Abstract
Increasing evidence suggests that postnatal events, such as handling or maternal separation, can produce long-term changes in brain function. These are often expressed as changes in the profile of endocrine or behavioral responses to stress. Changes in γ-aminobutyric acid type A receptors (GABARs), which mediate the majority of fast synaptic inhibition in adult brain, have been proposed as one potential mediator of these behavioral effects. In the current article, we use a combination of single-cell electrophysiology and antisense mRNA amplification to demonstrate permanent molecular and functional differences in GABARs within hippocampal dentate granule neurons after as few as two episodes of neonatal handling with brief maternal separation. Adult animals that as pups experienced handling with maternal separation maintained a more immature GABAR phenotype and exhibited increased activity in response to swim stress. These findings demonstrate the exquisite sensitivity of the developing GABAergic system to even subtle environmental manipulations and provide an unique molecular mechanism by which postnatal handling with maternal separation may alter stress-related behavior.
Keywords: development, glucocorticoid, dentate granule neurons, patch clamping, single-cell antisense mRNA amplification
The developing nervous system can be exquisitely sensitive to even minor perturbations in the environment. It has been recognized for decades that handling of neonatal rats could produce profound effects on later neuroendocrine and behavioral responses to stress (1, 2). Repetitive brief handling in neonatal rats has been shown to result in permanent alterations in hippocampal glucocorticoid (GC) receptors, decreased GC responses to stress in adulthood, and a relative protection against age-related hippocampal neuronal death and cognitive impairments (3). Adult animals handled as pups also demonstrate decreased expression of fear-related behaviors under stressful conditions (4–6). The molecular mechanisms underlying handling-induced behavioral and cognitive changes are not fully understood. γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in mammalian brain and regulates both endocrine and behavioral responses to stress (7–9). Benzodiazepines (BZs) and other drugs that potentiate GABA currents can be potent anxiolytics. Rats exposed to early-life handling have been found to have altered BZ receptor levels in several brain regions including brainstem nuclei, amygdala, and frontal cortex (5, 6), but handling effects on GABA type A receptor (GABAR) subunit expression and function in hippocampal neurons have not, to our knowledge, previously been reported.
GABARs are chloride ion channel-associated ligand-gated heteromeric receptors composed of five subunits, which are modulated by BZs, barbiturates, zinc, and neurosteroids. Many genetically distinct subunit subtypes, including α1–6, β1–4, γ1–3, δ, ε, π, θ, and ρ1–3, have been identified (10, 11). GABARs can be assembled in different subunit combinations, resulting in a striking structural heterogeneity in the brain. Subunit composition varies in different brain regions (12), during development, (13) and in various disease states such as epilepsy (14). Different subunit combinations confer GABARs with distinct functional and pharmacological properties. Studies of recombinant GABARs have shown that presence of a γ subunit is essential for BZ augmentation (15). The γ2-containing GABARs are further pharmacologically differentiated into types I and II BZ receptor subtypes based on their affinity for certain compounds such as CL218,872 and zolpidem (16). Type I BZ receptors with high affinity for zolpidem contain an α1 subunit (17, 18). GABARs containing α2, α3, or α5 subunits demonstrate type II BZ pharmacology (low affinity for zolpidem), and α4-containing receptors are BZ-insensitive (17). Both α and γ subunit variants can affect zinc sensitivity. Recombinant GABARs containing an α1 subunit are less sensitive to zinc inhibition than those containing other α subtypes (19, 20). GABARs lacking a γ subunit are potently blocked by zinc (20). GABAR desensitization kinetics are also influenced by receptor subunit variants, with α1-containing receptors demonstrating faster desensitization than receptors containing other α subunits (21). Findings in recombinant systems are supported by studies of native GABARs that demonstrate slow desensitization, robust zinc inhibition, and modest zolpidem augmentation when α1 subunit expression is low, such as during early development (13, 22), in temporal lobe epilepsy (14), and in α1 knockout animals (23).
As part of ongoing studies in our laboratory examining the effects of neonatal seizures on subsequent GABAR properties in hippocampal dentate granule neurons (DGNs), we made the observation that adult rats that had experienced two episodes of handling with maternal separation during the neonatal period had dramatically altered GABAR properties compared with nonhandled adults. The specific protocol under which this was observed involved removing pups from mother and home cage for 30 min on postnatal day 9 (P9) and 6 h on P10 [subsequently referred to as handling with maternal separation (HMS) 30 min/360 min or HMS-30/360]. Pups received injections of either lithium or saline on P9 and saline on P10 to permit comparison with pups that were injected with pilocarpine on P10 to induce seizures (data of pilocarpine treatment not shown). We hypothesized that HMS might alter developmental regulation of hippocampal GABARs and that these GABAR changes may contribute to changes in behavioral responses to stress described by others (2–6). The hippocampus is a major target for regulation of the hypothalamic–pituitary–adrenal axis and is an important component of the neuroanatomical circuit mediating stress responses. To test our hypothesis, we performed a detailed examination of the long-term effects of HMS-30/360 on postsynaptic GABAR function and subunit expression in DGNs. We also attempted to differentiate which components of the HMS-30/360 protocol were critical for producing the long-term alterations in GABARs and whether HMS-induced GABAR changes were associated with alterations in hippocampal-dependent spatial memory and behavioral stress responses.
Methods
Animal Handling. The Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia approved all of the protocols used in this study. Pregnant female Sprague–Dawley rats were received at gestational day 17–19 from Charles River Breeding Laboratories. One single mother was housed per cage in a 12-h light/dark cycle with access to food and water ad libitum. After delivery, pups remained with the mother until P9 when litters were culled of females. Each litter of four to eight male pups was then assigned to a treatment group: nonhandled or HMS-30/360. There was no difference in the average number of pups per litter between the groups. In nonhandled litters, the pups were never separated from mother. In HMS-30/360 litters, pups were held by a gloved investigator on P9, injected i.p. with lithium chloride (3 meq/kg) or saline, and then placed in a clean cage separate from mother for 30 min. The pups were then returned to mother for 18 h, and on P10 they were again handled and injected with saline i.p. (0.1 ml) and then placed in a clean cage separate from mother for 6 h. Lithium chloride- and saline-injected pups demonstrated no differences by any parameters when analyzed separately and were therefore pooled to form the handled group. Pups were weighed before and after each episode of HMS and weighed again at weaning. To control for possible effects of the injections and to differentiate the critical component(s) of the HMS-30/360 paradigm, two additional groups of handled animals were studied. Pups in the HMS-30/30 group were handled and separated from the mother for 30 min on P9, then again for 30 min at P10. Pups in the HMS-360 group were not touched on P9 but were handled and then separated for 6 h on P10. Neither group received any injections. After P10, none of the groups was handled and no bedding changes were made until weaning at P21. A total of 13 litters were studied for GABAR properties: four nonhandled litters (seven rats) and nine HMS litters [three saline-injected HMS-30/360 litters, 11 rats; three lithium chloride-injected HMS-30/360 litters, 17 rats; three litters with no injection (HMS-30/30 and HMS-360), 15 rats]. An additional five HMS-30/360 litters (12 rats) and five nonhandled litters (10 rats) were used for the behavioral studies. All of the pups were permitted to grow to adulthood and were studied at P90 or older.
Isolation of Neurons. DGNs were acutely isolated by using a modified protocol (24). Brains were dissected in chilled, oxygenated (100%) Hepes medium containing 155 mM NaCl, 3 mM KCl, 1 mM MgCl2, 3 mM CaCl2, 25 mM glucose, and 10 mM Hepes (pH 7.35). The dentate gyrus was dissected and cubed from hippocampal slice. The tissue then was digested at 30°C for 30 min in pure oxygen-bubbled saline containing 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 25 mM glucose, 20 mM Pipes, and 3 mg/ml protease XXIII (pH 7.2). After digestion, tissue cubes were rinsed and incubated in an enzyme-free medium. Neurons were isolated by trituration in Hepes buffer by using fire-polished glass pipettes. The cell suspension was then plated on a Petri dish and allowed to settle for 10–15 min before recording. DGNs were identified based on their size (10-μm diameter) and distinctive unipolar morphology (round or oval cell body with a single dendritic process).
Electrophysiology. Whole-cell patch-clamp techniques were used to record isolated DGNs. GABA-evoked current was recorded at room temperature in tetrodotoxin-containing (500 nM) Hepes solution. DGNs were voltage-clamped at –20 mV by using electrodes filled with an intrapipette solution containing 100 mM Trizma phosphate (dibasic), 28 mM Trizma base, 11 mM EGTA, 2 mM MgCl2, 0.5 mM CaCl2, 10 mM Mg ATP, and 20 mM Tris phosphocreatinine, and 1 unit/μl RNasin (pH 7.35), 290 mosM. Recording signals were amplified by using an Axopatch-1D amplifier and saved by using pclamp 8.01 software (Axon Instruments, Foster City, CA) for off-line analysis. A solution change time of <3 msec was accomplished with a step-perfusion device (Warner Instruments, Hamden, CT). GABA, zinc chloride, and zolpidem (Sigma) were applied to the cell for 2 sec.
mRNA Measurement. Relative expression of GABAR mRNAs within individual DGNs was measured by using the technique of single-cell antisense mRNA (aRNA) amplification as described (13, 14). After patch-clamp recording, neuronal contents were aspirated into the recording pipette and expelled into a microcentrifuge tube, and first-strand cDNA synthesis was performed by using avian myeloblastosis virus reverse transcriptase (Seikagaku America, Rockville, MD) and oligonucleotide T7. Double-stranded DNA was made by incubation with dNTPs, T4 DNA polymerase, and the Klenow fragment of DNA polymerase I (Roche Molecular Biochemicals). The single-stranded hairpin loop was removed with S1 nuclease; the ends of the double-stranded template were blunted with T4 DNA polymerase and the Klenow fragment of DNA polymerase I for 2 h, and then the cDNA was used for synthesis of amplified aRNA by using NTPs and T7 RNA polymerase (Epicentre Technologies, Madison, WI). The aRNA was then again synthesized into a single-stranded cDNA template for a second round of amplification. The final aRNA synthesis included 25 pmol of [α-32P]CTP (Perkin–Elmer).
Slot-Blot Preparation and Expression Profiles. Each slot blot contained 16 GABAR subunit cDNAs (α1–6, β1–3, γ1–3, δ, ε, π, and θ), β-actin (internal reference), glial fibrillary acidic protein (control for glial contamination), neurofilament-L (marker for neuronal phenotype), and pBluescript plasmid (background) cDNAs and was prehybridized for 12 h at 42°C in 5 ml of prehybridization solution as described (13, 14). The blots were then hybridized with the radiolabeled aRNA probe from an individual cell for 60 h (42°C). The blots were washed to a final concentration of 0.2× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) at 42°C for 30 min and then directly exposed for 4 h to a Molecular Dynamics PhosphorImage screen with a linear dynamic range over 5 orders of magnitude. Intensity of the autoradiographic signal was measured by 3D laser scanning densitometry by using imagequant software from Molecular Dynamics. To correct for nonspecific background hybridization, the hybridization value for pBluescript plasmid was subtracted from the hybridization values for the cDNAs on the blot.
Behavioral Testing. HMS-30/360 and nonhandled rats were evaluated for hippocampal-dependent spatial memory in the Morris water maze as adults (between P90 and P120) according to described protocols (25). Performance on this task has been shown to be altered in association with DGN lesions (26). A pool (160 cm in diameter × 50 cm high) was filled with water (25 ± 1°C) to a depth of 33 cm, and a 10 × 10-cm Plexiglas platform was positioned in the center of the northeast quadrant of the tank 1 cm below the water surface. The water was made opaque by addition of nontoxic white tempra paint to prevent visualization of the platform. Visual cues were placed around the room to enable the rats to learn the platform location. On the first day, animals were placed in a bucket of 26 ± 1°C water for 60 sec to habituate them to the water immediately before being placed in the tank for their first trial. On days 1–4, each rat underwent six trials per day for a total of 24 trials. The rats were permitted to remain on the platform for 30 sec for the first trial and then 15 sec for subsequent trials. Rats were permitted a 2-min rest period between trials, after which the next trial was started. If the rat did not find the platform in 120 sec, it was manually placed on the platform. Latencies to escape onto platform and swim speed were recorded.
Because previous studies have demonstrated an impact of early environmental effects on stress-related behaviors, the present study quantified behavioral responses to an inescapable stress (5-min forced swim). The behavioral profile in this model, which has been termed “behavioral despair,” is characterized by a shift from active to passive behavior and has been thought to model some aspects of affective disorders (27–29). Importantly, a role for GABAR in these behaviors has been implicated by actions of BZs, which potentiate those of antidepressants in this model (30). Rats were subjected to the forced swim at least 2 wk after the Morris water maze. Rats were placed in a cylindrical glass tank (46 cm high × 20 cm diameter) filled with water (25 ± 1°C) to a depth of 30 cm for 5 min. Behavior in the forced swim test was scored by using a time-sampling procedure identical to that previously reported (31). Behaviors were scored at the end of each 5-sec epoch as (i) immobility, defined by floating without struggling (i.e., the rat makes only those movements necessary to keep its head above the water); (ii) swimming, defined as swimming movements that result in a change in position of at least one-fourth of the cylinder circumference; (iii) diving, in which the entire body is submerged; and (iv) climbing, defined as making active movements with forepaws in and out of the water (usually directed against the cylinder walls) but not resulting in a significant change in relative position in the cylinder. Although diving was scored separately, it occurred relatively infrequently and was therefore added to swimming scores in the final analysis. For all behavioral testing, behavior was scored by an observer who was blind to the experimental condition.
Corticosterone Measurement. Corticosterone levels in serum were obtained from HMS-30/360 pups at 5 p.m. on P10 at the end of the 6-h separation and at an equivalent time in nonhandled pups. The pups were rapidly decapitated (within 30 sec of being removed from the cage and <2 min after the investigator first entered the room), and blood was obtained via trunk bleed. Serum was separated by centrifugation and shipped to Tallie Z. Baram (University of California, Irvine) for analysis of corticosterone levels by RIA according to her published protocols (32).
Analysis of Data. For electrophysiological data analysis and corticosterone measurements, statistical significance of differences between single values for two groups was evaluated by using the unpaired Student's t test or the Mann–Whitney Rank Sum test for groups with unequal variance. One-way ANOVA and Tukey's test for post hoc comparison were used for comparisons of multiple groups. Curves were fitted by using the Marquardt–Levenberg nonlinear least-squares algorithm (pclamp 8.01). One-way ANOVA with post hoc t tests with Bonferroni correction for multiple comparisons were used for statistical comparison of the mean relative expression of each subunit mRNA between the groups. For the Morris water maze data, the mean latency to platform for each day was compared between groups by using a two-way ANOVA evaluating for effect of time and treatment. For the forced swim test data, the mean incidence of each behavior was compared between groups by using a one-way factorial ANOVA.
Results
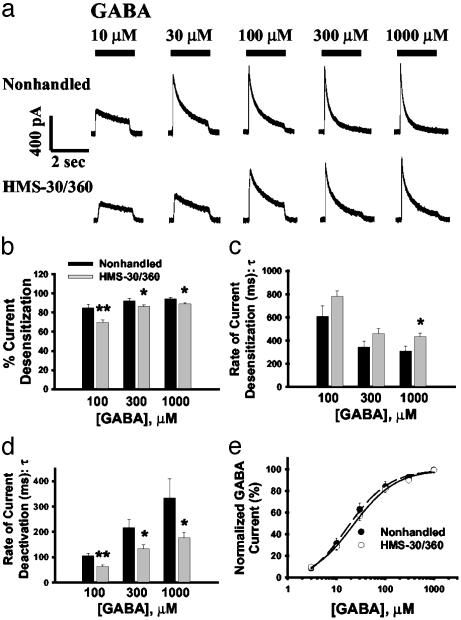
Neonatal HMS Alters GABAR Kinetics. Gross morphological examination of Timm's and cresyl-stained hippocampal sections from adult rats after HMS-30/360 at P10 revealed no cell loss or synaptic rearrangement (data not shown). The GABA-elicited current kinetics were examined after GABA application (see Methods). In response to GABA concentrations of 100–1,000 μM, there was significantly less current desensitization within the 2-sec period of GABA application in DGNs isolated from HMS-30/360 compared with nonhandled rats [Fig. 1 a and b; for all kinetic studies n = 12 cells (five rats) for HMS-30/360 and n = 14 cells (six rats) for nonhandled]. Further, the rate of GABA current desensitization was slower in DGNs from HMS-30/360 rats compared with nonhandled rats (Fig. 1c). Moreover, DGNs from nonhandled rats displayed longer current deactivation time constants than did HMS-30/360 animals (Fig. 1d). Functionally, the observed slower desensitization kinetics in HMS-30/360 DGNs, if manifested in the synapses, could result in an increased total charge transfer during GABAR activation, especially during repetitive stimulation. There was no significant difference in the GABA EC50 in DGNs isolated from HMS-30/360 rats (33.4 ± 7.2 μM) compared with that of nonhandled animals (27.9 ± 6.7 μM; Fig. 1e). There was also no significant difference in Hill coefficient in DGNs between these two groups (1.29 ± 0.09 for nonhandled and 1.22 ± 0.07 for HMS-30/360). GABA current density (defined as maximal GABA response divided by cell capacitance) was similar in the nonhandled and HMS-30/360 groups (176.6 ± 20.8 pA/pF for nonhandled and 167.2 ± 15.5 pA/pF for HMS-30/360; n = 18 cells from seven rats for each group).
Fig. 1.
HMS-30/360 altered GABA current kinetics without changing GABA efficacy or potency. (a) Representative traces for DGN responses to GABA concentrations of 10–1,000 μM in cells from a nonhandled control rat and a HMS-30/360 rat. (b) The percentage of GABA current desensitization within a 2-sec period of GABA application was decreased in the HMS-30/360, compared with nonhandled, DGNs. (c) The desensitization rate for GABA current was slower in the HMS-30/360 group compared with the nonhandled group as indicated by an increased current desensitization time constant (τ). (d) GABA current deactivation times were shortened in HMS-30/360, compared with nonhandled, groups. (e) Normalized GABA concentration–response curves demonstrated no significant difference in GABA EC50 between non-handled and HMS-30/360 groups (*, P < 0.05; **, P < 0.01; t test).
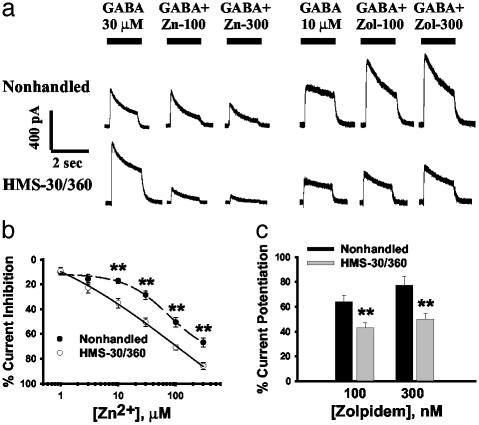
HMS-30/360 Alters GABAR Pharmacology. The most significant postnatal developmental changes in GABAR subunit expression in hippocampal DGNs involve the α1 and γ2 subunits (13), and we hypothesized that expression of these subunits was most likely to be altered by an early postnatal event such as HMS. We therefore examined the modulatory activity of zolpidem and zinc, which both have specific activity at these subunits. DGNs from HMS-30/360 rats showed both increased zinc inhibition of GABA currents and diminished augmentation of GABA currents by zolpidem compared with DGNs from nonhandled animals (Fig. 2). The concentration–response curve for zinc inhibition of GABA currents (Fig. 2b) demonstrated that zinc sensitivity was increased in DGNs from HMS-30/360 rats with the IC50 being significantly decreased (30.7 ± 5.8 μM; n = 23 cells from seven rats) compared with the nonhandled group (164.8 ± 29.5 μM; n = 16 cells from five rats; P < 0.001). There was also significantly less augmentation of GABA currents by zolpidem in DGNs isolated from HMS-30/360, compared with nonhandled, rats (Fig. 2c; n = 15 cells from three rats for nonhandled and 32 cells from seven rats for HMS-30/360). Based on the markedly increased sensitivity to zinc inhibition and decreased augmentation by zolpidem in HMS-30/360 DGNs, we predicted that these cells may express relatively lower amounts of α1 and/or γ2 mRNA compared with mRNA for other GABAR subunits.
Fig. 2.
HMS-30/360 altered GABAR pharmacology. (a) Representative responses of DGNs isolated from a nonhandled and a HMS-30/360 rat to 30 μM GABA application alone and coapplication with zinc (100 and 300 μM), and to 10 μM GABA application alone and coapplication with zolpidem (Zol; 100 and 300 nM). (b) Concentration–response curves for zinc inhibition of GABA current indicated that the zinc IC50 was significantly decreased in DGNs isolated from HMS-30/360, compared with nonhandled, rats. (c) GABA current potentiation by zolpidem, a type I BZ receptor-specific positive modulator, was significantly decreased in DGNs from the HMS-30/360 rats (t test; **, P < 0.01).
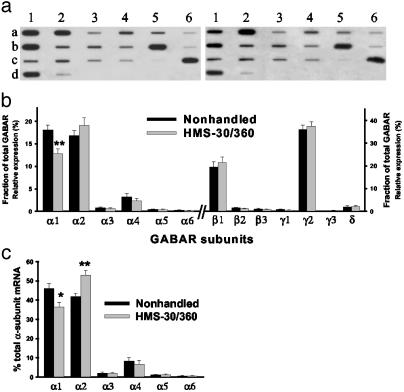
HMS-30/360 Alters GABAR Subunit Expression. To examine whether the changes in GABAR function and pharmacology were associated with altered GABAR subunit mRNA expression, single-cell aRNA amplification was performed on DGNs isolated from nonhandled (n = 24 cells from seven rats) and HMS-30/360 (n = 22 cells from eight rats) groups. Mean relative expression of α1 subunit mRNA in DGNs (as a percentage of total GABAR subunit expression in each cell) from HMS-30/360 rats was decreased to 67% of nonhandled values (Fig. 3b). This decrease in α1 subunit expression resulted in α2 becoming the predominant α subunit in DGNs from HMS-30/360 animals, whereas α1 was the predominant α subunit in nonhandled animals (Fig. 3c). No other significant changes in expression were seen in the other subunits. This finding suggests that the changes in GABAR kinetics and pharmacology seen in DGNs from HMS-30/360 rats may be secondary to a relative decrease in α1-containing GABARs and increase in α2-containing GABARs.
Fig. 3.
Changes in GABAR function are associated with decreased α1 mRNA expression. (a) aRNA expression profiles from single DGNs acutely isolated from nonhandled (Left) and HMS-30/360 (Right) rats. Slot blot demonstrates hybridization intensities of radiolabeled aRNA probe to a slot blot containing GABAR subunit cDNAs α1–6 (a1–a6), β1–3 (b1–b3), γ1–3 (b4–b6), δ, ε, π, and θ (c1–c4), glial fibrillary acidic protein (c5), neurofilament-L (c6), β-actin (d1), and pBluescript (d2). (b) Mean relative expression (± SEM) of GABAR subunit mRNA as a percentage of total GABAR mRNAs in DGNs isolated from HMS-30/360 vs. nonhandled rats. (c) Mean relative expression of each α-subunit mRNA as a percentage of total α subunit mRNA expression in DGN from HMS-30/360 vs. nonhandled rats (*, P < 0.05; **, P < 0.01, one-way ANOVA with post hoc t tests with Bonferroni correction for multiple comparisons).
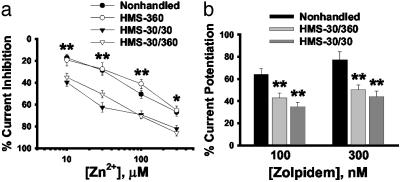
Brief HMS also Evokes GABAR Changes. Our HMS-30/360 paradigm involved both repeated neonatal handling and repeated maternal separation (30 min on P9 and 6 h on P10), as well as potentially stressful i.p. injections, all of which could potentially contribute to altered GABAR properties (5, 6, 33–35). To differentiate which of these environmental manipulations were critical for the long-term alterations in GABAR properties, we compared GABAR modulation in adult rats subjected to a single 6-h episode of handling and separation on P10 with no injections (HMS-360) or repeated episodes of handling with 30 min separations on P9 and P10 with no injections (HMS-30/30) with our HMS-30/360 paradigm. We found that a single prolonged episode of handling/separation (HMS-360) did not lead to long-term changes in modulation of GABA currents (Fig. 4; n = 18 cells from four rats). In contrast, two short handling/separation events (HMS-30/30) were sufficient to result in long-term alterations in both zinc and zolpidem modulation of GABA currents of similar magnitude to our HMS-30/360 paradigm (Fig. 4; n = 21 cells from four rats).
Fig. 4.
Repeated separation is critical for GABAR functional changes. (a) The long-term changes in GABAR zinc sensitivity observed in the HMS-30/360 group were similar to those observed after repeated brief separations (HMS-30/30) but not a single long separation (HMS-360). (b) The potentiation of zolpidem on GABA currents was also similar between both repeated separation groups (HMS-30/360 and HMS-30/30) and different from the nonhandled groups (*, P < 0.05; **, P < 0.01; one-way ANOVA and Tukey's test for post hoc comparison).
Effects of HMS-30/360 on Corticosterone Levels. Serum corticosterone levels were measured in HMS-30/360 pups at a single time point at the end of the 6-h separation on P10 and were not different between HMS-30/360 and nonhandled pups [3.63 ± 0.97 μg/dl for HMS-30/360 (n = 5) and 2.92 ± 0.42 μg/dl for nonhandled (n = 4)].
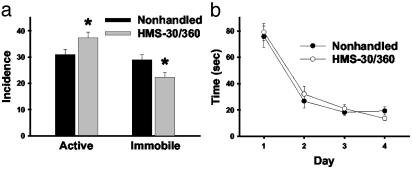
HMS Alters Stress-Related Behavior. To examine whether any behavioral or cognitive alterations occurred in association with the GABAR changes seen after HMS, nonhandled and HMS-30/360 rats were tested for stress-related behavior in response to a forced swim and for spatial memory by using the Morris water maze. Significant differences in stress-related behaviors were seen between the groups. HMS-30/360 rats exhibited a higher incidence of active behaviors (climbing and swimming) and a lower incidence of passive behavior (floating) compared with nonhandled rats (Fig. 5a). Interestingly, although the non-handled group exhibited an equal incidence of active and passive behaviors during the 5-min swim, the incidence of active behaviors predominated over passive behavior in the HMS-30/360 group [t(22) = d5.2, P < 0.001]. In contrast, HMS-30/360 did not alter the time course of learning in the Morris water maze procedure (Fig. 5b). A two-way ANOVA revealed a significant effect of time [F(3,88) = 29.7, P < 0.0001] but no significant effect of treatment [F(1,88) = 0.059] or interaction [F(3,88) = 0.072; n = 12 HMS-30/360 and 10 nonhandled rats] on the mean time to reach the platform. These findings indicate that the HMS-30/360 protocol produces long-term changes in behavioral stress responses but does not significantly alter hippocampal-dependent memory function.
Fig. 5.
HMS-30/360 alters stress-related behavioral responses but not spatial memory performance. (a) Quantification of behaviors of adult rats that were either handled/separated or nonhandled as pups during exposure to a forced swim. Bars represent the mean incidence of individual behaviors as described in Methods (± SEM). Active behavior was calculated as the sum of climbing and swimming. HMS-30/360 rats exhibited an increase in active behavior and decrease in immobility compared with nonhandled rats (*, P < 0.05, ANOVA). Additionally, the incidence of active behaviors compared with passive behaviors is higher in the HMS-30/360 group (P < 0.001) but equal in the non-handled group. (b) Activity of HMS-30/360 and nonhandled rats as adults in Morris water maze. The abscissa indicates the day of training, and the ordinate indicates the mean time required to reach the platform. A two-way ANOVA revealed a significant effect of time (P < 0.0001) but no significant effect of treatment or interaction.
Discussion
In the current study we show that two episodes of HMS during early postnatal development resulted in long-term changes in postsynaptic GABAR function and subunit expression in hippocampal DGNs that were associated with more active responses to stress in adulthood. Previous studies of the effects of neonatal handling have involved multiple repeated episodes of handling over periods of 14–25 days (3, 5, 6, 35). In this study, however, as few as two episodes of brief HMS were sufficient to produce permanent changes in hippocampal GABARs, suggesting that the GABAergic system is exquisitely sensitive to even minor environmental perturbations during postnatal development. Of additional note, prolonged separation was not necessary to produce this effect, and serum corticosterone levels were not significantly elevated at the end of our HMS-30/360 protocol (although they were only measured at a single time point and elevations occurring at other points in the protocol could have been missed). Further, we found no evidence of weight loss after the HMS nor any subsequent difference in growth between HMS-30/360 and nonhandled rats to suggest impaired nutrition played a role in either the molecular or behavioral changes seen. These results support the concept proposed by Meaney and coworkers (36, 37) that differences in maternal care after neonatal handling rather than the handling procedure itself may be the critical factor in “programming” subsequent behavioral responses to stress.
The present findings expand on work by others, suggesting that handling-induced behavioral changes may be mediated by alterations in the GABAergic system. Regional differences in GABAR binding (5, 6, 35) and γ2 subunit mRNA expression in forebrain, amygdala, and brainstem (6) after repeated episodes of neonatal handling have previously been reported. The specific GABAR subunit rearrangement found in hippocampal DGNs in the current study, however, suggests a potential molecular mechanism by which postnatal HMS may result in altered stress-related behavior. When α1 levels are diminished after HMS, relative levels of α2 mRNA are increased, and α2 becomes the predominant α subunit in DGNs (Fig. 3c). Recent evidence suggests that the anxiolytic effect of BZs is mediated by the GABAR α2 subunit (38), and elevated numbers of α2-containing GABARs may potentially enhance the GABAR response to anxiety and stress. Interestingly, the higher ratio of α2 to α1 subunit seen in adults after HMS is similar to the GABAR expression pattern seen in DGNs during early postnatal development (13), suggesting that neonatal HMS promotes maintenance of an immature GABAR phenotype. It must be noted, however, that although changes in stress-related behavior are occurring in the HMS animals concurrently with GABAR alterations in hippocampus, there is no demonstration of causality. It remains possible that the observed GABAR changes are an epiphenomenon unrelated to the behavioral change or that changes in GABARs in brain regions outside the hippocampus that were not examined are, in fact, critical for changes in behavior. Further studies will be needed to establish a causal connection between DGN GABAR alterations and stress-related behaviors.
The signaling pathways mediating HMS-induced changes in GABAR development are not fully elucidated. GCs and chronic stress are known to alter GABAR expression and pharmacology in adult animals (39–41), but their effects on GABARs during development are less clear. We found no acute differences in corticosterone levels after our HMS-30/360 protocol; however, as previously noted, we only looked at a single time point. Meaney et al. have reported long-term changes in GC receptor number (3) and expression of cAMP-inducible transcription factors (42) in hippocampus after neonatal handling, either of which could mediate differential GC effects on GABAR expression.
In summary, the current findings have several important implications for our understanding of the long-term effects of early-life environmental manipulations. First, the finding of HMS-induced GABAR changes in hippocampal DGNs suggests that the cellular and molecular consequences that may occur after neonatal handling extend beyond the recognized changes in the hypothalamic–pituitary–adrenal axis. Secondly, the association of GABAR changes in DGNs with changes in behavioral stress responses adds to our understanding of the array of cell networks and cell signaling mechanisms that may play a critical role in determining stress responsiveness. The decrease in GABAR α1 subunits and coincident increase in relative expression α2 subunits in DGNs of HMS animals with more active stress responses is particularly intriguing and suggests that the α2 subunit may play an important role in mediating endogenous stress and anxiety responses in addition to mediating the anxiolytic effects of exogenous BZs. Finally, as our initial observations on HMS effects were discovered in “control” animals in a study of seizure effects during early development, this study should serve as an important cautionary note for developmental researchers regarding the critical importance of identically handled controls in any experiments examining treatment effects on the immature brain.
Acknowledgments
We thank Dr. Tallie Z. Baram at the University of California, Irvine for performing the analysis of plasma corticosterone levels. This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-38595 (to A.R.B.-K.) and NS-38572 and NS-34203 (to D.A.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GABA, γ-aminobutyric acid; GABAR, GABA type A receptor; DGN, dentate granule neuron; aRNA, antisense mRNA; GC, glucocorticoid; BZ, benzodiazepine; HMS, handling with maternal separation; Pn, postnatal day n.
References
- 1.Levine, S. (1957) Science 126, 405–406. [DOI] [PubMed] [Google Scholar]
- 2.Levine, S., Haltmeyer, G. C., Karas, G. G. & Denenberg, V. H. (1967) Physiol. Behav. 2, 55–63. [Google Scholar]
- 3.Meaney, M. J., Aitken, D. H., van Berkel, C., Bhatnagar, S. & Sapolsky, R. M. (1988) Science 239, 766–768. [DOI] [PubMed] [Google Scholar]
- 4.Ladd, C. O., Huot, R. L., Thrivikraman, K. V., Nemeroff, C. B., Meaney, M. J. & Plotsky, P. M. (2000) Prog. Brain Res. 122, 81–103. [DOI] [PubMed] [Google Scholar]
- 5.Bodnoff, S. R., Suranyi-Cadotte, B., Quirion, R. & Meaney, M. J. (1987) Eur. J. Pharmacol. 144, 105–107. [DOI] [PubMed] [Google Scholar]
- 6.Caldji, C., Francis, D., Sharma, S., Plotsky, P. M. & Meaney, M. J. (2000) Neuropsychopharmacology 22, 219–229. [DOI] [PubMed] [Google Scholar]
- 7.Paulsen, O. & Moser, E. I. (1998) Trends Neurosci. 21, 273–278. [DOI] [PubMed] [Google Scholar]
- 8.Collinson, N., Kuenzi, F. M., Jarolimek, W., Maubach, K. A., Cothliff, R., Sur, C., Smith, A., Otu, F. M., Howell, O., Atack, J. R., et al. (2002) J. Neurosci. 22, 5572–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martijena, I. D., Rodriguez Manzanares, P. A., Lacerra, C. & Molina, V. A. (2002) Synapse 45, 86–94. [DOI] [PubMed] [Google Scholar]
- 10.Barnard, E. A., Skolnick, P., Olsen, R. W., Mohler, H., Sieghart, W., Biggio, G., Braestrup, C., Bateson, A. N. & Langer, S. Z. (1998) Pharmacol. Rev. 50, 291–313. [PubMed] [Google Scholar]
- 11.Bonnert, T. P., McKernan, R. M., Farrar, S., Le Bourdelles, B., Heavens, R. P., Smith, D. W., Hewson, L., Rigby, M. R., Sirinathsinghji, D. J. S., Brown, N., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 9891–9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisden, W., Laurie, D. J., Monyer, H. & Seeburg, P. H. (1992) J. Neurosci. 12, 1040–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks-Kayal, A. R., Shumate, M. D., Jin, H., Rikhter, T. Y., Kelly, M. E. & Coulter, D. A. (2001) J. Neurochem. 77, 1266–1278. [DOI] [PubMed] [Google Scholar]
- 14.Brooks-Kayal, A. R., Shumate, M. D., Jin, H., Rikhter, T. Y. & Coulter, D. A. (1998) Nat. Med. 4, 1166–1172. [DOI] [PubMed] [Google Scholar]
- 15.Pritchett, D. B., Sontheimer, H., Shivers, B. D., Ymer, S., Kettenmann, H., Schofield, P. R. & Seeburg, P. H. (1989) Nature 338, 582–585. [DOI] [PubMed] [Google Scholar]
- 16.Luddens, H., Korpi, E. R. & Seeburg, P. H. (1995) Neuropharmacology 34, 245–254. [DOI] [PubMed] [Google Scholar]
- 17.Pritchett, D., Luddens, H. & Seeburg, P. H. (1989) Science 245, 1389–1392. [DOI] [PubMed] [Google Scholar]
- 18.Criswell, H. E., McCown, T. J., Moy, S. S., Oxford, G. S., Mueller, R. A., Morrow, A. L. & Breese, G. R. (1997) Neuropharmacology 36, 1641–1652. [DOI] [PubMed] [Google Scholar]
- 19.White, G. & Gurley, D. (1995) NeuroReport 6, 461–464. [DOI] [PubMed] [Google Scholar]
- 20.Draguhn, A., Verdorn, T. A., Ewert, M., Seeburg, P. H. & Sakmann, B. (1990) Neuron 5, 781–788. [DOI] [PubMed] [Google Scholar]
- 21.Tia, S., Wang, J. F., Kotchabhakdi, N. & Vicini, S. (1996) Neuropharmacology 35, 1375–1382. [DOI] [PubMed] [Google Scholar]
- 22.Hutcheon, B., Morley, P. & Poulter, M. O. (2000) J. Physiol. (London) 522, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicini, S., Ferguson, C., Prybylowski, K., Kralic, J., Morrow, A. L. & Homanics, G. E. (2001) J. Neurosci. 21, 3009–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, S. S., Gong, Q. H., Hsu, F.-C., Markowitz, R. S., ffrench-Mullen, J. M. & Li, X. (1998) Nature 392, 926–930. [DOI] [PubMed] [Google Scholar]
- 25.Morris, R. (1984) J. Neurosci. Methods 11, 47–60. [DOI] [PubMed] [Google Scholar]
- 26.Jeltsch, H., Bertrand, F., Lazarus, C. & Cassel J. C. (2001) Neurobiol. Learn. Mem. 76, 81–105. [DOI] [PubMed] [Google Scholar]
- 27.Porsolt, R. D., Anton, G., Blavet, N. & Jalfre, M. (1978) Eur. J. Pharmacol. 47, 379–391. [DOI] [PubMed] [Google Scholar]
- 28.Weiss, J. M., Goodman, P. A., Losito, B. G., Corrigan, S., Charry, J. M. & Bailey, W. H. (1981) Brain Res. Rev. 3, 167–205. [Google Scholar]
- 29.Thierry, B., Steru, L., Chermat, R. & Simon, P. (1984) Behav. Neural Biol. 41, 180–189. [DOI] [PubMed] [Google Scholar]
- 30.Flugy, A., Gagliano, M., Cannizzaro, C., Novara, V. & Cannizzaro, G. (1992) Eur. J. Pharmacol. 214, 233–238. [DOI] [PubMed] [Google Scholar]
- 31.Detke, M. J., Rickels, M. & Lucki, I. (1995) Psychopharmacology 121, 66–72. [DOI] [PubMed] [Google Scholar]
- 32.Avishai-Eliner, S., Eghbal-Ahmadi, M., Tabachnik, E., Brunson, K. L., Baram, T. Z. (2001) Endocrinology 142, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sibug, R. M., Oitzl, M. S., Workel, J. O. & de Kloet, E. R. (2001) Brain Res. 912, 95–98. [DOI] [PubMed] [Google Scholar]
- 34.Sutanto, W., Rosenfeld, P., de Kloet, E. R. & Levine, S. (1996) Brain Res. Dev. Brain Res. 92, 156–163. [DOI] [PubMed] [Google Scholar]
- 35.Bolden, S. W., Hambley, J. W., Johnston, G. A. & Rogers, L. J. (1990) Neurosci. Lett. 111, 258–262. [DOI] [PubMed] [Google Scholar]
- 36.Caldji, C., Diorio, J. & Meaney, M. J. (2000) Biol. Psychiatry 48, 1164–1174. [DOI] [PubMed] [Google Scholar]
- 37.Caldji, C., Tannenbaum, B., Sharma, S., Francis, D., Plotsky P. M. & Meaney, M. J. (1998) Proc. Natl. Acad. Sci. USA 95, 5335–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Low, K., Crestani, F., Keist, R., Benke, D., Brunig, I., Benson, J. A., Fritschy, J. M., Rulicke, T., Bluethmann, H., Mohler, H., et al. (2000) Science 290, 131–134. [DOI] [PubMed] [Google Scholar]
- 39.Orchinik, M., Weiland, N. G. & McEwen, B. S. (1994) Mol. Cell. Neurosci. 5, 451–458. [DOI] [PubMed] [Google Scholar]
- 40.Orchinik, M., Carroll, S. S., Li, Y. H., McEwen, B. S. & Weiland, N. G. (2001) J. Neurosci. 21, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cullinan, W. E. & Wolfe, T. J. (2000) Brain Res. 887, 118–124. [DOI] [PubMed] [Google Scholar]
- 42.Meaney, M. J., Diorio, J., Francis, D., Weaver, S., Yau, J., Chapman, K. & Seckl, J. R. (2000) J. Neurosci. 20, 3926–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]