Abstract
Apical membrane antigen 1 (AMA-1) is a promising vaccine candidate for Plasmodium falciparum malaria. Antibodies against AMA-1 of P. falciparum (PfAMA-1) interrupt merozoite invasion into RBCs. Initially localized within the apical complex, PfAMA-1 is proteolytically processed and redistributed circumferentially on merozoites at about the time of their release and invasion into RBCs. An 83-kDa precursor form of PfAMA-1 is processed to 66-kDa and then to 48- and 44-kDa products. We show that, even at low concentrations, IgG antibodies against correctly folded recombinant PfAMA-1 cross-linked and trapped the 52-, 48-, and 44-kDa proteolytic products on merozoites. These products are normally shed into the culture medium. At higher concentrations antibodies inhibited invasion into RBCs and caused a reduction in the amount of 44- and 48-kDa products, both on merozoites and in the culture medium. A corresponding increase also occurred in the amount of the 66- and 52-kDa forms detected on the merozoites. These antibodies also prevented circumferential redistribution of AMA-1. In contrast, monovalent invasion-inhibitory Fab fragments caused accumulation of 66- and 52-kDa forms, with no cross-linking, trapping, or prevention of redistribution. Antibodies at low concentrations can be used as trapping agents for intermediate and soluble forms of AMA-1 and are useful for studying proteolytic processing of AMA-1. With this technique, it was confirmed that protease inhibitor chymostatin and Ca2+ chelators can inhibit the breakdown of the 66-kDa form. We propose that antibodies to AMA-1 capable of inhibiting erythrocyte invasion act by disrupting proteolytic processing of AMA-1.
Proteases play an important role in the process of host-cell invasion and intracellular development of important disease-causing pathogens, including viruses, bacteria, and protozoan parasites (1). The process of RBC invasion by the Plasmodium merozoite has been of considerable interest to malaria researchers. Parasite proteases assist invasion directly by modifying host RBC membrane or indirectly by proteolytic processing of other merozoite proteins, which in turn are involved in invasion (2). Merozoite surface protein 1 (MSP-1) of Plasmodium falciparum, an important vaccine candidate for malaria, is synthesized as an ≈200-kDa protein. As a result of several proteolytic cleavages during merozoite development, the ≈200-kDa protein is processed to a 19-kDa merozoite-bound molecule (MSP-119), which is believed to be of functional significance during invasion (3). Antibodies against MSP-119, which block invasion of merozoites into RBC, have also been shown to interrupt the crucial proteolytic step that gives rise to MSP-119 (4).
Apical membrane antigen 1 (AMA-1) is another important P. falciparum protein (PfAMA-1) being actively considered for vaccine development (5). Like MSP-1, PfAMA-1 is also synthesized as a precursor protein of 83 kDa (PfAMA-183) (5, 6). PfAMA-183 is localized in the apical complex (micronemes and rhoptries) (5–9) of the merozoite, where it is further processed to 66 kDa (PfAMA-166) by the removal of a short N-terminal prosequence (6, 10). At or around schizont rupture and merozoite invasion, PfAMA-166 translocates from within the apical complex to the surface of the merozoite (6, 8, 9). Once on the surface, PfAMA-166 is circumferentially redistributed and undergoes two C-terminal cleavages (either sequentially or independently), giving rise to 48- and 44-kDa soluble forms (PfAMA-148+44) (6, 10, 11). Processed forms containing the C-terminal end of PfAMA-1 have been detected on the ring forms (6, 10). Although the exact relationship among processing, translocation, redistribution, and shedding events of AMA-1 is not clear, their timing suggests involvement in merozoite invasion.
Recombinant P. falciparum AMA-1 protein induces antiparasitic antibodies, which inhibit parasite growth in vitro (12–14) and protect immunized animals against parasite challenge in vivo (15). In anticipation of a human safety and immunogenicity trial, we have manufactured GMP-grade recombinant AMA-1 protein from the P. falciparum 3D7 clone (16). Although this protein has not been tested in a non-human primate challenge model for P. falciparum malaria (because of the inability of the 3D7 parasite to infect monkeys), antibodies to this protein effectively inhibit invasion of the parasites in vitro (16). In the current study we report that inhibitory antibodies to AMA-1 affect its processing and localization on the merozoite.
Materials and Methods
Antibodies. Rabbit antibodies were raised against recombinant AMA-1 (449 aa of P. falciparum 3D7 clone, residues 83Gly–531Glu) by vaccinating with 100 μg of protein with Montanide ISA720 (Seppic Inc., Paris; s.c., three doses, 3 wk apart), and serum was collected 2 wk after the last immunization (16). Pooled or individual serum samples were used in the study. A pool of preimmune and adjuvant control rabbit sera served as control. IgGs were purified by using 1 ml of protein G column (Amersham Pharmacia). Fab fragments were prepared from IgG by papain digestion (17) by using ImmunoPure Fab kit (Pierce). Purity of the Fab fragments preparation was confirmed by SDS/PAGE. Polyclonal IgG against recombinant AMA-1 was labeled with biotin by using the EZ-link Biotinylation kit (Pierce). mAb 4G2dc1 reacts with a conformational epitope on the ectodomain of AMA-1 (9), and mAb 5.2 recognizes the MSP19 of P. falciparum (18).
AMA-1-Processing Assay. P. falciparum clone 3D7 cultures were prepared as described (19). Culture media included 10% heat-inactivated normal human serum in bicarbonate-containing RPMI medium 1640, containing final 0.42 mM Ca2+ and 0.40 mM Mg2+. Fifteen microliters of heat-inactivated rabbit serum (dialyzed against RPMI medium 1640 or used directly after heat inactivation) or purified IgG or Fab fragments was mixed with 135 μl of synchronized (19), Percoll-alanine-purified (20), midstage (approximately eight nuclei), >90%-pure schizonts (1 × 107 per ml) in a 48-well culture plate. PBS was used as a diluent if necessary. The plate was placed in a plastic bag, gassed with 5% O2/5% CO2/90% air, heat-sealed, and incubated at 37°C for ≈6 h (21). Aliquots from a control-culture flask were taken to monitor the percent rupture of schizonts by hemocytometer. After ≈90% schizonts had ruptured, the contents of each well were transferred to a microfuge tube and centrifuged at 10,000 × g for 5 min. The supernatant was aspirated, and the resulting parasite pellet was washed with 0.5 ml of chilled PBS and centrifuged as before. The supernatant was discarded, and 150 μl of 1× NuPAGE sample buffer (Invitrogen) was added to the tubes; samples were frozen at –30°C until they were analyzed. Soluble forms of AMA-1 were immunoprecipitated from the processing-assay culture supernatants or from culture supernatants of routinely maintained parasites by using MagnaBind Goat anti-Rabbit IgG-coated Magnetic Beads (Pierce).
Protease Inhibitors. Protease inhibitors were tested for their effect on AMA-1 processing. All inhibitors were from Sigma. PMSF, pepstatin, and N-p-tosyl-l-phenylalanine chloromethyl ketone (TPCK) were dissolved in 100% ethanol. Antipain, leupeptin, and 1,10-phenanthroline stocks were made in water. EDTA and EGTA stocks were made in PBS (pH adjusted to 7.2), 7-amino-1-chloro-3-tosylamido-2-heptanone (TLCK) was prepared in 1 mM HCl, and E64 and chymostatin were prepared in DMSO. All stocks were at 100× concentration. The protease inhibitor (1.5 μl) or its respective solvent control was added to 15 μl of PBS, and then 135 μl (1 × 107 per ml) of Percoll-purified midstage schizonts were added. The processing assay was carried out as described above. A parallel assay with protease inhibitors was carried out in two similarly prepared plates to which RBC were also added to a final 4% hematocrit. Parasites in the first plate were allowed to invade in suspension at 37°C, and thin smears were prepared after the rupture cycle for Giemsa staining and examination for the presence of protease inhibitor clusters of merozoites (22). In the second plate, parasites (135 μl of 5 × 106 per ml schizonts plus RBC at 4% hematocrit) were incubated overnight, and ring forms were quantitated by flow cytometry as a measure of parasite invasion (21).
PAGE and Western Blotting. Samples were briefly sonicated with a microtip sonicator, heated at 80°C for 2 min, and spun down; 16 μl was applied per well to a precast 4–12% gradient polyacrylamide gel (NuPAGE Bis-Tris, Invitrogen). Samples were run under nonreduced conditions unless specified. DTT at 50 mM was added to samples resolved under reduced conditions. Proteins from the gel were electrophoretically transferred to nitrocellulose membrane and blocked with 5% BSA in PBS containing 0.05% Tween 20 overnight at 4°C. AMA-1-specific bands were immunostained by incubating the blot with biotin-labeled polyclonal anti-AMA-1 IgG (2 mg/ml) at 1:1,500 dilution for 2 h. Reducing Western blots were immunostained with biotin-labeled polyclonal IgG against reduced and alkylated recombinant AMA-1. After washing with PBS-Tween, horseradish peroxidase-conjugated NeutrAvidin (Pierce) at 1:15,000 dilution was added for 1 h; the blot was washed and developed with SuperSignal West Pico Chemiluminescent substrate (Pierce), followed by x-ray film exposure (Kodak BioMax). Developed x-ray films were scanned, and band intensity was calculated by using imagequant 5.1 software (Molecular Dynamics).
AMA-1 Localization with Indirect Immunofluorescence Assay. Midstage schizonts were incubated with the antibodies in the same format as described in AMA-1-Processing Assay. At ≈70% schizont rupture samples were chilled, and a protease inhibitor mixture (catalog no. 554779, Pharmingen) was added to each sample. To have similar concentrations of the postimmune sera in the test and control wells, postimmune anti-AMA-1 sera (1:10) were added to wells corresponding to preimmune or negative serum control, and all samples were incubated on ice for another 1.5 h to allow the newly added antibodies to bind. This incubation was followed by centrifugation at 2,000 × g for 4 min, and the resulting pellet was washed with growth medium containing a 10% human serum and protease inhibitor mixture. The final pellet was suspended in 20 μl of wash buffer, and 1 μl was spotted for an immunofluorescence assay. Slides were fixed with acetone for 1 min and blocked with 10 mg/ml BSA in PBS for 30 min. Primary antibody was anti-MSP-1 mouse mAb 5.2 (1:1,000 of ascitic fluid); secondary antibodies were anti-rabbit FITC-conjugated antibody (1:300) and anti-mouse phycoerythrin conjugate (1:1,000) (all from Southern Biotechnology Associates). Both incubations were for 1 h. The slides were washed with PBS and mounted with Fluoromount G. Microscopy was done under UV light with a FITC filter for AMA-1 and a dual-cube FITC plus phycoerythrin filter for colocalizing AMA-1 and MSP-1. In the immunofluorescence assay for protease inhibitor-treated parasites, a 1:1,000 dilution of polyclonal anti-AMA-1 rabbit serum was added concurrently with mAb 5.2.
ELISA and Growth Inhibition Assay (GIA). ELISA and static GIA were done as described (16, 21).
Results
Processing of AMA-1 in the Presence of Anti-AMA-1. A processing assay was performed with preimmune (1:10) (noninhibitory by GIA), immune 1:10 (>85% inhibition by GIA), and immune 1:2,500 (noninhibitory by GIA) serum pools. The resulting parasite pellets were analyzed by Western blotting. Immunoprecipitated soluble AMA-1 fragments from culture supernatant of routinely maintained parasites, with polyclonal anti-AMA-1, were also analyzed on the same gel. As expected, two AMA-1-specific bands were detected under nonreduced conditions in merozoites released in the presence of preimmune serum (Fig. 1A, lane 1). These bands migrated at 73 and 62 kDa (under our electrophoretic conditions) and, as evidenced by reactivity to mAb 4G2dc1 (Fig. 3, lane c), correspond to the 83- and 66-kDa forms, respectively, of PfAMA-1 fragments described in the literature. In contrast, merozoites released in the presence of anti-AMA-1 sera showed two additional bands at 52 and 46 kDa, respectively (Fig. 1 A, lanes 2 and 3). The 52:46 band intensity ratio was higher at 1:10 dilution (Fig. 1 A, lane 2) than at 1:2,500 (Fig. 1 A, lane 3). The 52- and 46-kDa bands had mobility similar to the soluble forms of PfAMA-1 observed in the positive immunoprecipitation control (Fig. 1 A, lane 4). A recent publication (11) reported that AMA-1 fragments immunoprecipitated from culture supernatant migrate at 52 and 46 kDa under nonreduced conditions, and that the 46-kDa band constitutes comigrating 48- and 44-kDa soluble forms. The 46-kDa band observed in our processing assay also resolved into a 48- and 44-kDa band under reducing conditions (Fig. 1B, lanes 3 and 4). Hence, it was concluded that the 52- and 46-kDa bands observed on our processing assays under nonreduced conditions represent the 52- and 46-kDa bands observed by others (11), and, for the purpose of maintaining continuity with published data, we refer to the observed 73-, 62-, 52-, and 46-kDa bands as PfAMA-183, PfAMA-166, PfAMA-152, and PfAMA-148+44, respectively. Our data demonstrate that invasion-inhibitory anti-AMA-1 can trap an intermediate-form PfAMA-152 along with the soluble forms PfAMA-148+44 on the merozoite surface.
Fig. 1.
(A) Processing assay with immune serum pool at two dilutions. Each lane represents merozoites released from ≈1.4 × 105 schizonts: lane 1, preimmune (1:10 dilution); lanes 2 and 3, postimmune at 1:10 and 1:2,500 dilutions, respectively; lane 4, soluble AMA-1 fragments immunoprecipitated from culture supernatant (representative of ≈5 × 106 rupturing schizonts, assuming 100% recovery). (B) Processing assay samples corresponding to lanes 3 and 4 of A run under reduced conditions and immunostained with biotin-labeled IgG against reduced and alkylated AMA-1 protein. (C) Immunoprecipitation from culture supernatants of the processing assay containing 1:10 preimmune (lane 1), 1:10 postimmune (lane 2), and 1:2,500 postimmune serum (lane 3). PfAMA-1-specific bands and molecular mass marker positions (Multimark, Invitrogen) are shown with arrows.
Fig. 3.
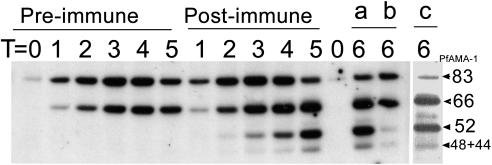
Effect of anti-AMA-1 antibodies on AMA-1 processing during schizont maturation and merozoite release. The processing assay was performed with 1:10 dilution of preimmune or postimmune pools. Samples were drawn at five times (T1–T5 corresponding to 0–90% rupture; see Results). To rule out immunoprecipitation from culture supernatant, postimmune serum was added to a preimmune serum-containing well (at T5, keeping the final dilution 1:10), and incubation continued at 37°C for another 30 min (lane a, postimmune sample at T6; lane b, preimmune control incubated with the postimmune serum at T6). Lane c corresponds to the sample in lane a analyzed for reactivity to AMA-1-specific mAb 4G2dc1.
In the same experiment the shed fragments of AMA-1 were immunoprecipitated from culture supernatant and analyzed under nonreducing conditions. The 46-kDa band was detected in the supernatant from parasites incubated with preimmune (Fig. 1C, lane 1) and 1:2,500 immune (Fig. 1C, lane 3) sera, but it was absent in the supernatant from parasites incubated with 1:10 immune serum (Fig. 1C, lane 2). The results indicate that, at inhibitory concentration, antibodies to AMA-1 inhibit the formation and shedding of PfAMA-148+44 from merozoites.
An individual inhibitory rabbit serum was tested in a processing assay at 20-, 30-, 90-, 270-, 810-, and 2,400-fold dilutions, respectively (Fig. 2). Four distinct effects of antibodies on AMA-1 processing were observed. First, PfAMA-152 and PfAMA-148+44 were trapped on the merozoites. Second, PfAMA-152 and PfAMA-148+44 appeared to have a precursor–product relationship (linear correlation between increasing and decreasing band intensity). Third, the formation of PfAMA-148+44 appeared to be inhibited by antibodies, and the ratio of PfAMA-152 to PfAMA-148+44 was higher at higher concentrations of the inhibitory sera. Fourth, a high concentration of inhibitory anti-AMA-1 sera led to substantial accumulation of the PfAMA-166 (Fig. 2; compare 1:20 and 1:270 dilutions), suggesting processing inhibition of PfAMA-166.
Fig. 2.
Processing assay showing dose–response of an individual inhibitory rabbit serum on AMA-1 processing. Final serum dilutions used in the assay were 1:2,400, 1:810, 1:270, 1:90, 1:30, and 1:20.
Kinetics and Specificity of the Processing Assay. AMA-1 is synthesized and processed during schizont development and rupture (6–11). To determine whether the processing assay can detect the synthesis and processing of AMA-1, schizonts were allowed to rupture in the presence of inhibitory immune serum pool at a 1:10 dilution (Fig. 3). A pool of control preimmune serum was also incubated at identical concentration. Schizont rupture was monitored by hemocytometer counts. No difference was observed in the rupture kinetics of schizonts in pre- or postimmune sera. Starting at time point 0 (T0) (corresponding to 0% rupture) sample sets were drawn at T1 (2.25 h; 30% rupture), T2 (3 h; 40%), T3 (4.25 h; 60%), T4 (5.5 h; 76%), and T5 (6.5 h; 87%) and analyzed by Western blotting under nonreduced conditions. In the preimmune control lanes, PfAMA-183 and PfAMA-166 were detected, whereas, in the lanes corresponding to immune serum, additional PfAMA-152 and PfAMA-148+44 bands were seen. The relative intensity of PfAMA-152 and PfAMA-148+44 remained unchanged over time. To rule out immunoprecipitation of AMA-1 from the culture supernatant, immune serum was added (at T5) to one of the preimmune wells and incubation continued at 37°C for an additional 30 min (T6). PfAMA-152 and PfAMA-148+44 bands in this control lane were much weaker (lane b) than when immune sera were present from the beginning (lane a), suggesting that the assay detects proteolytic products of cross-linked, membrane-bound AMA-1 molecules. Reactivity to mAb 4G2dc1 was further used to confirm the identity of the observed bands (lane c).
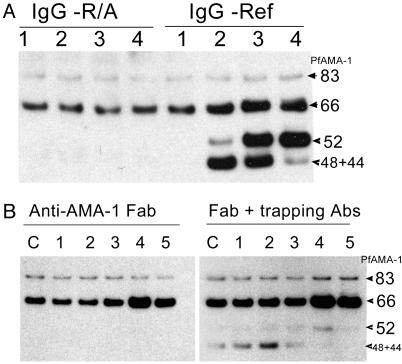
IgG prepared from an inhibitory serum pool of rabbits immunized with refolded recombinant AMA-1 (16), and IgG isolated from noninhibitory serum of rabbits immunized with reduced and alkylated recombinant AMA-1, were tested in a processing assay. Table 1 shows ELISA titers and GIA activity of the two IgG pools at three dilutions. Fig. 4A shows that, as with whole sera, purified IgG also caused processing inhibition and trapping. Although antibodies against reduced and alkylated protein tested positive on ELISA, these antibodies tested negative on the GIA and processing assay. It has been reported that most of the inhibitory epitopes on recombinant AMA-1 were disulfide bond-dependent (16, 23); the same appears to be the case with processing inhibition and trapping.
Table 1. ELISA titers and percent inhibition in a GIA of the IgG-Ref and IgG-R/A.
| IgG sample* (3.5 mg/ml)
|
ELISA titer (OD405 = 1)
|
GIA at three dilutions, %
|
||
|---|---|---|---|---|
| 10 | 100 | 1,000 | ||
| Ref-AMA-1 | 116,539 | 71 | 10 | -1 |
| R/A AMA-1 | 44,483 | 4 | 5 | 1 |
GIA was performed at three dilutions with the same samples of IgG used in the processing assay (Fig. 4A).
Ref-AMA-1, refolded AMA-1; R/A AMA-1, reduced and alkylated AMA-1.
Fig. 4.
(A) Processing assay showing the effects of IgG against reduced and alkylated AMA-1 (IgG-R/A) and refolded AMA-1 (IgG-Ref) proteins. IgG concentrations in lanes 1–4 were 0.00035, 0.0035, 0.035, and 0.35 mg/ml, respectively. (B) Processing assay showing the effect of anti-AMA-1 Fab fragments. Fab fragment concentrations in lanes 1–5 were 0.000014, 0.00014, 0.0014, 0.014, and 0.14 mg/ml, respectively. C indicates media control. A parallel assay with trapping antibodies (1:2,500 postimmune pool) is also shown.
Monovalent Fab fragments of inhibitory IgG failed to show trapping (Fig. 4B). Equimolar concentration of intact anti-AMA-1 IgG showed a high level of trapping, indicating that antigen cross-linking may be important for trapping. The 66-kDa form accumulated in the presence of high concentration of Fab fragments (Fig. 4B, lanes 4 and 5), similar to the observation with intact IgG. To determine the effect of Fab fragments on PfAMA-152 processing, 1:2,500 dilution of the AMA-1 serum pool was added to the assay as a trapping agent. (This dilution trapped efficiently, but showed no inhibition of PfAMA-166 or PfAMA-152 processing.) As observed with intact IgG, PfAMA-152 appeared only at high Fab concentration (Fig. 4B, lane 4), whereas, at lower concentrations, PfAMA-148+44 was seen. The intensities of the trapped PfAMA-152 and PfAMA-148+44 were much lower than those observed with intact IgG, probably because of competition between Fab fragments (nontrapping) and the intact antibodies (trapping). In a comparative GIA, IgG at 0.37 mg/ml showed 81% inhibition, whereas purified Fab fragments of the same IgG sample (at 0.28 mg/ml; approximate equimolar antigen binding sites) showed 78% inhibition of parasite invasion. Control IgG and Fab fragments showed no inhibition. The anti-AMA-1 Fab preparation used in the GIA showed a profile similar to Fig. 4B, lanes 4 and 5, whereas the Fab fragments of preimmune IgG appeared similar to the control lane c on a processing assay. The inhibition of invasion by Fab fragments is consistent with the previous observations with Plasmodium knowlesi (24).
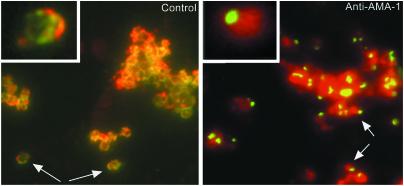
AMA-1 Localization Assay. AMA-1 was located apically and circumferentially on merozoites released in the presence of the preimmune control pool (1:10 dilution). These are the expected locations of AMA-1 on free merozoites (Fig. 5; control). In contrast, AMA-1 on the merozoites released in the presence of immune pool (1:10 dilution) was located apically with little or no circumferential distribution (Fig. 5; anti-AMA-1). This effect was clearly seen with up to 1:1,000 dilution of the serum pool. No apical restriction was seen in the presence of inhibitory concentrations of Fab fragments. Hence, it appears that apical restriction was associated with cross-linking and trapping.
Fig. 5.
Double immunofluorescence image, with a dual-cube filter, of free merozoites released in the presence of preimmune (Left, 1:10 dilution) or postimmune (Right, 1:10 dilution) serum. The preimmune sample was incubated with 1:10 postimmune sera for 1.5 h on ice after rupture. Slides were acetone-fixed, and AMA-1 was visualized by staining with FITC-conjugated anti-rabbit (green fluorescence), and the merozoite surface was demarcated by staining with P. falciparum MSP-1-specific mAb 5.2 and anti-mouse phycoerythrin (red fluorescence). (Insets) Enlarged view of a single merozoite.
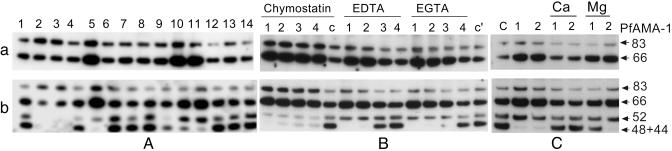
Processing of AMA-1 in the Presence of Protease Inhibitors and Cation Chelators. Inhibitors of serine proteases (antipain, PMSF, TLCK, TPCK, leupeptin, and chymostatin), cysteine proteases (antipain, leupeptin, chymostatin, and E64), cation-dependent proteases (1,10-phenanthroline, EDTA, and EGTA), and aspartic proteases (pepstatin) were used to determine the nature of proteases involved in AMA-1 processing. The assay was also performed in the presence of trapping antibodies (1:2,500 dilution of rabbit anti-AMA-1 sera pool). The ability of these protease inhibitors to block schizont rupture or RBC invasion was studied in parallel experiments. Inhibitors 1,10-phenanthroline, TPCK, TLCK, and PMSF interrupted schizont maturation, as observed by the presence of midstage schizonts on Giemsa-stained thin smears. Protease inhibitor clusters of merozoites were observed on Giemsa-stained slides of E64, chymostatin, and leupeptin. In the AMA-1-processing assay none of the inhibitors showed significant accumulation of the PfAMA-183 precursor accompanied by reduced PfAMA-166 (Fig. 6Aa). PfAMA-166, however, was found to accumulate in the presence of chymostatin, with a corresponding decrease in the intensity of trapped PfAMA-152 and PfAMA-148+44 (Fig. 6 Ab, lane 5, and B). Chymostatin did not block schizont development, but protease inhibitor clusters of merozoites were observed on Giemsa-stained smears. Chymostatin at 100 μM showed >90% inhibition of merozoite invasion. Cation-chelating agents EDTA and EGTA also caused accumulation of PfAMA-166 (Fig. 6 Aa, lanes 10 and 11, and B); however, unlike chymostatin, where the formation of both PfAMA-152 and PfAMA-148+44 were inhibited, EDTA and EGTA inhibited the formation of only PfAMA-148+44 (Fig. 6 Ab, lanes 10 and 11, and B). EDTA and EGTA did not affect schizont rupture at 1 mM, but showed ≈40% and ≈20% inhibition of RBC invasion, respectively. Addition of Ca2+ to both EDTA and EGTA lanes reversed the accumulation of PfAMA-166, accompanied by increase in the level of PfAMA-148+44 (Fig. 6 Ca and b). Although the addition of Mg2+ reversed the EDTA-induced inhibition, it had no effect on the EGTA-induced inhibition. (EGTA is a poor chelator of Mg2+.) We did not observe apical restriction of AMA-1 by immunofluorescence assay on merozoites released in the presence of any of the inhibitors mentioned above.
Fig. 6.
(a) Processing assay in the presence of protease inhibitors. (b) Identical assay performed in the presence of trapping antibodies (1:2,500 AMA-1 immune serum pool). (A) Lane 1, PMSF (200 μM); lane 2, TLCK (100 μM); lane 3, TPCK (100 μM); lane 4, leupeptin (100 μM); lane 5, chymostatin (100 μM); lane 6, antipain (100 μM); lane 7, E64 (10 μM); lane 8, pepstatin (5 μM); lane 9, 1,10-phenanthroline (1 mM); lane 10, EDTA (1 mM); lane 11, EGTA (1 mM); lane 12, ethanol control; lane 13, DMSO control; lane 14, PBS control. (B) Dose–response of chymostatin, EDTA, and EGTA on AMA-1 processing. Concentrations of inhibitors used from lanes 1–4 were: chymostatin, 100, 50, 25, and 12.5 μM; EDTA and EGTA, 2, 1, 0.5, and 0.25 mM, respectively; lane c, DMSO control; lane c′, PBS control. (C) Processing assay showing the effect of 1 mM MgCl2 or 1 mM CaCl2 added to reverse the EDTA and EGTA (1 mM each) mediated processing inhibition. Lane 1, EDTA; lane 2, EGTA; lane c, PBS control.
Discussion
This article describes the observations of the effects of invasion-inhibitory anti-AMA-1 antibodies on parasite AMA-1 processing and redistribution. Bivalent IgG and monovalent Fab fragments that block invasion were found to cause significant accumulation of the PfAMA-166 and PfAMA-152 forms on the merozoite. In addition, bivalent IgG showed cross-linking of three soluble forms of AMA-1 (i.e., PfAMA-152, PfAMA-148, and PfAMA-144) on the merozoite, and inhibited the circummerozoite redistribution and shedding of PfAMA-1.
The expression of AMA-1 on the parasite is vital for its survival (25). The biological function of AMA-1 is not known, nor is it known whether processing, redistribution, and shedding are necessary for its function. However, the timing of these events and their similarity to activation by proteolytic processing of other molecules of pathogens (1, 2) suggest a role in merozoite invasion into RBCs. Like MSP-1, only a processed C-terminal product of PfAMA-1 was detected in successfully invaded ring forms (6, 10), further indicating that processing may be linked to the function of AMA-1. The shedding of PfAMA-144&48 is unlikely to be an artifact of merozoite degradation in the absence of RBCs for invasion, because PfAMA-144&48 forms were reported to be as abundant in culture media with RBCs for invasion as in those without (11). The processing of PfAMA-166 in the absence of RBCs suggests that processing does not require invasion of RBCs. On the other hand, the four reagents that inhibited the processing of PfAMA-166 (immune IgG and Fab, chymostatin, and EDTA/EGTA) also inhibited invasion. This correspondence suggests a cause-and-effect relationship but does not rule out other possible mechanisms of action of antibodies, given the complexity of the process of RBC invasion.
Steric hindrance caused by binding of antibodies to AMA-1 on merozoites (either within the schizont or outside) may limit access to protease recognition sites inhibiting its processing. Inhibition of circumferential redistribution by bivalent antibodies may also physically prevent AMA-1 from coming in contact with membrane-bound “sheddases” (11). The data with monovalent Fab fragments, however, suggest that processing inhibition of PfAMA-166 and the appearance of PfAMA-152 caused by steric hindrance may be the major factor.
Proteolytic sites that generate PfAMA-152 are not known (11). Based on size, PfAMA-152 may have resulted from a cleavage within the transmembrane domain (11). PfAMA-152 may be loosely embedded in the membrane, such that small amounts of it could be shed, while the rest undergoes further processing. Antibodies can cross-link this molecule on the merozoites, which is how it is detected in the processing assay. Although we do not have direct evidence that PfAMA-152 is further processed, serial-dilution experiments with antibodies (Fig. 2) suggest a precursor–product relationship between the merozoite-bound PfAMA-152 and PfAMA-148+44, which would indicate that PfAMA-152 is a transitional intermediate formed during the processing of PfAMA-166. However, little or no PfAMA-152 was seen on merozoites rupturing in the absence of immune sera, suggesting that PfAMA-152 is a product of anomalous processing of PfAMA-166, as suggested by others (11). Hence, the production of PfAMA-152 on the merozoites may be an additional mechanism of antibody action. In fact, the ratio of trapped PfAMA-152/PfAMA-148+44 band intensity directly correlated with the ability of a serum sample to inhibit invasion in a GIA (Fig. 4A and Table 1). Based on our observations, we suggest alternative pathways for proteolysis of PfAMA-166 (Fig. 7), one in which PfAMA-152 is a normal intermediate and the other in which PfAMA-166 directly forms PfAMA-144+48.
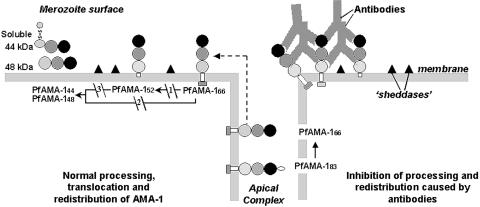
Fig. 7.
Proposed model of AMA-1 processing. The PfAMA-1 molecule is represented by a prosequence (open oval), three subdomains based on the predicted disulfide bond structure (see ref. 29) (dark domain I-, medium domain II-, and light domain III-colored circles), transmembrane domain (clear rectangle), and cytoplasmic domain (gray rectangle). Putative membrane-bound sheddases are represented by filled triangles. PfAMA-148 and PfAMA-144 represent the soluble forms of AMA-1. PfAMA-144 is represented in its native conformation with a disulfide bond (—S S—) connecting the 44-kDa form to its proteolytic fragment (see ref. 11). Arrows indicate the substrate (start) and products (end) of each putative enzymatic step. Dashed arrow represents translocation of PfAMA-166 from the apical complex to the surface (apical end). Our data suggest that PfAMA-1 processing on merozoites includes a 52-kDa form, which is either a normal intermediate or a product of anomalous processing that may further be processed to PfAMA-148+44. In this hypothetical model, proteolytic steps 1 and 2 are expected to be chymostatin-sensitive, whereas steps 2 and 3 are EGTA-sensitive. All three proteolytic steps and the redistribution of AMA-1 appear to be sensitive to anti-AMA-1 antibodies. (Left) Normal processing, translocation, and redistribution of AMA-1. (Right) Antibody-mediated processing inhibition, cross-linking, and trapping of AMA-1 fragments.
S—) connecting the 44-kDa form to its proteolytic fragment (see ref. 11). Arrows indicate the substrate (start) and products (end) of each putative enzymatic step. Dashed arrow represents translocation of PfAMA-166 from the apical complex to the surface (apical end). Our data suggest that PfAMA-1 processing on merozoites includes a 52-kDa form, which is either a normal intermediate or a product of anomalous processing that may further be processed to PfAMA-148+44. In this hypothetical model, proteolytic steps 1 and 2 are expected to be chymostatin-sensitive, whereas steps 2 and 3 are EGTA-sensitive. All three proteolytic steps and the redistribution of AMA-1 appear to be sensitive to anti-AMA-1 antibodies. (Left) Normal processing, translocation, and redistribution of AMA-1. (Right) Antibody-mediated processing inhibition, cross-linking, and trapping of AMA-1 fragments.
The enzymes that cleave PfAMA-166 are suggested to be divalent cation-dependent serine proteases (11), which is consistent with our findings that chymostatin and EGTA inhibited this cleavage but E64 had no effect. However, our findings with PMSF, TLCK, and TPCK were not interpretable because they showed significant inhibition of schizont development or rupture at concentrations that appeared to inhibit AMA-1 processing. We did not see inhibition of surface redistribution of AMA-1 in the presence of chymostatin, which prevents PfAMA-166 processing. This finding is consistent with previous findings that, soon after its formation, PfAMA-166 redistributes on the merozoite surface (6).
Our data suggest that antibodies to AMA-1 can affect the processing of native AMA-1 at concentrations achievable after vaccination. Development of a vaccine based on AMA-1 would benefit from a reliable correlate of immunity. Future studies with AMA-1 antibodies from non-human primates protected against parasite challenge (15) or human immune individuals (12) are needed to establish whether these observations directly correlate with protection. In addition to MSP-1 and AMA-1, several other vaccine-candidate P. falciparum antigens such as SERA (26), RAP-1 (27), and Pfs230 (28) are known to be stage-specifically processed. It remains to be seen whether antiparasitic antibodies against these antigens also inhibit their processing and localization.
Acknowledgments
We thank Dr. Alan W. Thomas (Biomedical Primate Research Centre, Rijswijk, The Netherlands) for mAb 4G2dc1; American Type Culture Collection for mAb 5.2; Doug Smoot (Naval Medical Research Center, Silver Spring, MD) for flow cytometry; Drs. Jeff Lyon (Walter Reed Army Institute of Research), Ripley W. Ballou (GlaxoSmithKline, Rixensart, Belgium), and Mike Blackman (National Institute of Medical Research, Mill Hill, U.K.) for critical suggestions. Funding for this project was received from the U.S. Department of Defense under an In-house Lab Independent Research Project.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMA-1, apical membrane antigen 1; PfAMA-1, AMA-1 of Plasmodium falciparum; GIA, growth or invasion inhibition assay; TPCK, N-p-tosyl-l-phenylalanine chloromethyl ketone; TLCK, 7-amino-1-chloro-3-tosylamido-2-heptanone; MSP-1, merozoite surface protein-1; Tn, time point n.
References
- 1.Dunn, B. M., ed. (1991) Proteases of Infectious Agents (Academic, New York).
- 2.Blackman, M. J. (2000) Curr. Drug Targets 1, 59–83. [DOI] [PubMed] [Google Scholar]
- 3.Holder, A. A., Guevara Patino, J. A., Uthaipibull, C., Syed, S. E., Ling, I. T., Scott-Finnigan, T. & Blackman, M. J. (1999) Parassitologia (Rome) 41, 409–414. [PubMed] [Google Scholar]
- 4.Blackman, M. J., Scott-Finnigan, T. J., Shai, S. & Holder, A. A. (1994) J. Exp. Med. 180, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson, M. G., Marshall, V. M., Smythe, J. A., Crewther, P. E., Lew, A., Silva, A., Anders, R. F. & Kemp, D. J. (1989) Mol. Cell. Biol. 9, 3151–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narum, D. L. & Thomas, A. W. (1994) Mol. Biochem. Parasitol. 67, 59–68. [DOI] [PubMed] [Google Scholar]
- 7.Crewther, P. E., Culvenor, J. G., Silva, A., Cooper, J. A. & Anders, R. F. (1990) Exp. Parasitol. 70, 193–206. [DOI] [PubMed] [Google Scholar]
- 8.Healer, J., Crawford, S., Ralph, S., McFadden, G. & Cowman, A. F. (2002) Infect. Immun. 70, 5751–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocken, C. H., van der Wel, A. M., Dubbeld, M. A., Narum, D. L., van de Rijke, F. M., van Gemert, G. J., van der Linde, X., Bannister, L. H., Janse, C., Waters, A. P. & Thomas, A. W. (1998) J. Biol. Chem. 273, 15119–15124. [DOI] [PubMed] [Google Scholar]
- 10.Howell, S. A., Withers-Martinez, C., Kocken, C. H., Thomas, A. W. & Blackman, M. J. (2001) J. Biol. Chem. 276, 31311–31320. [DOI] [PubMed] [Google Scholar]
- 11.Howell, S. A., Wells, I., Fleck, S. L., Kettleborough, C., Collins, C. & Blackman, M. J. (2003) J. Biol. Chem. 278, 23890–23898. [DOI] [PubMed] [Google Scholar]
- 12.Hodder, A. N., Crewther, P. E. & Anders, R. F. (2001) Infect. Immun. 69, 3286–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy, M. C., Wang, J., Zhang, Y., Miles, A. P., Chitsaz, F., Saul, A., Long, C. A., Miller, L. H. & Stowers, A. W. (2002) Infect. Immun. 70, 6948–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocken, C. H., Withers-Martinez, C., Dubbeld, M. A., van der Wel, A., Hackett, F., Valderrama, A., Blackman, M. J. & Thomas, A. W. (2002) Infect. Immun. 70, 4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stowers, A. W., Kennedy, M. C., Keegan, B. P., Saul, A., Long, C. A. & Miller, L. H. (2002) Infect. Immun. 70, 6961–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutta, S., Lalitha, P. V., Ware, L. A., Barbosa, A., Moch, J. K., Vassell, M. A., Fileta, B. B., Kitov, S., Kolodny, N., Heppner, D. G., et al. (2002) Infect. Immun. 70, 3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlow, E. & Lane, D., eds. (1988) Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 626–629.
- 18.Chang, S. P., Kramer, K. J., Yamaga, K. M., Kato, A., Case, S. E. & Siddiqui, W. A. (1988) Exp. Parasitol. 67, 1–11. [DOI] [PubMed] [Google Scholar]
- 19.Haynes, J. D. & Moch, J. K. (2002) Methods Mol. Med. 72, 489–497. [DOI] [PubMed] [Google Scholar]
- 20.Kanaani, J. & Ginsburg, H. (1989) J. Biol. Chem. 264, 3194–3199. [PubMed] [Google Scholar]
- 21.Haynes, J. D., Moch, J. K. & Smoot, D. S. (2002) Methods Mol. Med. 72, 535–554. [DOI] [PubMed] [Google Scholar]
- 22.Lyon, J. A. & Haynes, J. D. (1986) J. Immunol. 136, 2245–2251. [PubMed] [Google Scholar]
- 23.Anders, R. F., Crewther, P. E., Edwards, S., Margetts, M., Matthew, M. L., Pollock, B. & Pye, D. (1998) Vaccine 16, 240–247. [DOI] [PubMed] [Google Scholar]
- 24.Thomas, A. W., Deans, J. A., Mitchell, G. H., Alderson, T. & Cohen, S. (1984) Mol. Biochem. Parasitol. 13, 187–199. [DOI] [PubMed] [Google Scholar]
- 25.Triglia, T., Healer, J., Caruana, R. S., Hodder, A. N., Anders, R. F., Crabb, B. S. & Cowman, A. F. (2000) Mol. Microbiol. 38, 706–718. [DOI] [PubMed] [Google Scholar]
- 26.Mitamure, L. J., Fox, B. A., Bzik, D. J. & Horii, T. (2002) Parasitol. Int. 51, 343–352. [DOI] [PubMed] [Google Scholar]
- 27.Harnyuttanakorn, P., McBride, J. S., Donachie, S., Heidrich, H. G. & Ridley, R. G. (1992) Mol. Biochem. Parasitol. 55, 177–186. [DOI] [PubMed] [Google Scholar]
- 28.Williamson, K. C., Fujioka, H., Aikawa, M. & Kaslow, D. C. (1996) Mol. Biochem. Parasitol. 78, 161–169. [DOI] [PubMed] [Google Scholar]
- 29.Hodder, A. N., Crewther, P. E., Matthew, M. L., Reid, G. E., Moritz, R. L., Simpson, R. J. & Anders, R. F. (1996) J. Biol. Chem. 271, 29446–29452. [DOI] [PubMed] [Google Scholar]