutNLS1+S271A, or mutNLS1,2,3 5-LO/GFP, with localization determined as described in Experimental Procedures. Data are means ± SE of n = 4 independent transfections per construct (*, P < 0.05 vs. WT; ns, not statistically different). (D) LTB4 synthesis. Transfected cells were stimulated with 10 μM A23187 and 10 μMAA at 37°C for 15 min, and LTB4 in conditioned media was measured by ELISA. Data are means ± SE (n = 4) (*, P < 0.05 vs. WT; ns, not statistically different). (Inset) Protein expression of the examined constructs assessed by immunoblotting. Results are from one experiment and are representative of three.
The effect of the combined mutNLS1+S271A mutation of 5-LO/GFP on localization was essentially identical to that observed when all three NLSs on 5-LO/GFP were mutated (mutNLS1,2,3; Fig. 2B), which has been described (14). Quantitatively, both sets of combined mutations were similar in reducing the percent of cells having nuclear accumulation and in increasing the percent with strong cytoplasmic localization of 5-LO/GFP (Fig. 2C). It should be noted that in cells expressing combined mutations, like those expressing WT 5-LO/GFP, ≈30% of cells had a balanced nuclear/cytoplasmic distribution (data not shown). These cells may be explained, at least in part, by the loss of compartmentalization associated with nuclear envelope breakdown during mitosis, as well as delayed reestablishment of gradients postmitotically, as shown (13). As a result, these two sets of combined mutations eliminated the nuclear accumulation of 5-LO typically seen in 3T3 cells, although some nuclear 5-LO persisted in mitotic and postmitotic cells even with the combined mutations.
LTB4 production was decreased by >90% in 3T3 cells overexpressing mutNLS1+S271A 5-LO/GFP (149 ± 22 pg/ml), vs. cells overexpressing WT 5-LO/GFP (2,316 ± 148 pg/ml), when stimulated with calcium ionophore plus exogenous AA (Fig. 2D). Cells overexpressing mutNLS1,2,3 5-LO/GFP showed a comparable reduction in LTB4 synthesis (124 ± 14 pg/ml). The protein expression levels of WT, mutNLS1+S271A, and mutNLS1,2,3 5-LO/GFP in transfected cells used for LTB4 synthesis were comparable (Fig. 2D Inset). These results, together with those obtained with the single mutants NLS1 and S271A, clearly indicated a correlation between the extent of 5-LO nuclear localization in resting cells and LTB4 synthetic capacity after stimulation.
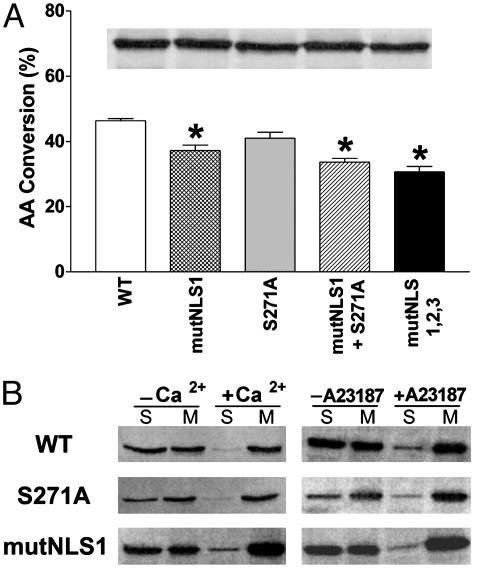
Different Molecular Constructs Do Not Differ in Enzymatic Activity or Membrane Translocation. As noted above, the reduced capacity for LTB4 synthesis in the different molecular constructs of 5-LO could not be attributed to differences in the amounts of expressed protein. It seemed possible that reduced LTB4 production could reflect disruption in tertiary structure due to the mutations, or the presence of a cytosolic inhibitor of 5-LO (25, 26). Cell-free assays of 5-LO activity showed that the S271A mutation did not significantly decrease enzymatic activity (Fig. 3A), consistent with a recent report (27). Mutations involving changes in NLS1 significantly reduced activity, although this effect was clearly less than the reductions in cellular LTB4 synthesis. Again, immunoblot analysis verified that comparable amounts of expressed protein were evaluated during the cell-free assay (Fig. 3A Inset). These results indicated that reduced LTB4 synthesis in cells expressing mutated 5-LO could not be attributed to loss of 5-LO enzymatic activity. The results also suggested that differences in LTB4 production could not be caused by potential inhibitors in the cytoplasm, as cell lysates retained high levels of 5-LO activity across all constructs.
Fig. 3.
Effect of mutations of 5-LO/GFP on enzymatic activity and membrane association. (A) Cell-free enzymatic activity. Cell lysates from 3T3 cells expressing the different 5-LO/GFP constructs were tested for their capacity to convert AA to 5-hydroperoxyeicosatetraenoic acid (5-HPETE)/5-hydroxyeicosatetraenoic acid (5-HETE), using comparable amounts of 5-LO protein (indicated by immunoblot evaluation, Inset). Data are means ± SE of n = 3 independent transfections per construct (*, P < 0.05 vs. WT). (B)Ca2+-dependent membrane translocation of expressed 5-LO/GFP proteins both in vitro and in intact cells. 3T3 cells expressing WT, S271A, or mutNLS1 5-LO/GFP were fractionated with or without Ca2+ or stimulated with or without 10 μM A23187 (20 min, 37°C) before fractionation. Cells were fractionated into soluble (S) and microsomal (M) fractions and were examined by immunoblotting using antibodies against GFP based on equal loading (10 μg per well) of total protein.
Previous studies have suggested that activation of p38 MAPK can stimulate membrane association of 5-LO (10), potentially through phosphorylation on Ser-271 (9). This notion suggested the possibility that reduced capacity for LTB4 synthesis in the different molecular constructs might result from impaired membrane association of 5-LO. The effects of the S271A substitution and the NLS1 mutation on membrane association were tested by both cell-free and intact cell approaches. When 3T3 cells, transiently transfected with WT 5-LO/GFP, were fractionated into soluble and microsomal membrane fractions in the absence of calcium, 5-LO was detected in both fractions (Fig. 3B). When calcium was present during fractionation, significantly less 5-LO/GFP was in the soluble fraction and more was in the membrane fraction, demonstrating calcium-dependent membrane association. Similar results were observed for 5-LO/GFP containing either the S271A substitution or the NLS1 mutation (Fig. 3C). When, alternatively, whole cells were stimulated with the calcium ionophore A23187 for 20 min and then fractionated, WT 5-LO/GFP was found to decrease in the soluble fraction and increase in the membrane fraction, as compared with unstimulated cells (Fig. 3B). Again, similar results were observed for 5-LO/GFP containing either the S271A substitution or the NLS1 mutation (Fig. 3B). This finding demonstrated that neither mutation significantly impaired membrane translocation of 5-LO, and this could not explain the difference in LTB4 generation.
Different Pools of 5-LO Translocate to Different Intracellular Membranes and Differ in Access to Substrate. The ability of WT and altered 5-LO/GFP to translocate to membranes was also directly visualized in intact cells stimulated with ionophore. After stimulation, WT 5-LO/GFP moved from its predominantly intranuclear site to the inner membrane of the nuclear envelope, resulting in a characteristic ring of fluorescence (Fig. 4A), as shown (28, 29). Stimulation of cells expressing the S271A form of 5-LO/GFP resulted in some cells with translocation like that of WT 5-LO/GFP (Fig. 4B). However, in other cells in the same population, 5-LO/GFP translocated primarily to membrane structures surrounding the nucleus, which appeared to fill the cytoplasmic compartment and were largely distinct from the nuclear envelope (Fig. 4B). Similar patterns of translocation were observed with the NLS1 mutant of 5-LO/GFP (data not shown). Stimulation of cells expressing mutNLS1+S271A 5-LO/GFP (Fig. 4C) or mutNLS1,2,3 5-LO/GFP (data not shown) resulted in redistribution to cytoplasmic and perinuclear membranes, with only a small portion actually ringing the nucleus. These results showed that intranuclear 5-LO translocated to a different site, the inner membrane of the nuclear envelope, than did cytoplasmic 5-LO. Because intranuclear 5-LO generates more LTB4 than cytoplasmic 5-LO, it would seem that the nuclear envelope must be a better site for 5-LO action than the other membranes accessed by cytoplasmic 5-LO.
Fig. 4.
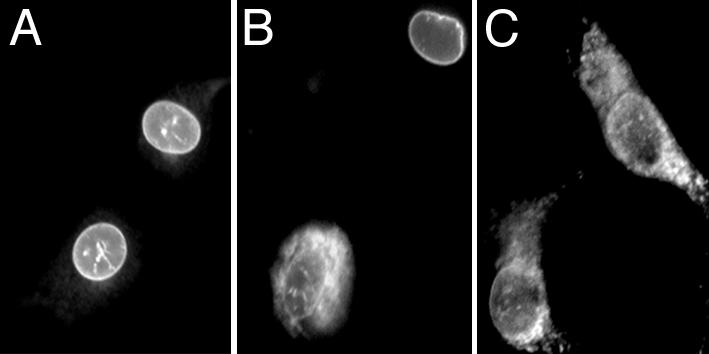
Nuclear and cytosolic 5-LO translocate to different subcellular sites after activation. Transfected cells were stimulated with 10 μM A23187 (15 min, 37°C) and fluorescently imaged. Representative fields of cells expressing activated WT 5-LO/GFP (A), S271A 5-LO/GFP (B), and mutNLS1+S271A 5-LO/GFP (C) are shown.
Previous studies have indicated that exogenous AA moves rapidly to the nucleus (30), as well as lipid bodies (31). This finding suggested that exogenously derived AA, as used in our metabolic experiments, might flow preferentially to nuclear 5-LO at the nuclear envelope. To test this, AA was fluorescently labeled with PDAM, which reacts spontaneously with the carboxyl group of the fatty acid (23). Live cells incubated with AA/PDAM were imaged continuously with phase contrast and fluorescence microscopy. In untransfected 3T3 cells incubated with 10 μM AA/PDAM for 15 min, fluorescence was largely restricted to perinuclear membranes as well as the nuclear envelope, as indicated by a bright ring around the nucleus (Fig. 5 B, E, and G). Many cells also had very bright, punctate staining suggestive of lipid bodies (Fig. 5B), as have been described in 3T3 cells (32). Time-matched imaging of cells before AA/PDAM addition showed minimal cellular autofluorescence (Fig. 5C). These results demonstrated that exogenous AA moves selectively and rapidly to nuclear and perinuclear membranes, as well as lipid body-like sites.
Fig. 5.
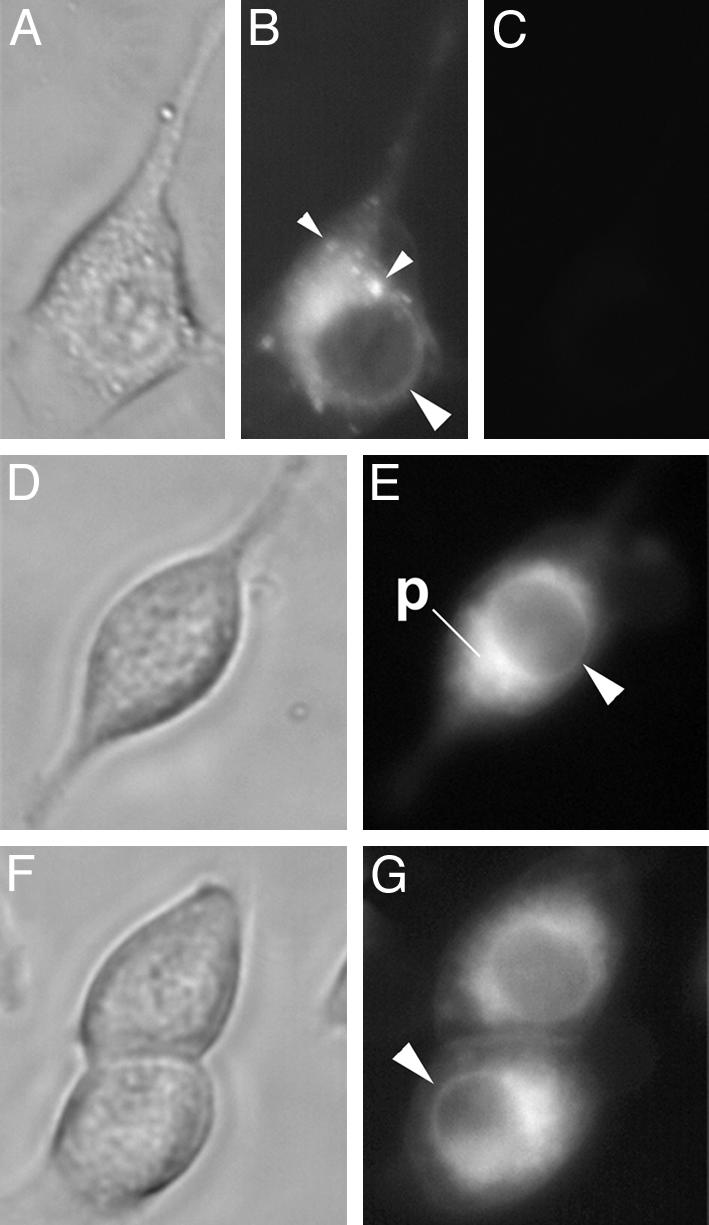
Exogenous AA accumulates at the perinuclear and nuclear membranes. AA/PDAM (10 μM) was added to 3T3 cells, and cells were imaged by phase contrast (A, D, and F) and fluorescent (B, E, and G) microscopy after 15 min. Time-matched fluorescent imaging of cells before addition of AA/PDAM (C) indicated minimal autofluorescence. Large arrowheads (B, E, and G) indicate nuclear envelope; small arrowheads (B) indicate punctate fluorescence suggestive of lipid bodies. p, perinuclear membranes. Sets of images are from independent experiments.
To determine whether added AA colocalizes with activated 5-LO/GFP, AA/PDAM was added to transfected cells before or after cell stimulation. As noted above, the variant of GFP used in these studies is poorly excited by the UV light used to excite PDAM. Again, AA/PDAM was observed to be at the nuclear and perinuclear membranes and also in lipid bodies (Fig. 6 A, C, and E), regardless of the localization of 5-LO/GFP. Before activation, WT 5-LO/GFP localization was distinctly different from that of AA/PDAM (Fig. 6 A and B). After activation with calcium ionophore, WT 5-LO/GFP localization strongly overlapped (Fig. 6 C and D). In contrast, the localization of activated (mutNLS1+S271A) 5-LO/GFP only partially overlapped with that of AA/PDAM (Fig. 6 E and F). These results colocalized the added substrate with the activated WT enzyme. The similar pattern observed for AA/PDAM with activated WT 5-LO/GFP suggested that exogenous AA may be more efficiently delivered to activated nuclear 5-LO than to activated cytosolic 5-LO.
Fig. 6.
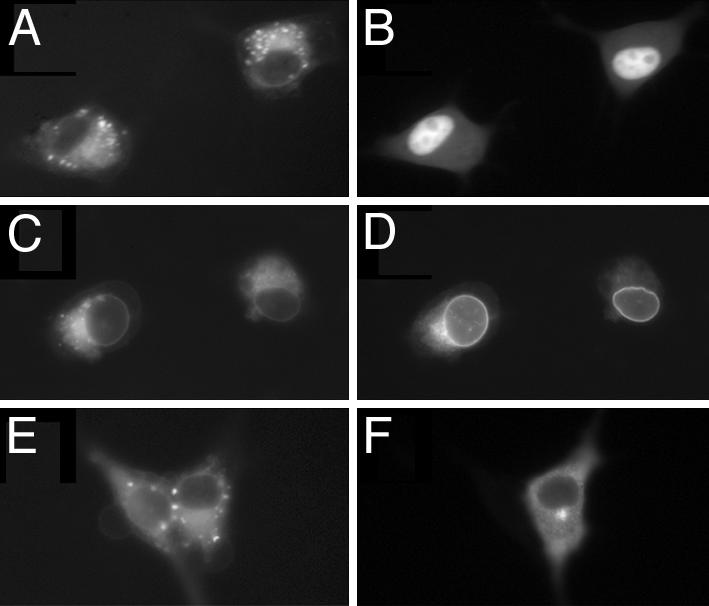
Exogenous AA colocalizes strongly with activated WT 5-LO/GFP. Cells were transfected with WT or (mutNLS1+S271A) 5-LO/GFP; AA/PDAM was added before (A and B) or after (C–F) cell stimulation with A23187. (A) AA localization in cells expressing WT 5-LO/GFP before activation. (B) 5-LO/GFP localization in cells shown in A.(C) AA localization in cells expressing WT 5-LO/GFP and activated before AA addition. (D) 5-LO/GFP localization in cells shown in C. (E) AA localization in cells expressing (mutNLS1+S271A) 5-LO/GFP and activated before AA addition. (F) 5-LO/GFP localization in cells shown in E.
Our results demonstrate unequivocally that the positioning of 5-LO in cells before cell stimulation can determine LTB4 synthetic capacity upon cell stimulation. This positioning seems to be critical because it allows 5-LO to interact with specific membranes; intranuclear 5-LO associates with the inner membrane of the nuclear envelope, and cytoplasmic 5-LO with endoplasmic reticulum and the outer nuclear membrane. Previous studies, using immunoelectron microscopy, have demonstrated these differences in destinations for activated intranuclear (33) vs. cytoplasmic 5-LO (21). However, it is only now that the significance of these differences has been revealed.
We also show that exogenous AA moves to the nuclear envelope and colocalizes with activated nuclear 5-LO. Early evidence showed that exogenous AA moves rapidly to the nucleus (30); recent studies suggest how this might happen. All fatty acids diffuse rapidly across membranes by a flip-flop mechanism (34). Medium and long chain fatty acids can then be bound by liver fatty acid-binding protein (34, 35) and rapidly shuttled into the nucleus (36). The result of this directed transport is selective accumulation of medium and long chain fatty acids at the nuclear envelope (35). Liver fatty acid-binding protein directly associates, within the nucleus, with peroxisome proliferator-activated receptor (37). Thus, AA may be carried by liver fatty acid-binding protein to the interior of the nucleus, where it can then interact with intranuclear 5-LO at the inner nuclear membrane.
Whereas exogenous AA may preferentially target the nucleus, we find that, under the conditions used here, some AA also moves to extranuclear membranes and lipid bodies. Thus, some substrate is available for metabolism by cytosolic 5-LO, and other factors must also contribute to the differences in LTB4 generation between cytosolic and nuclear 5-LO. For example, calcium, ATP, and oxidants affect 5-LO activity (1), and their levels may be more optimal within the nucleus than in the cytoplasm. Alternatively, export and secretion of nuclear-derived product may be more efficient than that of cytoplasm-generated LTB4. That is, export of LTB4 is a carrier-mediated process (39), and perhaps a fatty acid-binding protein positioned in the nucleus mediates export of the 5-LO product. The enzyme LTA4 hydrolase, which produces LTB4 from the 5-LO end product LTA4, is typically cytoplasmic (38), a localization that should favor LTB4 synthesis by cytosolic 5-LO. We suggest the possibility that carrier-mediated LTB4 secretion requires a fatty acid-bind protein to bind LTA4 in the nucleus, transport it to LTA4 hydrolase in the cytoplasm for conversion to LTB4, and then deliver the product to the plasma membrane for release. Although there appears to be no evidence to date for fatty acid-binding proteins mediating LTB4 export, the presence of a product-export mechanism that complements the substrate-import system could explain enhanced LTB4 secretion by cells with nuclear 5-LO.
The finding that Ser-271 plays a role in determining the subcellular localization of 5-LO in resting cells is surprising. Recent studies suggested that phosphorylation of Ser-271 serves to increase 5-LO activity (9) and nuclear membrane association of activated 5-LO (40). Also, activation of p38 MAPK, which presumably leads to phosphorylation of Ser-271 by activating MAPK-activated protein kinase 2 (8), increases nuclear association of 5-LO (10). Increased nuclear association might result from either increased membrane association or nuclear import, or both. Our data indicate that Ser-271 is not necessary for membrane association of 5-LO and that it does not regulate import of 5-LO through NLS1. We would suggest that Ser-271 affects LTB4 generation through its effect on the subcellular localization of 5-LO.
Our data also provide no evidence that Ser-271 directly affects 5-LO activity. We show that mutation of Ser-271 does not affect 5-LO cell-free activity (Fig. 3). Also, mutation of Ser-271 does not affect LTB4 synthesis when subcellular localization of 5-LO is unchanged (compare LTB4 synthesis by mutNLS1 vs. S271A, Fig. 1, and by mutNLS1,2,3 vs. mutNLS1+S271A, Fig. 2). In these experiments, cells were stimulated with the calcium ionophore A23187, which is a potent activator of p38 MAPK (27) and MAPK-activated protein kinase 2 (8, 9). Furthermore, cells were provided with AA, which promotes phosphorylation of Ser-271 in vitro (9) and also activates p38 MAPK (27). The apparent lack of effect of Ser-271 mutation on LTB4 synthesis, particularly when cells were activated in ways shown to optimally activate p38 MAPK and MAPK-activated protein 2, as well as phosphorylate Ser-271 in vitro, further argues that Ser-271 is more important in regulating LTB4 generation through its effects on 5-LO localization, rather than by increasing enzyme activity.
The link between 5-LO localization and LTB4 generation has biological implications. For example, nuclear localization of 5-LO would be anticipated to be important for driving immune and inflammatory responses involving increased LTB4 generation. This interpretation is supported by the observations that alveolar macrophages and mast cells, which line the mucosa, have nuclear 5-LO (33, 41) and that in neutrophils nuclear import of 5-LO occurs during their recruitment into sites of inflammation (11, 18). Our results also underline the alternative consequence that localization of 5-LO in the cytoplasm would be a relevant mechanism for minimizing LTB4 generation. For example, nuclear export of 5-LO might be an important step in resolving the inflammatory process. Further elucidation of factors that drive import and export of 5-LO, then, will have biological significance.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AI43574, R21 AI48141, and R01 HL50496.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 5-LO, 5-lipoxygenase; AA, arachidonic acid; MAPK, mitogen-activated protein kinase; LT, leukotriene; NLS, nuclear localization signal; PDAM, 1-pyrenyldiazomethane.
References
- 1.Funk, C. D. (2001) Science 294, 1871–1875. [DOI] [PubMed] [Google Scholar]
- 2.Bailie, M., Standiford, T., Laichalk, L., Coffey, M., Strieter, R. & Peters-Golden, M. (1996) J. Immunol. 157, 5221–5224. [PubMed] [Google Scholar]
- 3.Chen, N., Restivo, A. & Reiss, C. S. (2001) J. Neuroimmunol. 120, 94–102. [DOI] [PubMed] [Google Scholar]
- 4.Chen, X., Sheller, J., Johnson, E. & Funk, C. (1994) Nature 372, 179–182. [DOI] [PubMed] [Google Scholar]
- 5.Goulet, J., Snouwaert, J., Latour, A., Coffman, T. & Koller, B. (1994) Proc. Natl. Acad. Sci. USA 91, 12852–12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters-Golden, M. & McNish, R. (1993) Biochem. Biophys. Res. Commun. 196, 147–153. [DOI] [PubMed] [Google Scholar]
- 7.Brock, T. G., McNish, R. W. & Peters-Golden, M. (1995) J. Biol. Chem. 270, 21652–21658. [DOI] [PubMed] [Google Scholar]
- 8.Werz, O., Klemm, J., Samuelsson, B. & Radmark, O. (2000) Proc. Natl. Acad. Sci. USA 97, 5261–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werz, O., Szellas, D., Steinhilber, D. & Radmark, O. (2002) J. Biol. Chem. 277, 14793–14800. [DOI] [PubMed] [Google Scholar]
- 10.Werz, O., Burkert, E., Samuelsson, B., Radmark, O. & Steinhilber, D. (2002) Blood 99, 1044–1052. [DOI] [PubMed] [Google Scholar]
- 11.Brock, T. G., McNish, R. W., Bailie, M. B. & Peters-Golden, M. (1997) J. Biol. Chem. 272, 8276–8280. [DOI] [PubMed] [Google Scholar]
- 12.Brock, T. G., Anderson, J. A., Fries, F. P., Peters-Golden, M. & Sporn, P. H. S. (1999) J. Immunol. 162, 1669–1676. [PubMed] [Google Scholar]
- 13.Hanaka, H., Shimizu, T. & Izumi, T. (2002) Biochem. J. 361, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, S. M., Luo, M., Peters-Golden, M. & Brock, T. G. (2003) J. Biol. Chem. 278, 10257–10263. [DOI] [PubMed] [Google Scholar]
- 15.Jones, S. M., Luo, M., Healy, A. M., Peters-Golden, M. & Brock, T. G. (2002) J. Biol. Chem. 277, 38550–38556. [DOI] [PubMed] [Google Scholar]
- 16.Cowburn, A. S., Holgate, S. T. & Sampson, A. P. (1999) J. Immunol. 163, 456–465. [PubMed] [Google Scholar]
- 17.Hsieh, F. H., Lam, B. K., Penrose, J. F., Austen, K. F. & Boyce, J. A. (2001) J. Exp. Med. 193, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brock, T. G., Maydanski, E., McNish, R. W. & Peters-Golden, M. (2001) J. Biol. Chem. 276, 35071–35077. [DOI] [PubMed] [Google Scholar]
- 19.Sweitzer, T. D., Love, D. C. & Hanover, J. A. (2000) Curr. Top. Cell. Regul. 36, 77–94. [DOI] [PubMed] [Google Scholar]
- 20.Healy, A. M., Peters-Golden, M., Yao, J. P. & Brock, T. G. (1999) J. Biol. Chem. 274, 29812–29818. [DOI] [PubMed] [Google Scholar]
- 21.Woods, J. W., Evans, J. F., Ethier, D., Scott, S., Vickers, P. J., Hearn, L., Charleson, S., Heibein, J. A. & Singer, I. I. (1993) J. Exp. Med. 178, 1935–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouzer, C. A. & Kargman, S. (1988) J. Biol. Chem. 263, 10980–10988. [PubMed] [Google Scholar]
- 23.Nimura, N., Kinoshita, T., Yoshida, T., Uetake, A. & Nakai, C. (1988) Anal. Chem. 60, 2067–2070. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi, Y., Tomita, T., Senda, M., Hirai, A., Terano, T., Tamura, Y. & Yoshida, S. (1986) J. Chromatogr. 357, 199–205. [DOI] [PubMed] [Google Scholar]
- 25.Chen, C. J., Huang, H. S., Lee, Y. T., Yang, C. Y. & Chang, W. C. (1997) Biochem. J. 327, 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffey, M. J., Wilcoxen, S. E., Sporn, P. H. & Peters-Golden, M. (1998) Prostaglandins Other Lipid Mediat. 56, 103–117. [DOI] [PubMed] [Google Scholar]
- 27.Burkert, E., Szellas, D., Radmark, O., Steinhilber, D. & Werz, O. (2003) J. Leukocyte Biol. 73, 191–200. [DOI] [PubMed] [Google Scholar]
- 28.Chen, X.-S., Zhang, Y.-Y. & Funk, C. (1998) J. Biol. Chem. 273, 31237–31244. [DOI] [PubMed] [Google Scholar]
- 29.Christmas, P., Fox, J. W., Ursino, S. R. & Soberman, R. J. (1999) J. Biol. Chem. 274, 25594–25598. [DOI] [PubMed] [Google Scholar]
- 30.Neufeld, E. J., Majerus, P. W., Krueger, C. M. & Saffitz, J. E. (1985) J. Cell Biol. 101, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weller, P. F., Monahan-Earley, R. A., Dvorak, H. F. & Dvorak, A. M. (1991) Am. J. Pathol. 138, 141–148. [PMC free article] [PubMed] [Google Scholar]
- 32.Dvorak, A. M., Morgan, E. S., Tzizik, D. M. & Weller, P. F. (1994) Int. Arch. Allergy Immunol. 105, 245–250. [DOI] [PubMed] [Google Scholar]
- 33.Woods, J. W., Coffey, M. J., Brock, T. G., Singer, I. I. & Peters-Golden, M. (1995) J. Clin. Invest. 95, 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ek-Von Mentzer, B. A., Zhang, F. & Hamilton, J. A. (2001) J. Biol. Chem. 276, 15575–15580. [DOI] [PubMed] [Google Scholar]
- 35.Huang, H., Starodub, O., McIntosh, A., Kier, A. B. & Schroeder, F. (2002) J. Biol. Chem. 277, 29139–23151. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence, J. W., Kroll, D. J. & Eacho, P. I. (2000) J. Lipid Res. 41, 1390–1401. [PubMed] [Google Scholar]
- 37.Wolfrum, C., Borrmann, C. M., Borchers, T. & Spener, F. (2001) Proc. Natl. Acad. Sci. USA 98, 2323–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haeggstrom, J. Z. (2000) Am. J. Respir. Crit. Care Med. 161, S25–S31. [DOI] [PubMed] [Google Scholar]
- 39.Lam, B., Gagnon, L., Austen, K. F. & Soberman, R. J. (1990) J. Biol. Chem. 265, 13438–13441. [PubMed] [Google Scholar]
- 40.Werz, O., Klemm, J., Samuelsson, B. & Radmark, O. (2001) Blood 97, 2487–2495. [DOI] [PubMed] [Google Scholar]
- 41.Chen, X.-S., Naumann, T. A., Kurre, U., Jenkins, N. A., Copeland, N. G. & Funk, C. D. (1995) J. Biol. Chem. 270, 17993–17999. [DOI] [PubMed] [Google Scholar]