Abstract
The structure of ecological communities reflects a tension among forces that alter populations. Marine ecologists previously emphasized control by locally operating forces (predation, competition, and disturbance), but newer studies suggest that inputs from large-scale oceanographically modulated subsidies (nutrients, particulates, and propagules) can strongly influence community structure and dynamics. On New Zealand rocky shores, the magnitude of such subsidies differs profoundly between contrasting oceanographic regimes. Community structure, and particularly the pace of community dynamics, differ dramatically between intermittent upwelling regimes compared with relatively persistent down-welling regimes. We suggest that subsidy rates are a key determinant of the intensity of species interactions, and thus of structure in marine systems, and perhaps also nonmarine communities.
Many ecological processes determine the structure and dynamics of communities and ecosystems. Theoretical and experimental advances have led to a growing awareness that different processes operate at different spatial and temporal scales (1, 2). Capturing the full richness of ecosystem dynamics thus requires studies ranging across a wide range of scales and allowing effective evaluation of the relative impact of the relevant factors. Doing so can be challenging. The logistics are daunting, especially in using the power of experimentation over large spatial scales. One solution, the “comparative-experimental” approach (3), involves replicated local-scale experimentation at multiple sites spanning larger scales, coupled with local-scale repeated sampling of factors that vary at characteristically larger scales.
In its early development, marine community ecology focused on the dynamic consequences of primarily local-scale processes such as species interactions and physical disturbance (4). More recently, marine and nonmarine ecologists alike have documented the influence on communities of larger-scale phenomena including subsidies of materials and propagules transferred between adjacent ecosystems (5–8). Although evidence for the importance of subsidies is growing, questions remain about their impact, generality, magnitude, and interdependence and the physical and biotic mechanisms that underlie them.
Here we use the comparative-experimental approach to address the role of large-scale oceanographic phenomena in structuring communities on New Zealand rocky shores. Based on earlier results (6, 9), we predicted that intertidal community structure and dynamics would reflect the coastal oceanographic regime. We hypothesized that the influences of oceanographically modulated subsidies (propagules, as a cause of increases in population density of benthic species, and the concentration of phytoplankton and detritus, as food for filter feeders) would be high with upwelling and low with downwelling.
Study System
Previous research on the west and east coasts of the South Island of New Zealand (pairs of sites 100–500 m apart on each coast) revealed striking differences in community dynamics and structure (3, 10). The high and midzones at both sets of sites were similar and dominated by barnacles (high) and mussels (mid); the lower shores were dominated by mussels and kelp on the east coast and bare rock and algal turf on the west coast. Recruitment and growth of sessile invertebrates and the effect and rate of predation were low on the east coast and high on the west coast.
These patterns and between-coast oceanographic differences (10–15) suggested the hypothesis that intercoastal differences in these intertidal communities were determined by coastal (0–5 km from shore) oceanography. We predicted that the patterns summarized above would reflect dynamics detectable at larger geographic scales and would vary with upwelling. Specifically, we predicted that decreased upwelling would result in decreased subsidies, and therefore decreased abundances of prey and their predators, and, through feedback effects in the food web, decreased impacts of predators on prey. Our approach to testing these predictions was to identify workable sites spaced relatively evenly along the upwelling gradient and to implement intensive, identical studies of community structure, subsidies, and species interactions at each site.
Oceanographic Conditions
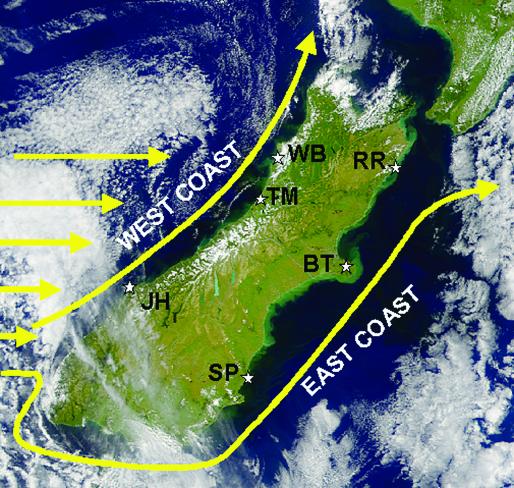
The Tasman Current approaches the South Island from the west and splits into the northeastward-flowing Westland Current and the Southland Current, which flows around the southern end of the island and then is guided by the Subantarctic Convergence northeastward along the east coast (10, 16) (Fig. 1). Satellite imagery suggests that the northwest coast [north of ≈43°S, 170°E (13)] has an “intermittent” upwelling regime, with alternations between upwelling (visible as cold water close to shore and warm water offshore) and downwelling (visible as more uniform temperatures along an offshore axis). Further, water temperatures quantified onshore revealed several upwelling events in spring–summer 1994–1995 (10). On the east coast, available satellite imagery and intertidal water temperatures revealed little or no upwelling. Hence, west-coast circulation should alternate between northward and offshore vs. southward and onshore transport of larvae and particulates with periodic pulses of nutrients, whereas east-coast circulation should be primarily northward and onshore, leading to minimal nutrient inputs and little phytoplankton growth. Although persistent shoreward transport in downwelling regions should deliver larvae to the coast, rates should be low because, without upwelling, little phytoplankton would be available to support larval populations.
Fig. 1.
Representative SeaWiFS image of the South Island, October 23, 2002, showing the Tasman Current flowing eastward and the resulting Westland and Southland Currents. The study sites are as shown. Note the eddy (light green) to the northeast of BT on the Banks Peninsula. [Image provided by ORBIMAGE (Copyright 2003, Orbital Imaging Corporation) and processing by NASA Goddard Space Flight Center.]
Methods
Study Sites. The west-coast sites were Woodpecker Bay (WB), Twelve-Mile Beach (TM), 50 km south of WB, and Jackson Head (JH), 295 km south of TM (Fig. 1), and the east-coast sites were Raramai (RR), Box Thumb (BT), 145 km south of RR, and Shag Point (SP), 260 km south of BT. Study periods were October 1999–March 2000; October 2000; March and October–December 2001; January, March, and October 2002; and January 2003.
Community Structure. Percent cover of sessile organisms was estimated by using phototransects. Transect tapes 30 m in length were placed parallel to the edge of the water in the center of the vertical range of each zone. At 3-m intervals, a quadrat (0.5 × 0.5 m) was placed on the landward side of the tape and photographed (10 quadrats per transect). To quantify organisms concealed by algae, photographs were taken before and after displacing the canopy to the side. Each quadrat was subdivided into a grid of 5 × 5 subquadrats, each of which occupied 4% of the area of the quadrat. Estimates of each space occupant were made visually from the photos and are accurate to ≈1% cover (17).
Sea-star (primarily Stichaster australis) and whelk (Thais orbita and Haustrum haustorum) densities in the low zone were estimated in 1 × 5-m belt transects (five to eight) through the mid-low portion of the shore. Sea-star wet mass and arm length (madreporite to tip of an opposite arm) and whelk shell length were measured in the field.
Oceanographic Conditions. We quantified intertidal air and seasurface temperatures using Onset Tidbits (Onset Computer Corporation, Pocasset, MA), accurate to 0.5°C. Replicate pairs of loggers set to record at hourly intervals were fastened to the substratum at +1.0-m tide height. An sas program (SAS Institute Inc., Cary, NC) that combined temperature data, tide-chart data, and time of recording was used to differentiate air and water temperatures.
Monthly and daily upwelling indices (January 1990–June 2002) were obtained from the Pacific Fisheries Environmental Laboratory (www.pfel.noaa.gov) to quantify average upwelling conditions by site and upwelling event duration, respectively. Coordinates (in degrees of latitude and longitude, respectively) used to retrieve indices were: WB, 41.5 S, 171.5 E; TM, 42.5 S, 170.5 E; JH, 44.5 S, 167.5 E; SP, 47.5 S, 171.5 E; BT, 45.5 S, 173.5 E; and RR, 43.5 S, 175.5 E. Event duration is a critical measure because phytoplankton blooms require ≈5+ days to develop (18–20). Using daily records, we quantified the length of upwelling and downwelling events and also evaluated the correspondence between events lasting >5 days and intertidal temperature changes.
Nutrients and particulates were quantified every 2–4 weeks from October 1999 to March 2000 [chlorophyll a (chl-a)] and December 2001 to January 2002 (chl-a, total particulates). Samples were taken by filling acid-washed opaque plastic (high-density polyethylene) 250-ml bottles at the edge of the water at low tide. Nutrient samples (50 ml) were filtered through 25-mm combusted Whatman glass-fiber filters with a pore size of 0.7 μm. The filtrate and filter were transported to the laboratory on ice and frozen for later quantification of nitrate, nitrite, phosphate, and silicate on a Lachat autoanalyzer (21) or chl-a by using a Turner Designs (Sunnyvale, CA) fluorometer (model TD-700). Particulate concentrations (ash-free dry mass) were quantified three times during December 2001 and January 2002 by filtering five replicate 200-ml water samples on preashed, preweighed glass-fiber filters, drying the filters, weighing, ashing, and reweighing each sample.
Subsidies and Ecological Processes. We quantified recruitment and growth of sessile invertebrates, colonization rate, rates of interactions among sessile organisms, and predation rate. Barnacle recruitment was quantified by using settlement plates [10 × 10-cm, 5-mm-thick polyvinylchloride plates coated with Saf-T-Walk, a rubbery plastic with a textured surface (22)]. Cyprids and metamorphs were identified and counted under a dissecting microscope. Mussel recruitment was quantified by using plastic-mesh ovoids that mimic the filamentous algal substrata to which settling mussel larvae attach (“Tuffys”) (10, 23). In the laboratory, recruits were detached from the Tuffys by dissolving their byssal attachments in bleach for 5 min, shaking the jar, and sieving (53 μm) and then were counted under a microscope. Recruit collectors (eight of each) were replaced monthly at each site.
Barnacle growth rates were estimated by using pitted 10 × 10-cm settlement plates (10). Each plate had an 8 × 8 grid of 1-mm-diameter pits drilled on one surface. Barnacles settle preferentially in depressions, allowing each to grow unimpeded by crowding from other barnacles. Plates were deployed from October 1999 to March 2000. Most pits were occupied by barnacles by December 1999.
Rates of predation, colonization, and competition were obtained experimentally. Predation rates were quantified in cage (stainless-steel mesh, 15 × 15 × 5 cm) exclosure experiments. Twenty mussels (Mytilus galloprovincialis), 3–4 cm in length, were translocated to each 15 × 15-cm plot in the low intertidal, held down with plastic mesh until they had reattached (≈2 months), and subjected to one of four treatments in each of five replicates. Marked plots (+predators and –mesh) were marked by screws but had no mesh barriers. Roofs (+predators and +mesh) were covered by mesh (15 × 15 × 5 cm) fastened to the rock on two sides and open on two sides. Exclosures (–predators and +mesh) were protected from benthic predators from all sides, and whelk enclosures (+2 whelks and +mesh) were protected from sea stars by complete mesh cages. Enclosures tested predation rates by whelks independent of sea-star predation rates. Mussel survival was determined every 2–4 weeks from November 1999 to March 2000. Total predation was the difference in survival of mussels in exclosures vs. marked plots (survival did not differ between marked plots and roofs; repeated measures ANOVA, multivariate analysis, P ≥ 0.34). Whelk predation was the difference in survival between exclosures (no predators) and enclosures (+whelks only).
Colonization of sessile biota (mostly barnacles, mussels, and algae) was estimated by clearing plots and monitoring changes in cover over time. Total cover was estimated visually in digital photographs taken at monthly (first 5 months) to 6-month intervals from November 1999 to March 2002. The rate of colonization in each of 10 plots per site was estimated as the slope of a regression through the time series of total percent cover, estimated as 100 minus percent bare space. The rate of competitive overgrowth of barnacles by mussels was estimated as the slope of decline in barnacle cover due to displacement by mussels in five cage exclosures per site.
Data Analysis. In all analyses, data were tested for adherence to assumptions of normality, independence of error terms, and equality of variance. In most cases, log(lnx + 1) transformation was necessary to meet the assumptions. We analyzed oceanic regimes in two ways: by coast (east vs. west), to reflect the upwelling regime as suggested by the Bakun indices, and by larval transport regime (high, intermediate, and low), to reflect the expected magnitude of larval transport. Nested ANOVA tested for differences between coasts (upwelling regimes) or larval transport regimes and among sites nested within coasts or transport regimes. Correlation coefficients determined the degree of association among different factors.
Results
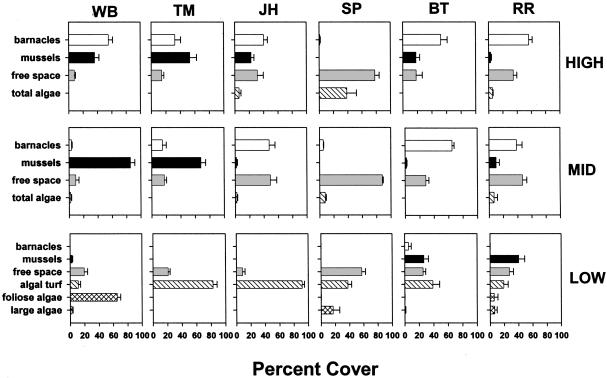
Community Structure. Community structure varied dramatically among the six sites (Fig. 2). High and midzones were dominated by barnacles and mussels and mussels, respectively, at the two northwest sites but were characterized by barnacles at JH, BT, and RR and by bare space at SP. Low zones were characterized by macroalgae at all but the two northerly east-coast sites where mussels dominated or codominated. Available space was generally higher at all east-coast sites and at JH. Sea stars (S. australis, predators of mussels and barnacles) were abundant on the west and rare on the east coast (Fig. 4C) (10, 24). On the west coast, average sea-star size (mean g of live mass ± 1 SE), often a reflection of food availability (23), decreased from north to south (185.6 ± 14.4, 100.7 ± 2.7, 30.5 ± 1.4 at WB, TM, and JH, respectively). Whelks (T. orbita and H. haustorum), often important carnivores elsewhere (25), were sparse everywhere, ranging among sites from 0 to 0.4/0.25 m2, with no apparent relation to oceanographic conditions.
Fig. 2.
Abundance (mean percent cover + SE) of barnacles, mussels, macroalgae, and “free” space (bare or algal-crust-occupied rock surface).
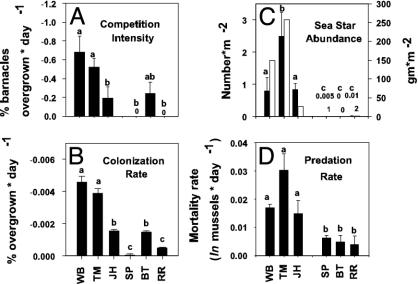
Fig. 4.
Rates of mussel–barnacle competition (n = 5) (A), recolonization of cleared plots (n = 15) (B), predation on transplanted mussels by all predators (total, mostly sea stars + whelks) and by whelks alone (whelk) (n = 5) (D), and density (□, n = 5) and wet mass (▪) of the sea star S. australis (C). Bars with different lowercase letters adjacent to them differ at P < 0.05.
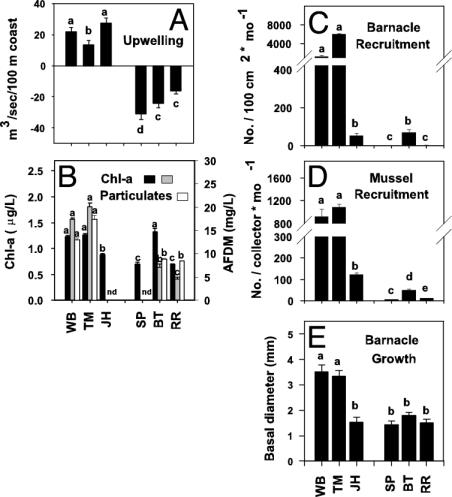
Oceanographic Conditions. Monthly mean upwelling indices (mean + 1 SE of monthly indices) indicate that the west coast is upwelling-dominated, whereas the east coast is downwelling-dominated (Fig. 3A). In daily temperature records from 1999 to 2002, west-coast upwelling events (upwelling conditions of ≥5 days) occurred more than twice as often as downwelling events (≥5 days) (percent upwelling vs. percent downwelling: WB/TM, 38.8 vs. 19.7; JH, 43.4 vs. 17.0). Opposite trends occurred on the east coast (SP, 13.6 vs. 40.4; BT, 12.7 vs. 39.0; RR, 7.9 vs. 39.2). Upwelling events were more likely to be associated with sharp temperature drops on the west than on the east coast. At WB, TM, and JH, 66.7% (of 15 events), 86.7% (of 15), and 64.7% (of 17) were associated with drops of 2–3°C. On the east coast, few upwelling events of ≥5 days occurred (two, one, and two each at SP, BT, and RR, respectively), and only three of these five were associated with appreciable temperature drops (one at BT and two at RR). Thus, both large-scale upwelling indices and direct measurement of the thermal environment onshore suggest distinctly different upwelling regimes on the west and east coasts.
Fig. 3.
Mean (+1 SE) of 1990–2002 Bakun upwelling indices (A); average chl-a concentration 1999–2002 (black bars) and chl-a (gray bars) and total particulates (white bars) in December 2001 and January 2002 (n = 5 each) (B); and rates of recruitment (n = 8 each) (C and D) and barnacle growth (n = 5) (E). Bars with different lowercase letters adjacent to them differ at P < 0.05. In nested ANOVA, upwelling differed between coasts (1,4 df, P = 0.0005) and among sites (4,894 df, P = 0.0019). (B) Chl-a and particulates differed among sites [site (coast) and site (transport regime), P < 0.0001 for both). Overall average chl-a and 2001–2002 chl-a, respectively, did not differ with either coast (P > 0.41, P > 0.16) or transport regime (P = 0.11, P = 0.34), but particulates were marginally greater on the west coast (P = 0.06).
Subsidies and Ecological Processes. The magnitudes of most processes were greater at west-coast sites (Figs. 3 and 4). Upwelling variability was positively associated with recruitment, colonization, sea-star abundance, and total predation rate but not with chl-a concentration, barnacle growth, or competition (Table 1). Barnacle recruitment, but not mussel recruitment, was correlated with chl-a concentration. Basal trophic-level processes (barnacle growth, competition, and colonization) were positively correlated with recruitment (Table 1), suggesting that these rates may be driven by larval availability. Total predation rate and sea-star abundance were positively correlated with recruitment but not with chl-a, barnacle growth, competition, or colonization rate (Table 1). Particulate concentration was quantified at too few sites to be included in the correlation analysis.
Table 1. Correlation coefficients (above diagonal) and probabilities (below diagonal) for associations between standardized (coefficient of variation) upwelling indices, oceanic subsidies, basal level processes, and top-down factors.
| Subsidies
|
Basal level processes
|
Top-down factors
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Upwelling (CV) | Chl-a | Barnacle recruitment | Mussel recruitment | Prey recruitment | Barnacle growth | Competition intensity | Colonization rate | Sea star density | Total predation rate |
| Upwelling | × | 0.59 | 0.85 | 0.92 | 0.88 | 0.72 | 0.77 | 0.83 | 0.89 | 0.93 |
| Chl-a | 0.22 | × | 0.86 | 0.77 | 0.82 | 0.74 | 0.74 | 0.77 | 0.50 | 0.49 |
| Barnacle recruitment | 0.03 | 0.03 | × | 0.98 | na | 0.90 | 0.87 | 0.94 | 0.85 | 0.83 |
| Mussel recruitment | 0.01 | 0.07 | 0.0006 | × | na | 0.90 | 0.91 | 0.97 | 0.86 | 0.85 |
| Prey recruitment | 0.02 | 0.047 | na | na | × | 0.91 | 0.88 | 0.95 | 0.88 | 0.86 |
| Barnacle growth | 0.11 | 0.09 | 0.01 | 0.01 | 0.01 | × | 0.96 | 0.97 | 0.74 | 0.70 |
| Competition intensity | 0.08 | 0.09 | 0.02 | 0.01 | 0.02 | 0.003 | × | 0.98 | 0.65 | 0.64 |
| Colonization rate | 0.04 | 0.07 | 0.004 | 0.002 | 0.003 | 0.001 | 0.0006 | × | 0.77 | 0.75 |
| Sea star density | 0.02 | 0.32 | 0.03 | 0.026 | 0.02 | 0.09 | 0.16 | 0.07 | × | 0.99 |
| Total predation | 0.008 | 0.32 | 0.04 | 0.03 | 0.03 | 0.12 | 0.17 | 0.09 | 0.0002 | × |
Coefficients in italic type and probabilities in bold-faced type indicate significant correlations. n = 6 (site means) for all comparisons. All means were In-transformed before analysis. na, Not applicable; CV, coefficient of variation. Whelk predation was not correlated to any factor.
In the upwelling regime analysis, mussel recruitment, predation, and sea-star density were higher on the west coast, and recruitment, barnacle growth, competition, colonization, and sea-star density differed among sites within each coast (Table 2 and Figs. 3 and 4). Neither chl-a nor particulates differed with coast, but both differed among sites (Fig. 3). The lack of between-coast differences between these latter processes seems partly a consequence of low power; with more sites, the trends may have achieved statistical significance.
Table 2. Nested ANOVA on ecological processes with respect to coast (west vs. east) or transport regime (high, intermediate, and low) (fixed) and site (coast) or site (transport regime) (random).
| Process | F (coast) | F [site (coast)] | F (regime) | F [site (regime)] |
|---|---|---|---|---|
| Barnacle recruitment | 6.240.07 | 377.7<0.0001 | 46.30.006 | 41.8<0.0001 |
| Mussel recruitment | 13.900.02 | 126.0<0.0001 | 59.90.004 | 17.9<0.0001 |
| Barnacle growth | 3.350.14 | 11.81<0.0001 | 76.50.002 | 0.520.67 |
| Competition rate | 5.560.08 | 4.160.011 | 40.260.007 | 0.460.71 |
| Colonization rate | 6.880.059 | 34.4<0.0001 | 71.50.003 | 2.570.06 |
| Predation rate | 29.50.006 | 1.300.30 | 2.150.26 | 5.960.004 |
| Whelk predation rate | 4.600.09 | 0.620.65 | 0.750.54 | 1.190.34 |
| Sea star density | 13.630.02 | 2.720.045 | 3.010.19 | 4.990.005 |
Effects of coast and transport regime were tested over the nested term mean squares. Numerator and denominator degrees of freedom (df), respectively, were 1,4 for tests of coast and 2,3 for tests of transport regime; error df ranged from 20 to 84. Probabilities are superscripted; bold P values are statistically significant.
Discussion
Coastal Ecosystem Dynamics. Our results support the hypothesis that oceanographically induced gradients in the supplies of particulate food and propagules mediate patterns of community structure through bottom-up effects on species interactions. Our prediction that community processes such as recruitment and predation would be higher on the upwelling-dominated west coast and lower on the downwelling-dominated east coast were supported by our measurements and experiments testing these factors. Inspection of the data, however, suggested a somewhat more complicated interpretation than this simple upwelling-vs.-downwelling difference. Patterns of mussel and barnacle recruitment, colonization rate, and competition rate, for example, suggested the existence of three clusters of sites, not two. Specifically, rates of these factors tended to be high at the two northerly sites (WB and TM) on the west coast, intermediate at JH on the southwest coast and BT on the central east coast, and low at the northern and southern east-coast sites (RR and SP) (Figs. 3 and 4).
What additional effects might these three clusters of sites represent? Because rates of recruitment, colonization, and competition may all depend in part on rates of transport of larvae to shore, we reconsidered how larval transport might be modified by other effects. First, as suggested by satellite imagery, JH seems dominated oceanographically by the divergence of the Tasman Current into the Westland and Southland Currents (Fig. 1). Thus, the primary supply of subsidizing factors at this site most likely comes from offshore, more-depleted waters rather than upstream coastal sources as at TM and WB further to the north in the Westland Current. We hypothesize that this is why JH has lower rates of recruitment, colonization, and competition and lower phytoplankton concentrations than WB and TM despite its occurrence in an upwelling regime (Fig. 3). Second, although BT, similar to RR and SP, is in a downwelling region, its higher magnitudes of recruitment, colonization, and competition are also consistent with the occurrence of relatively higher transport. In this case, this is likely due to a persistent eddy that forms to the north of the Banks Peninsula (Fig. 1; see also ref. 26). We hypothesize that this eddy concentrates larvae and phytoplankton, leading to higher rates of inputs of these factors despite the occurrence of this site in a downwelling-dominated regime.
If we reanalyze the results, grouping them by larval transport regime (high = WB and TM; intermediate = JH and BT; and low = SP and RR), the among-site heterogeneity evident in the upwelling-vs.-downwelling analysis is reduced greatly [Table 2, regime and site (regime) analysis]. In this analysis, recruitment, competition, and colonization all decreased clinally along the gradient of decreasing transport (Table 2 and Figs. 3 and 4). Note, however, that recruitment, predation, and sea-star density still differed between sites within regime, suggesting that other differences may have an influence as well.
Species interactions (competition and predation) were most intense at the two high-transport sites on the upwelling coast (WB and TM) where prey recruitment and food for sessile prey were higher. The weakest species interactions occurred at the two low-transport sites (SP and RR) where bottom-up inputs were least. Competition was intermediate at the two intermediate transport sites, JH and BT. In contrast, predation rates, which differed so strongly with upwelling regime, did not vary with a more finely subdivided transport regime.
Predation rates, Stichaster abundances, and Stichaster size structures (another sea star, Coscinasterias calamaria, is primarily subtidal in distribution and is sparse at all sites) suggest that top-down effects respond to oceanographic regime in at least two ways. First, sea-star abundances and predation rates were high on the west coast and low on the east coast. These differences and the presence of small, presumably recently recruited sea stars (which, similar to mussels and barnacles, broadcast their gametes into the plankton) at all west-coast but no east-coast sites suggest that upwelling regime-related differences in sea-star propagule supply influenced sea-star abundance and predation rates. Second, prey supply evidently modifies these patterns. The north–south decrease in sea-star size on the west coast (see Results) is associated with a similar decrease in mussel abundance (Fig. 2) and recruitment (Fig. 3D), suggesting an effect of food supply on sea-star biomass (Fig. 4C). Although prey supply is intermediate at BT (Fig. 3D), the apparent absence of sea-star recruits at this site precludes the establishment of a sea-star population despite a relatively high abundance of prey (Fig. 2).
Community structure reflected these variable linkages between bottom-up inputs and top-down effects. Invertebrate prey and their predators were most abundant (Figs. 2 and 4) where subsidies were strongest. Mussel abundance was particularly sensitive to these variations. Mussel density was greatest where recruitment was highest and in zones above the sea-star foraging range (WB and TM high and midzones) or where predation was low (BT and RR low zones). Mussel density was lowest where either recruitment was low (JH and east-coast high and midzones and SP low zone) or predation was strong (west-coast low zone).
Oceanographic Mechanisms. Phytoplankton concentrations differed among sites but not as strikingly as recruitment. Further, compared with some coastal areas, concentrations were consistently low and did not vary consistently with upwelling regimes (Fig. 3) (3, 6). These observations suggest that processes underlying phytoplankton dynamics differ from those driving patterns of recruitment.
Our analyses suggest that larval transport is likely to be driven by coastal circulation patterns that result from upwelling on the west coast and downwelling on the east coast but with local modification by headlands or current pattern. Both coasts experience frequent wind-generated shifts in current direction, but the regime on the west coast is more likely to favor high recruitment rates. Upwelling occurs more often on the west coast and at frequencies that favor offshore accumulation of larvae at upwelling fronts (see Results) and subsequent delivery of these larvae shoreward during relaxation or downwelling (27). Downwelling is the prevalent condition of the east coast (Fig. 3A). With a low frequency of short-duration upwelling events on this coast, there are few opportunities for the occurrence of nutrient-pulse-fueled phytoplankton blooms. Thus availability of food for larvae should be low, as is suggested by the lower concentrations of particulates on the east coast (Fig. 3C).
But if upwelling prevails on the west coast, why aren't there dense phytoplankton blooms or at least higher concentrations of phytoplankton? In coastal upwelling systems, the supply of inorganic nutrients is strongly coupled to the transport of cold water from depth. Comparatively warmer water temperatures in New Zealand (12–17°C) relative to Oregon (7–13°C) evident in our long-term monitoring records point to reduced nutrient supply to innershelf systems on either coast of the South Island. The lack of increased phytoplankton blooms on the west coast further suggests that factors additional to nutrient supply are also important in regulating innershelf productivity. Stratification, advective losses, pelagic food-web structure, and trace-element limitation can offer additional constraints on coastal phytoplankton growth. The information needed to evaluate these alternatives is unavailable, but each could be examined with available methods and technology.
Scale. Many have emphasized the importance of “scaling up” or better matching the scales of our understanding of community dynamics to the relevant environmental scales. In the sea, oceanographic scales can range up to thousands of kilometers, whereas ecological scales are typically several orders of magnitude less (4). Because determining mechanism is critical to understanding phenomenology, scaling up of ecological research, where possible, should employ the comparative-experimental method, whereby identically designed experiments and measurements are carried out at multiple locations along larger environmental scales (3, 28–30).
Conclusions
We conclude that larval transport and recruitment are driven by coastal oceanography. Recruitment is highest in intermittent upwelling regions, intermediate at sites in other oceanographic conditions (e.g., gyres and eddies) that tend to concentrate larvae, and lowest in downwelling regions lacking gyres or eddies. Unexpectedly, the concentration of phytoplankton and detritus (as food for filter feeders) was associated less strongly with upwelling regime. We believe that unlike results reported elsewhere (6), larval transport and oceanic productivity are decoupled in New Zealand.
We hypothesized that the importance of subsidies would vary with upwelling. Our results suggest that this indeed is the case but that additional factors are also important. Our study indicates that on New Zealand shores, the “pace” of life, or the rate of key ecological processes, is strongly linked to oceanographic conditions through the differing ways oceanic waters influence the magnitude of key subsidies. Through propagule delivery, oceanographic regimes determine the abundances of important prey, which drives how quickly space is colonized and probably how intensely space occupiers compete for space. Particle delivery rates may also modify space occupation by influencing growth rates of sessile invertebrates. This in turn influences community structure and prey availability by altering the outcome of competition, which is partly a consequence of growth rates of competitors. Finally, high abundances of prey may influence predator abundance by increasing survival of predator recruits, increasing predator growth rates, and by determining the “characteristic” body size of the larger predators in the population. Through such influences, predation rate can be driven by oceanographic subsidies. Similar relationships between bottom-up/top-down linkages and material flows between ecosystems have been described in other aquatic and terrestrial environments (31, 32), suggesting a general pathway of ecological organization.
Acknowledgments
We thank D. Schiel of the University of Canterbury (Christchurch, New Zealand) for comments, hosting our research, and providing logistical support; and E. Sanford for comments. R. Murdoch of NIWA (National Institute of Water and Atmosphere, Wellington, New Zealand) provided access to satellite imagery. Research was supported by the Andrew W. Mellon Foundation, the David and Lucile Packard Foundation, the R. W. and B. C. Lundeen Fund, the Wayne and Gladys Valley Foundation, National Science Foundation Predoctoral fellowships (to T.L.F., M.E.S.B., H.L., and M.S.W.), and Fulbright and Natural Sciences and Engineering Research Council Predoctoral fellowships (to R.R.).
Abbreviations: WB, Woodpecker Bay; TM, Twelve-Mile Beach; JH, Jackson Head; RR, Raramai; BT, Box Thumb; SP, Shag Point; chl-a, chlorophyll a.
See commentary on page 11927.
References
- 1.Pascual, M. & Ellner, S. P. (2000) Ecology 81, 2767–2780. [Google Scholar]
- 2.Levin, S. A. (1992) Ecology 73, 1943–1967. [Google Scholar]
- 3.Menge, B. A., Sanford, E., Daley, B. A., Freidenburg, T. L., Hudson, G. & Lubchenco, J. (2002) Ecol. Res. 17, 1–16. [Google Scholar]
- 4.Menge, B. A. (1992) Ecology 73, 755–765. [Google Scholar]
- 5.Gaines, S. D. & Roughgarden, J. (1985) Proc. Natl. Acad. Sci. USA 82, 3707–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menge, B. A., Daley, B. A., Wheeler, P. A., Dahlhoff, E., Sanford, E. & Strub, P. T. (1997) Proc. Natl. Acad. Sci. USA 94, 14530–14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano, S. & Murakami, M. (2001) Proc. Natl. Acad. Sci. USA 98, 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polis, G. A. & Hurd, S. D. (1996) Am. Nat. 147, 396–423. [Google Scholar]
- 9.Bustamante, R. H., Branch, G. M., Eekhout, S., Robertson, B., Zoutendyk, P., Schleyer, M., Dye, A., Hanekom, N., Keats, D., Jurd, M. & McQuaid, C. (1995) Oecologia 102, 189–201. [DOI] [PubMed] [Google Scholar]
- 10.Menge, B. A., Daley, B. A., Lubchenco, J., Sanford, E., Dahlhoff, E., Halpin, P. M., Hudson, G. & Burnaford, J. L. (1999) Ecol. Monogr. 69, 297–330. [Google Scholar]
- 11.Stanton, B. R. (1976) N. Z. J. Mar. Freshwater Res. 10, 445–467. [Google Scholar]
- 12.Stanton, B. R. & Moore, M. I. (1992) N. Z. J. Mar. Freshwater Res. 26, 339–358. [Google Scholar]
- 13.Vincent, W. F., Howard-Williams, C., Tildesley, P. & Butler, E. (1991) N. Z. J. Mar. Freshwater Res. 25, 21–42. [Google Scholar]
- 14.Grieg, M. J., Ridgway, N. M. & Shakespeare, B. S. (1988) N. Z. J. Mar. Freshwater Res. 22, 391–400. [Google Scholar]
- 15.McKendry, I. G., Sturman, A. P. & Owens, I. F. (1988) N. Z. J. Mar. Freshwater Res. 22, 91–100. [Google Scholar]
- 16.Knox, G. A. (1975) in Biogeography and Ecology in New Zealand, ed. Kuschel, G. (W. Junk, The Hague, The Netherlands), pp. 353–403.
- 17.Dethier, M. N., Graham, E. S., Cohen, S. & Tear, L. M. (1993) Mar. Ecol. Prog. Ser. 96, 93–100. [Google Scholar]
- 18.Huppert, A., Blasius, B. & Stone, L. (2002) Am. Nat. 159, 156–171. [DOI] [PubMed] [Google Scholar]
- 19.Gross, M. G. (1990) Oceanography: A View of the Earth (Prentice–Hall, Englewood Cliffs, NJ).
- 20.Smetacek, V. & Passow, U. (1990) Limnol. Oceanogr. 35, 228–234. [Google Scholar]
- 21.Atlas, E. L., Hager, S. W., Gordon, L. I. & Park, P. K. (1971) A Practical Manual for the Use of the Technicon Auto Analyzer in Sea Water Nutrient Analysis (Dept. of Oceanography, Oregon State Univ., Corvallis).
- 22.Farrell, T. M., Bracher, D. & Roughgarden, J. (1991) Limnol. Oceanogr. 36, 279–288. [Google Scholar]
- 23.Menge, B. A., Berlow, E. L., Blanchette, C. A., Navarrete, S. A. & Yamada, S. B. (1994) Ecol. Monogr. 64, 249–286. [Google Scholar]
- 24.Paine, R. T. (1971) Ecology 52, 1096–1106. [Google Scholar]
- 25.Menge, B. A. (2000) J. Exp. Mar. Biol. Ecol. 250, 257–289. [DOI] [PubMed] [Google Scholar]
- 26.Carter, L. & Herzer, R. H. (1979) N. Z. Oceanogr. Inst. Mem. 83, 1–33. [Google Scholar]
- 27.Roughgarden, J., Pennington, J. T., Stoner, D., Alexander, S. & Miller, K. (1991) Acta Oecol. 12, 35–51. [Google Scholar]
- 28.Dayton, P. K. (1971) Ecol. Monogr. 41, 351–389. [Google Scholar]
- 29.Menge, B. A. (1976) Ecol. Monogr. 46, 355–393. [Google Scholar]
- 30.Menge, B. A. (1991) Oecologia 88, 1–8. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter, S. R., Cole, J. J., Hodgson, J. R., Kitchell, J. F., Pace, M. L., Bade, D., Cottingham, K. L., Essington, T. E., Houser, J. N. & Schindler, D. E. (2001) Ecol. Monogr. 71, 163–186. [Google Scholar]
- 32.Polis, G. A., Anderson, W. B. & Holt, R. D. (1997) Annu. Rev. Ecol. Syst. 28, 289–316. [Google Scholar]