Abstract
Smooth muscle cell proliferation around small pulmonary vessels is essential to the pathogenesis of pulmonary hypertension. Here we describe a molecular mechanism and animal model for this vascular pathology. Rodents engineered to express angiopoietin 1 (Ang-1) constitutively in the lung develop severe pulmonary hypertension. These animals manifest diffuse medial thickening in small pulmonary vessels, resulting from smooth muscle cell hyperplasia. This pathology is common to all forms of human pulmonary hypertension. We demonstrate that Ang-1 stimulates pulmonary arteriolar endothelial cells through a TIE2 (receptor with tyrosine kinase activity containing IgG-like loops and epidermal growth factor homology domains) pathway to produce and secrete serotonin (5-hydroxytryptamine), a potent smooth muscle mitogen, and find that high levels of serotonin are present both in human and rodent pulmonary hypertensive lung tissue. These results suggest that pulmonary hypertensive vasculopathy occurs through an Ang-1/TIE2/serotonin paracrine pathway and imply that these signaling molecules may be targets for strategies to treat this disease.
The establishment and remodeling of blood vessels is governed by a chemical dialogue between endothelial and smooth muscle cells. This cell-to-cell communication is regulated by paracrine signals between muscle-secreted peptides and transmembrane tyrosine kinase receptors on endothelial cells (1). It is widely believed that endothelial cells, by the secretion of factors, modulation of cell-surface proteins, or both, then regulate the proliferation of smooth muscle cells in their vicinity, leading to the formation of mature blood vessels. Angiopoietin 1 (Ang-1) is a muscle-secreted ligand that plays a critical role in vasculogenesis and is an important modulator of both physiologic and pathologic angiogenesis (2). Ang-1 activity is modulated by the endothelial-specific receptor TIE2 (a receptor with tyrosine kinase activity containing IgG-like loops and epidermal growth factor homology domains) (3). Although several downstream effects of Ang-1–TIE2 binding have been characterized (4), those that contribute to blood vessel structure are unknown. Several studies have suggested that Ang-1 regulates endothelial-cell recruitment of muscle cells to encase and stabilize primitive endothelial tubes (5). Mice lacking Ang-1 or TIE2 die in utero from defects in vascular development, including severe vascular abnormality of the lungs (6). Endothelial cells of these mice were arrayed into tubes, but vessels lacked muscular investment, had few branches, and displayed minimal graduation in size. In addition, observations in humans have revealed that venous malformations are associated with mutations in TIE2 (7), suggesting that Ang-1 stabilizes vessel development by stimulating muscle cells to proliferate around nascent endothelial tubes, creating mature arterial structures.
Most forms of pulmonary hypertension have no known cure, and this disease is the cause of at least 1% of all deaths in the United States each year (8). Pulmonary hypertension is characterized by excessive muscular investment of pulmonary arterioles, which is caused by smooth muscle cell polyclonal hyperplasia (9). This process is progressive and diffuse and eventually results in obliteration of the distal pulmonary arterial tree. Muscularization of small pulmonary vessels occurs in all forms of human pulmonary hypertension, including idiopathic disease and pulmonary hypertension induced by thromboembolism, drugs, and pulmonary shunt (10). The mechanism of this organ-specific vasculopathy is unknown. Because pulmonary hypertension is a disease of excessive muscularization of pulmonary arterioles, we hypothesized that this process may be due to aberrant expression of the Ang-1 gene product in the lung. Recently we have shown that Ang-1 is constitutively expressed in the lungs of patients with different etiologies of pulmonary hypertension and is absent in normal adult lung tissue (11). These results suggested that Ang-1 correlated with the pulmonary hypertensive phenotype but did not prove that it plays a causal role in the disease process.
To establish whether up-regulation of the Ang-1 gene product causes pulmonary hypertension, we created an animal model of this disease based on tissue-specific alteration of the expression of this gene in the lung. We also characterized the downstream signaling events of constitutive Ang-1 expression in the lung essential to the generation of this vasculopathy. Our observations suggest that pulmonary hypertension is caused by an Ang-1/TIE2/serotonin feedback pathway between endothelial and smooth muscle cells, and consequently they have important implications for the treatment and prevention of this disease.
Methods
Gene Delivery, Hemodynamic Measurements, and Angiography. Adenoviruses (stereotype 5; ref. 12) with the cytomegalovirus promoter driving either the mouse Ang-1 or the Escherichia coli lacZ gene were constructed. Viral stocks had <1 replication-competent adenovirus per 109 genomic particles. Injections of 100 μl of PBS, 109 plaque-forming units of Adeno-Ang-1 in PBS, or 109 plaque-forming units of Adeno-lacZ in PBS were made into the right ventricular outflow tract of hearts of adult Fischer rats (15 animals per group) through a left thoracotomy while occluding the right and left pulmonary veins for 5 sec. At serial time points, pulmonary artery and systemic blood pressures were transduced from the main pulmonary artery or aorta. For angiography, the pulmonary artery was flushed with 10 ml of PBS. Animals were killed by exsanguination through the abdominal aorta. Lungs were perfused through the pulmonary artery with 0.25 ml of MicroFil, a liquid silicon-based polymer (Flow Tech, Carver, MA), for 1 min by using an infusion pump. Lungs were bathed in ethanol, placed in methyl salicylate, and photographed.
Human and Rodent Tissue Processing and Histologic Assessment. Human pulmonary artery and systemic blood pressures were measured by a Swan–Ganz catheter and arterial line. After sternotomy or thoracotomy, a 4-cm biopsy was taken from the upper or middle lobe of either the right or the left lung before cardiopulmonary bypass and during ventilation with 100% oxygen. Tissue was collected from five patients with primary nonfamilial pulmonary hypertension and three patients with scleroderma undergoing transplant, five infants with pulmonary hypertension from ventricular septal defects undergoing corrective repair, and five patients with thromboembolic pulmonary hypertension undergoing pulmonary endarterectomy. Pulmonary hypertensive patients had pulmonary artery systolic pressure >50 mmHg (1 mmHg = 133 Pa) and pulmonary vascular resistance >600 dynes·sec·cm–5 (1 dyne = 10 μN). Tissue was collected from 10 patients without pulmonary hypertension (pulmonary artery systolic pressure <20 mmHg, pulmonary vascular resistance <120 dynes·sec·cm–5) undergoing lung resection for benign nodules. All patients had given consent for lung biopsy. Hematoxylin- and eosin-stained human and rodent sections were examined by light microscopy by a pathologist to grade severity of vasculopathy.
Culture of Human Pulmonary Arteriolar Endothelial and Smooth Muscle Cells. Human pulmonary arteriolar endothelial cells (HPAEC) and human pulmonary smooth muscle cells were isolated from 500- to 1500-μm-diameter arterioles from normotensive lung tissue (13, 14). Methods of cell isolation, primary cell culturing, and subculturing are detailed in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Ang-1 Protein Purification and Treatment of Endothelial Cells. Ang-1 cDNA was subcloned in pTrcHis2A (Invitrogen), a plasmid encoding a carboxyl-terminal polyhistidine-epitope sequence, and recombinant Ang-1 (rAng-1), produced in bacteria. rAng-1 was purified over an Ni–NTA (nickel–nitrilotriaceticacid)-affinity column (Qiagen, Valencia, CA) and dialyzed in 20 mM Tris·HCl (pH 8.0)/250 mM NaCl/10% glycerol. Protein size and purity were verified by Coomassie blue staining and immunoblotting. Biological activity of rAng-1 was confirmed by induction of TIE2 receptor kinase activity in 293T cells transfected with a TIE2 expression plasmid. After subculturing for three passages, HPAEC were incubated for 2 h in serum-free medium 199 [endothelial cell media (ECM)] and treated with 50 ng/ml rAng-1 for 6 h. After incubation, media were collected for smooth muscle cell treatment and measurement of serotonin content. Three experiments were performed, each consisting of 10 independent endothelial cell cultures treated with rAng-1 and 10 independent endothelial cell cultures treated with diluent alone. A concentration of 50 ng/ml rAng-1 was chosen based on dose–response experiments assessing rAng-1 concentration in relation to endothelial cell serotonin production and secretion.
Smooth Muscle Cell Growth Assays. Human pulmonary artery smooth muscle cells (passages 3 and 4) were seeded at 5 × 104 cells per 25-mm-diameter well, serum-starved in medium 199 for 16 h, and treated with 1 ml of conditioned medium from HPAEC with or without 1 nM to 20 μM fluoxetine (Sigma–Aldrich). For smooth muscle cell treatment, 1 μM fluoxetine was chosen based on an IC50 of 500 nM fluoxetine for inhibiting smooth muscle cell proliferation in earlier dose–response experiments. One hour later, three regions on each well were photographed to establish a baseline for cell counting. After 18 h of incubation, 1 μCi (1 Ci = 37 GBq) of [3H]thymidine was added to each well, and incubation was continued for 18–30 h. Before processing for thymidine incorporation, the same areas were photographed and the change in cell number was determined. Incorporation of [3H]thymidine was measured by using a Beckman Coulter scintillation counter. Four independent [3H]thymidine/cell-count experiments were performed, with each assay done in triplicate. Values for each group were averaged and presented as mean ± SEM.
Supporting Information. RNA, protein, immunohistochemistry, in situ hybridization, serotonin ELISA, and statistical methods are described in Supporting Text.
Results
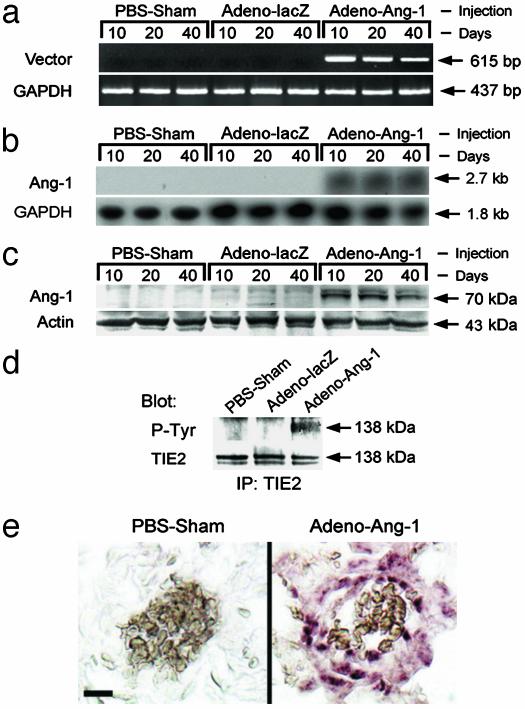
Constitutive Lung Expression of Ang-1. To determine whether constitutive expression of Ang-1 in the lung could cause pulmonary hypertension, we injected Adeno-Ang-1, Adeno-lacZ, or an equal volume of PBS (PBS carrier) into the right ventricular outflow tract (just below the pulmonary valve) of anesthetized Fischer rats. RT-PCR analysis at 10, 20, and 40 days after injection confirmed that vector-specific Ang-1 transcripts were present in animal lungs injected with Adeno-Ang-1 but were not detected in lungs injected with Adeno-lacZ or PBS carrier (Fig. 1a). By Northern blot analysis, steady-state levels of Ang-1 mRNA were detected in lungs of animals injected with Adeno-Ang-1 but were not detected in lung tissue from animals injected with Adeno-lacZ or PBS carrier (Fig. 1b). Western blot analysis revealed that Ang-1 was strongly detected in lungs injected with Adeno-Ang-1 but was not detected in the lungs of control animals at 10, 20, and 40 days after gene delivery (Fig. 1c). Transgene Ang-1 mRNA and protein expression were lost by 45 days after gene delivery.
Fig. 1.
Constitutive lung expression of Ang-1 in animals whose pulmonary circulations were perfused with Adeno-Ang-1. (a) The pattern of Ang-1 expression in rodent lung samples was studied by PCR using primers specific to the virally transduced Ang-1 mRNA (Vector). Tissue was analyzed 10, 20, and 40 days after injection of Adeno-Ang-1, Adeno-lacZ, or PBS alone (PBS-Sham). (b) Northern blot demonstrating that lungs of animals injected with the Adeno-Ang-1 construct had detectable steady-state levels of Ang-1 mRNA, whereas control lung specimens did not. (c) Western blot showing Ang-1 protein in lung tissue infected with Adeno-Ang-1 virus. Ang-1 protein was undetectable in animals injected with either Adeno-lacZ or PBS. (d) Immunoblot analysis of anti-TIE2 immunoprecipitates from rodent lung tissue, demonstrating that constitutive Ang-1 expression induced TIE2 receptor tyrosine phosphorylation but did not modulate TIE2 protein levels. (Upper) Immunoprecipitated (IP) with anti-TIE2 antibody, blotted with anti-phosphotyrosine (P-Tyr) antibody. (Lower) IP with anti-TIE2, blotted with anti-TIE2. (e) Detection of recombinant viral mRNA by in situ hybridization. Lung tissue from rats injected with PBS demonstrated no staining when a virus-specific antisense RNA probe was used. Conversely, virus-specific mRNA was detected in animals infected with Adeno-Ang-1 in lung vessel walls. (Scale bar represents 10 μm.)
Virally produced Ang-1 transcripts were detected at low levels by RT-PCR in the liver but not in myocardium, brain, skeletal muscle, intestine, testes, or kidney. Serum Ang-1 protein was not detected in the Adeno-Ang-1 rats or in the two control groups by Western blot analysis at any time point after gene delivery (data not shown).
Ang-1 Induces TIE2 Receptor Phosphorylation in the Lung. To determine whether Ang-1 signaled through a TIE2 pathway, we examined whether TIE2 phosphorylation occurred in the lungs of rats constitutively expressing the Ang-1 gene product. Although steady-state levels of TIE2 protein were similar in lung specimens from Adeno-Ang-1-, Adeno-lacZ-, and PBS-injected animals, we found that TIE2 phosphorylation was increased in the lungs of animals treated with Adeno-Ang-1 (Fig. 1d). This result confirmed that Ang-1 delivered by viral vector was functionally active in vivo.
Localization of Virally Transduced Ang-1 Expression in the Lung. To investigate the cell specificity of virally delivered genes in the lung, we performed in situ hybridization using a probe specific to the 3′ untranslated region of the Ang-1 genes in our viral constructs. Lung specimens from animals injected with Adeno-Ang-1 showed staining for viral-specific transcripts produced in the vessel wall of small pulmonary arteries and arterioles (Fig. 1e). Virally transduced Ang-1 transcripts were identified in small pulmonary vessels up to 40 days after gene delivery. PBS-injected lungs demonstrated no in situ hybridization to our vector-specific probe.
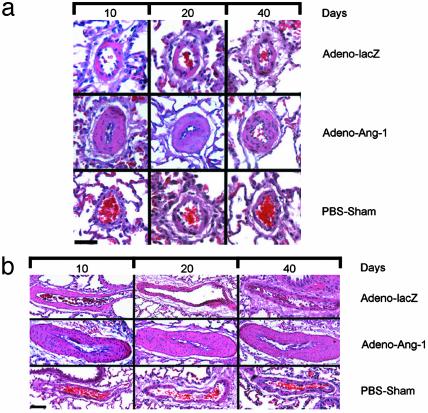
Ang-1 Induces Pulmonary Arteriolar Muscular Hyperplasia. Animals constitutively expressing high levels of Ang-1 protein in the lung from injection of the Adeno-Ang-1 virus showed diffuse pathologic changes consistent with advanced pulmonary hypertension (Fig. 2). This Ang-1-induced lung pathology was independent of different viral preparations. Lungs from Adeno-Ang-1 animals demonstrated severe muscular hyperplasia in the medial layer of pulmonary arteries and arterioles measuring <500 μm in diameter. Small vessel medial hyperplasia was present in >80% of the blood vessels examined at 10 days and >90% of the blood vessels examined at 20 and 40 days after Adeno-Ang-1 injection and occurred throughout all lobes. By micrometer analysis, small pulmonary vessels on average increased their vessel wall diameter by 400% and increased the number of myocytes per vessel wall area by 300%. One-third of pulmonary arterioles examined in animals constitutively expressing the virally delivered Ang-1 gene were occluded from severe medial hyperplasia at each time point. This small-vessel pathology persisted for the life of the animal. Despite diffuse small-vessel occlusion and stenosis from excessive muscularization, few plexiform lesions were found in animals overexpressing Ang-1 in the lung. There was no lymphocytic infiltration in or around the pulmonary vessels with this pathology. Rats injected in the right ventricular outflow tract with either Adeno-lacZ or PBS carrier demonstrated normal lung vascular histology.
Fig. 2.
Lung vascular pathology in Ang-1-induced rat pulmonary hypertension. (a) Pulmonary arterioles 40 μm in diameter from animals 10, 20, and 40 days after injection with Adeno-Ang-1, Adeno-lacZ, or PBS alone (PBS-Sham). Adeno-Ang-1 animal lungs (middle horizontal series of micrographs in a and b) showed diffuse pulmonary medial arteriolar thickening. (Scale bar represents 25 μm.) (b) Pulmonary arterioles 200 μm in diameter from animals 10, 20, and 40 days after injection with Adeno-Ang-1, Adeno-lacZ, or PBS. (Scale bar represents 150 μm.)
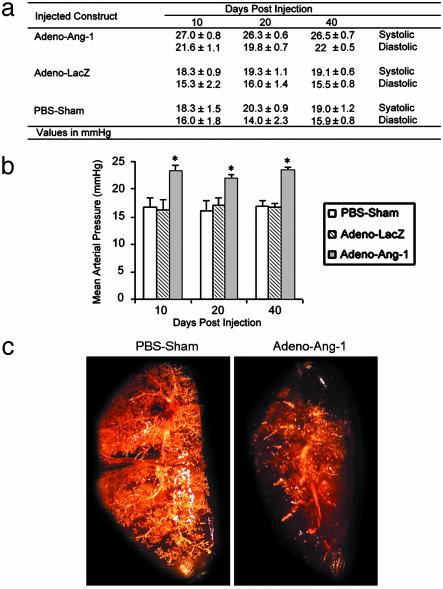
Ang-1 Induces Clinical Pulmonary Hypertension. Each animal constitutively expressing Ang-1 at 10, 20, and 40 days demonstrated a significant increase in pulmonary artery systolic and diastolic pressures (Fig. 3a) that was reproducible and averaged over four measurements taken during a 10-min period before the animal was killed. This represented a selective elevation in pulmonary artery pressures, because systemic arterial blood pressures were unchanged compared with baseline measurements taken before gene transfer. Mean pulmonary artery pressures for animals expressing virally delivered Ang-1 were elevated significantly (P < 0.01) compared with pressures measured in control animals (Fig. 3b).
Fig. 3.
Pulmonary artery pressures and angiography in animals with Ang-1-induced pulmonary hypertension. (a) Averaged pulmonary arterial pressures in rats injected with Adeno-Ang-1, Adeno-lacZ, or PBS at 10, 20, and 40 day intervals after gene transfer (four readings for each animal over a 10-min period, five animals for each group at each time point). Values are reported as number ± SEM. (b) Mean pulmonary arterial pressures in rats injected with Adeno-Ang-1, Adeno-lacZ, or PBS at 10, 20, and 40 day intervals after gene transfer. Each bar represents four measurements taken in each of five animals per group ± SEM. *, P < 0.01 versus the two control animal groups. (c) Pulmonary angiograms using the MicroFil cast technique of rodent lungs treated previously with either PBS carrier or Adeno-Ang-1. Note the absence of peripheral vessel staining in the Adeno-Ang-1-treated lung with large-vessel blunting.
Rodents expressing high levels of Ang-1 in the lung had pulmonary angiograms demonstrating severe small-vessel pruning similar to that seen in human pulmonary hypertension (Fig. 3c). A continuous in vivo MicroFil polymer perfusion technique (15) that distends vessels evenly and results in reproducible assessments was used to image the pulmonary vascular tree. Control animals demonstrated vessel branching and vascular blush throughout the lung parenchyma, including the periphery of the lung. In contrast, lungs with measured pulmonary hypertension after Adeno-Ang-1 injection showed normal to slightly enlarged hilar vessels with minimal peripheral angiographic blush. The lack of vessels seen in the lung periphery with this technique was consistent with the fact that most pulmonary arterioles ≤500 μm in diameter (more than four branch divisions from the main pulmonary artery) were nearly occluded. A similar angiographic pattern is seen in the human form of this disease (16).
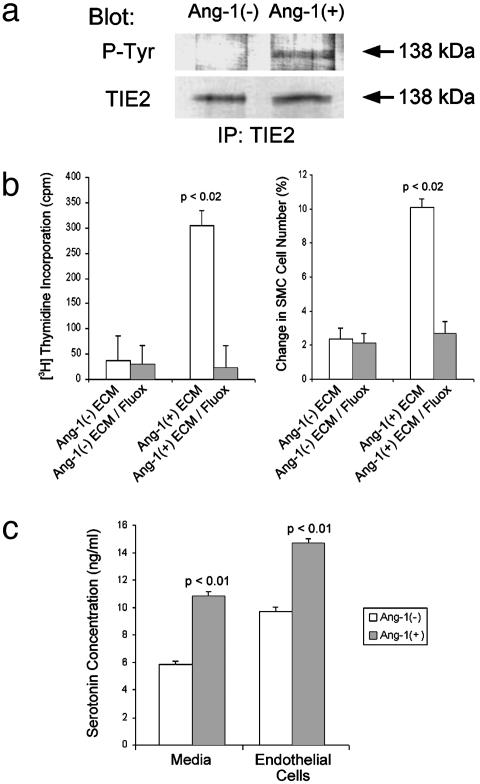
Ang-1-Treated Primary Pulmonary Arteriolar Endothelial Cells Produce and Release Serotonin. Our animal experiments suggested that constitutive Ang-1 levels in the lung induce small vessel smooth muscle cell proliferation. Therefore, we hypothesized that Ang-1 signaling through the TIE2 receptor in pulmonary endothelial cells would result in release of a paracrine smooth muscle cell growth factor. To test this hypothesis, we determined whether Ang-1-stimulated subcultured HPAEC would release a growth factor specific for pulmonary smooth muscle cells. Purified rAng-1 protein was added to serum-starved HPAEC in culture. HPAEC treated with rAng-1 consistently demonstrated TIE2 receptor phosphorylation, whereas untreated HPAEC did not (Fig. 4a). Serum-free media derived from rAng-1-treated HPAEC stimulated the proliferation of human pulmonary arteriolar smooth muscle cells in culture as measured by [3H]thymidine incorporation and cell count (Fig. 4b). These data suggested that pulmonary arteriolar endothelial cells secrete a specific smooth muscle cell growth factor in response to rAng-1 stimulation.
Fig. 4.
Ang-1-treated human pulmonary endothelial cells release a potent smooth muscle cell growth factor: serotonin. (a) Immunoblot analysis of anti-TIE2 immunoprecipitates from subcultured pulmonary arteriolar endothelial cells either treated or not treated with Ang-1 protein. Cells incubated with Ang-1 protein demonstrated TIE2 receptor phosphorylation, whereas untreated cells did not (Upper). Protein levels of TIE2 were similar in the two groups (Lower). (Upper) Immunoprecipitated (IP) with anti-TIE2 antibody, blotted with anti-phosphotyrosine (P-Tyr) antibody. (Lower) IP with anti-TIE2, blotted with anti-TIE2. (b) Stimulation of pulmonary smooth muscle cell proliferation by serum-free medium from Ang-1-treated endothelial cells is blocked by a serotonin transporter inhibitor. (Left)[3H]Thymidine incorporation in smooth muscle cells treated with (i) medium from endothelial cells not treated with Ang-1 [Ang-1(–)ECM], (ii) medium from endothelial cells not treated with Ang-1 to which 1 μM fluoxetine was added [Ang-1(–)ECM/Fluox], (iii) medium from endothelial cells treated with Ang-1 [Ang-1(+)ECM], or (iv) medium from endothelial cells treated with Ang-1 to which 1 μM fluoxetine was added [Ang-1(+)ECM/Fluox]. (Right) Percent change in smooth muscle cell count after treatment with the same media combinations as in Left.(c) Pulmonary endothelial cells treated with 50 ng/ml Ang-1 produce and secrete serotonin. Graph depicts results of ELISA quantitation of serotonin from cells treated with or without Ang-1 (right bars) and from media taken from cells treated with or without Ang-1 (left bars).
Because previous reports suggested that serotonin (5-hydroxytryptamine) plays a role in smooth muscle cell proliferation in the vasculature of other organs (17), we tested whether the growth factor produced by rAng-1-activated HPAEC was indeed serotonin. Serum-free medium taken from rAng-1-treated HPAEC contained elevated levels of serotonin by ELISA, compared with serum-free medium from unstimulated HPAEC (Fig. 4c). To distinguish whether rAng-1-treated HPAEC were producing and/or secreting serotonin, we assayed serotonin levels in rAng-1-treated and untreated HPAEC as well as in media derived from these cells. We found that Ang-1-stimulated HPAEC both harbor and secrete large amounts of serotonin compared with baseline unstimulated cells (Fig. 4c).
To associate cause and effect (i.e., serotonin is the growth factor responsible for pulmonary arteriolar smooth muscle cell proliferation in culture), we treated serum-free human pulmonary arteriolar smooth muscle cells with serum-free media from HPAEC stimulated with rAng-1 both in the presence and the absence of 1 μM fluoxetine {N-methyl-γ-[4-(trifluoromethyl)-phenoxy]benzenepropamin}, an inhibitor of serotonin uptake by smooth muscle cells. Smooth muscle cells treated for 36–48 h with medium from rAng-1-stimulated HPAEC showed enhanced cellular proliferation (from 5 × 104 cells per well to 5.5 × 104 cells per well, a 5-fold increase in cell number compared with control wells) as assayed by cell count and [3H]thymidine incorporation (Fig. 4b). In contrast, pulmonary arteriolar smooth muscle cells treated with the same medium plus fluoxetine did not proliferate. This result was reproducible between repeat cellular experiments, different proliferation assays ([3H]thymidine incorporation and cell count), and a wide range of fluoxetine concentrations. Fluoxetine had no effect on baseline levels of smooth muscle cell division seen when smooth muscle cells were treated with medium from unstimulated HPAEC (Fig. 4b).
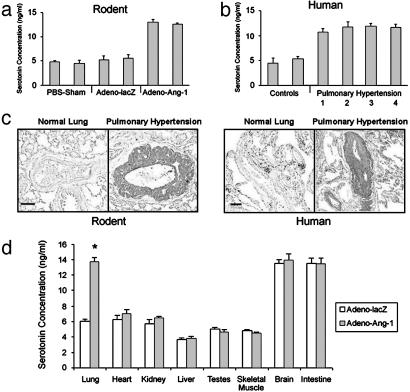
High Levels of Serotonin in the Wall of Small Pulmonary Arterioles in Rodent and Human Pulmonary Hypertension. Our cell culture experiments suggested that the smooth muscle cell hyperplasia seen in small lung vessels in pulmonary hypertension may be the result of serotonin production and/or release by endothelial cells within these vessels. Therefore, we assayed lung tissue from our Ang-1 rodent pulmonary hypertension model as well as human lung tissue derived from pulmonary hypertensive patients for the presence of increased serotonin levels. Rats with constitutive lung Ang-1 expression and clinical pulmonary hypertension had increased levels of serotonin in their lung tissue compared with controls (Fig. 5a). Similarly, lung tissue from patients with a variety of etiologies of pulmonary hypertension also manifest high serotonin levels compared with normotensive human lung tissue (Fig. 5b).
Fig. 5.
High levels of serotonin in rodent and human pulmonary hypertensive lung tissue. (a) ELISA quantitation of serotonin in lungs from animals injected with Adeno-Ang-1 (pulmonary hypertension group) compared with serotonin levels in lungs from rodents injected with Adeno-lacZ or PBS (normotensive control groups). (b) ELISA quantitation of serotonin in lung samples taken from humans with pulmonary hypertension resulting from chronic thromboembolism (bar 1), pulmonary overcirulation from ventricular septal defects (bar 2), scleroderma (bar 3), and idiopathic nonfamilial disease (bar 4), compared with lung specimens obtained from patients without pulmonary hypertension (controls). (c) Immunohistochemical staining of lung tissue with anti-serotonin antibody shows that rodents with Ang-1-induced pulmonary hypertension have serotonin within the vascular wall of pulmonary vessels ≤500 μm in diameter (Left), whereas serotonin staining is not detected in normal rat lung tissue. Human tissue stained with anti-serotonin antibody demonstrates serotonin in the wall of small pulmonary vessels in pulmonary hypertensive lung tissue only (Right), without staining in lung specimens from patients without pulmonary hypertension. (Scale bars represent 50 μm.) (d) ELISA quantitation of serotonin in organs of animals with pulmonary hypertension induced by Adeno-Ang-1, compared with animals without pulmonary hypertension injected with control Adeno-lacZ virus. *, P < 0.01 compared with lung control group.
In rat and human pulmonary hypertensive lung tissue, serotonin-specific staining was detected in the intima and media of pulmonary vessels measuring ≤500 μm in rats and ≤800 μm in humans (Fig. 5c). Endothelial-specific staining was not due to platelet aggregation at the vessel wall. Staining of successive lung sections with anti-serotonin and anti-CD31 antibodies confirmed that serotonin was detected in endothelium, rather than in adherent blood cells. This vascular localization of serotonin-specific staining was seen only in pulmonary hypertensive lung tissue (pulmonary artery systolic pressure >25 mmHg in rats, and >50 mmHg in humans) and was absent from normotensive human or rodent lung specimens. Low levels of cytoplasmic serotonin-specific staining were also seen in bronchial neuroendocrine cells in the epithelial layer of small bronchioles, consistent with previous pathological reports (18).
To characterize whether augmented serotonin levels were specific only to lung tissue in pulmonary hypertension, we compared the organ-specific levels of serotonin between normal and Ang-1-induced pulmonary hypertensive rats (Fig. 5d). Baseline levels of serotonin were similar in heart, kidney, liver, testes, skeletal muscle, brain, and intestine between control and pulmonary hypertensive animals. In contrast, lung levels of serotonin were more than doubled in animals with constitutive pulmonary Ang-1 expression. These results suggest that the pulmonary hypertensive phenotype is defined by Ang-1-induced high levels of serotonin in the lung (and not other organs).
Discussion
The genesis of pulmonary hypertension is a complex vascular process involving alterations in vessel wall anatomy in the lung. In the earliest phases of pulmonary hypertension, the distal pulmonary vessels are histologically normal but capable of exaggerated vasoconstriction. As the disease progresses, morphologic changes in the distal pulmonary arterioles occur, resulting in muscular hyperplasia of the medial layer of these vessels. This vessel wall thickening obstructs arteriolar blood flow by direct luminal compression and is responsible for the irreversible high pressures in the pulmonary vascular tree. Little is known about the molecular signals that generate this process. Recently, a subset of patients with inherited idiopathic pulmonary hypertension have been found to have heterozygous mutation of the bone morphogenetic protein receptor type 2 gene (19); however, the dose-dependent role of this type β transforming growth factor receptor-like protein in the lung is unknown.
Our goal has been to understand how small vessels in the lung develop the progressive vasculopathy of pulmonary hypertension. In this disease, arteriolar obstruction results from pathologic smooth muscle cell proliferation in the walls of these vessels. Worsening pulmonary hypertension is pathologically correlated with progressive luminal clogging. As a result of the work reported here, we have four major conclusions. First, high steady-state levels of Ang-1 in the rodent lung, particularly at the level of the arteriolar wall, lead to the clinical development of, and pathologic changes associated with, pulmonary hypertension. This result, which implies that deregulation of a single gene product may trigger the genesis of pulmonary hypertension, serves as a starting point for the molecular dissection of this disease.
The pulmonary hypertensive phenotype in our animals was not due to the vector, the route of administration, or the carrier buffer, as indicated by the fact that control rats had morphologically normal lung tissue. High steady-state levels of Ang-1 induced by viral injection into the pulmonary circulation led to the clear-cut development of medial hyperplasia in pulmonary arterioles, resulting in vascular obstruction. This pathology persisted after transgene expression had ceased. We therefore believe that supranormal concentrations of Ang-1 in the pulmonary vasculature facilitate the development of profuse muscular encasement of small pulmonary blood vessels. This gene-specific phenotype is specific to arterioles and did not affect pulmonary veins or large pulmonary arteries. Muscularization of pulmonary vessels correlated with increased pulmonary arterial pressures, consistent with what is seen in human pulmonary hypertension. These findings directly demonstrate postnatal in vivo bioactivity in the lung associated with Ang-1.
Second, we have created an animal model to study the treatment, prevention, and molecular mechanisms responsible for pulmonary hypertension. This model is particularly important given that there is no cure for this disease and that it affects millions of individuals in the United States each year. Although several animal models based on chemically induced inflammation or on hypoxia have been used to study pulmonary hypertension, none mimics the human disease accurately (20). The advantage of our animal model, based on overexpression of a single gene in the lung, is that it induces elevated pulmonary artery pressures and vessel muscular thickening similar to the human disease, without relying on chemically induced lung inflammation or hypoxia.
Third, we link a common pattern of gene expression in different forms of human pulmonary hypertension to the constellation of gene expression that we have identified in our rodent model for the disease. Recent work from our group has demonstrated that Ang-1 is constitutively expressed at mRNA and protein levels in the lungs of patients with many types of pulmonary hypertension, making it a sensitive molecular marker for the presence and severity of disease (11). Endothelial cells lining small pulmonary vessels of patients with pulmonary hypertension also showed elevated levels of TIE2 receptor phosphorylation, consistent with what we see experimentally in animals overexpressing Ang-1.
Finally, we demonstrate that Ang-1 induces pulmonary arteriolar endothelial cells to produce and release the muscle-specific growth factor, serotonin. Specifically, we show that HPAEC treated in culture with Ang-1 protein produce and release serotonin as well as demonstrate that high steady-state serotonin levels are found in the lungs of rats with pulmonary hypertension induced by constitutive Ang-1 expression. Our animal model for pulmonary hypertension mimics human pulmonary hypertension in that high serotonin levels in the lung are seen in many human forms of this disease, particularly at the level of the arteriolar vascular wall.
In rat pulmonary artery smooth muscle cells, the mitogenic effect of serotonin requires internalization of this indoleamine by a high-affinity transporter (21). Pulmonary artery smooth muscle cells possess both a single membrane-bound transporter and several serotonin receptors, which are chemically distinct from each other (22). Smooth muscle cell proliferation is stimulated by serotonin binding and internalization by the transporter protein (23). The receptors for serotonin, 5-HT1B and 5-HT2B, have also been suggested to play a role in vascular smooth muscle cell proliferation in rodent models of pulmonary hypertension (22, 24). We show that administration of fluoxetine, a serotonin transporter inhibitor, completely blocked the proliferative effect of serotonin released by endothelial cells treated with Ang-1. These results imply that the growth factor secreted by Ang-1-stimulated endothelial cells is indeed serotonin and that the smooth muscle cell hyperplasia seen in pulmonary hypertensive lungs is the result of molecular signaling between endothelial and smooth muscle cells.
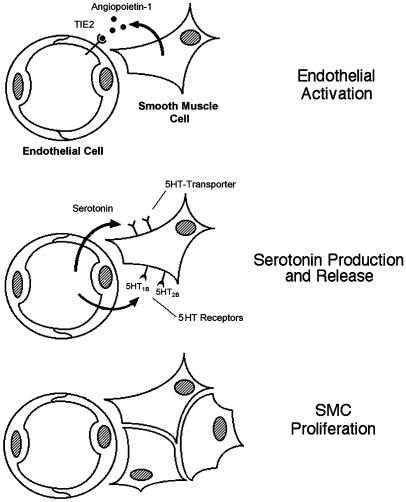
In summary, we find that high steady-state levels of Ang-1 in the lung induce pulmonary hypertension and are the stimulus for cell–cell signaling that culminates in organ-specific vascular smooth muscle cell mitogenesis. Our results suggest that Ang-1 signals pulmonary vascular endothelial cells through a TIE2 pathway to produce and secrete serotonin, effecting a paracrine feedback axis that results in vascular smooth muscle cell proliferation (Fig. 6). These data support a molecular mechanism where Ang-1/TIE2/serotonin crosstalk between vascular endothelial and smooth muscle cells is responsible for the generation of pulmonary hypertension. Further understanding of the effect of Ang-1 in the pulmonary artery tree may reveal clues for treating pulmonary hypertension and modulating vascular wall biology in this organ system.
Fig. 6.
Proposed model for vascular smooth muscle cell (SMC) hyperplasia in pulmonary hypertension. Ang-1 released by SMCs binds the TIE2 receptor and induces endothelial cells (Top) to synthesize and release serotonin (Middle). Endothelial-derived serotonin acts as a mitogen by binding a specific transporter protein on the surface of SMCs, inducing the SMCs to proliferate (Bottom). In normotensive lung, Ang-1 is not produced.
Supplementary Material
Acknowledgments
This work was supported by the Charles B. Wang Foundation and National Institutes of Health Grant R01 HL70852 01 (to P.A.T.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ang-1, angiopoietin 1; HPAEC, human pulmonary arteriolar endothelial cells; rAng-1, recombinant Ang-1.
References
- 1.Folkman, J. & D'Amore, P. A. (1996) Cell 87, 1153–1155. [DOI] [PubMed] [Google Scholar]
- 2.Hayes, A. J., Huang, W. Q., Mallah, J., Yang, D., Lippman, M. E. & Li, L. Y. (1999) Microvasc. Res. 58, 224–237. [DOI] [PubMed] [Google Scholar]
- 3.Davis, S., Aldrich, T. H., Jones, P. F., Acheson, A., Compton, D. L., Jain, V., Ryan, T. E., Bruno, J., Radziejewski, C., Maisonpierre, P. C., et al. (1996) Cell 87, 1161–1169. [DOI] [PubMed] [Google Scholar]
- 4.Jones, N. & Dumont, D. J. (2000) Cancer Metastasis Rev. 19, 13–17. [DOI] [PubMed] [Google Scholar]
- 5.Davis, S. & Yancopoulos, G. D. (1999) Curr. Top. Microbiol. Immunol. 237, 173–185. [DOI] [PubMed] [Google Scholar]
- 6.Suri, C., Jones, P. F., Patan, S., Bartunkova, S., Maisonpierre, P. C., Davis, S., Sato, T. N. & Yancopoulos, G. D. (1996) Cell 87, 1171–1180. [DOI] [PubMed] [Google Scholar]
- 7.Vikkula, M., Boon, L. M., Carraway, K. L., III, Calvert, J. T., Diamonti, A. J., Goumnerov, B., Pasyk, K. A., Marchuk, D. A., Warman, M. L., Cantley, L. C., et al. (1996) Cell 87, 1181–1190. [DOI] [PubMed] [Google Scholar]
- 8.Smith, C. M., Beasley, G. C., Cheng, Y., Ormond, D. B. & Spain, P. (2000) in The U.S. Scientific Registry of Transplant Recipients and the Organ Procurement and Transplantation Network: Transplant Data 1990–1999 (U.S. Dept. of Health and Human Services, Richmond, VA), pp. 351–410.
- 9.Katzenstein, A. L. & Askin, F. B. (1982) in Surgical Pathology of Non-Neoplastic Lung Disease, ed. Bennington, J. L. (Saunders, Philadelphia), Vol. 13, pp. 281–313. [PubMed] [Google Scholar]
- 10.Rabinovitch, M. (1996) in Glenn's Thoracic and Cardiovascular Surgery, eds. Baue, A., Geha, A., Hammond, G., Laks, H. & Naunheim, K. (Appleton & Lange, Stamford, CT), 6th Ed., Vol. 2, pp. 1047–1071. [Google Scholar]
- 11.Du, L., Sullivan, C. C., Chu, D., Cho, A. J., Kido, M., Wolf, P. L., Yuan, J. X., Deutsch, R., Jamieson, S. W. & Thistlethwaite, P. A. (2003) N. Engl. J. Med. 348, 500–509. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Foix, A. M., Coats, W. S., Baque, S., Alam, T., Gerard, R. D. & Newgard, C. B. (1992) J. Biol. Chem. 267, 25129–25134. [PubMed] [Google Scholar]
- 13.Yuan, J. X., Aldinger, A. M., Juhaszova, M., Wang, J., Conte, J. V., Jr., Gaine, S. P., Orens, J. B. & Rubin, L. J. (1998) Circulation 98, 1400–1406. [DOI] [PubMed] [Google Scholar]
- 14.Lou, J. N., Mili, N., Decrind, C., Donati, Y., Kossodo, S., Spiliopoulos, A., Ricou, B., Suter, P. M., Morel, D. R., Morel, P., et al. (1998) In Vitro Cell. Dev. Biol. Anim. 34, 529–536. [DOI] [PubMed] [Google Scholar]
- 15.Coral-Vazquez, R., Cohn, R. D., Moore, S. A., Hill, J. A., Weiss, R. M., Davisson, R. L., Straub, V., Barresi, R., Bansal, D., Hrstka, R. F., et al. (1999) Cell 98, 465–474. [DOI] [PubMed] [Google Scholar]
- 16.Bergin, C. J., Sirlin, C. B., Hauschildt, J. P., Huynh, T. V., Auger, W. R., Fedullo, P. F. & Kapelanski, D. P. (1997) Radiology (Easton, Pa.) 204, 695–702. [DOI] [PubMed] [Google Scholar]
- 17.Sharma, S. K., Del Rizzo, D. F., Zahradka, P., Bhangu, S. K., Werner, J. P., Kumamoto, H., Takeda, N. & Dhalla, N. S. (2001) Ann. Thorac. Surg. 71, 1856–1864. [DOI] [PubMed] [Google Scholar]
- 18.Colby, T. V. & Yousem, S. (1997) in Histology for Pathologists, ed. Sternberg, S. (Lippincott–Raven, Philadelphia), 2nd Ed., pp. 433–458.
- 19.Deng, Z., Morse, J. H., Slager, S. L., Cuervo, N., Moore, K. J., Venetos, G., Kalachikov, S., Cayanis, E., Fischer, S. G., Barst, R. J., et al. (2000) Am. J. Hum. Genet. 67, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Suylen, R. J., Smits, J. F. & Daemen, M. J. (1998) Am. J. Respir. Crit. Care Med. 157, 1423–1428. [DOI] [PubMed] [Google Scholar]
- 21.Eddahibi, S., Humbert, M., Fadel, E., Raffestin, B., Darmon, M., Capron, F., Simonneau, G., Dartevelle, P., Hamon, M. & Adnot, S. (2001) J. Clin. Invest. 108, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Launay, J. M., Herve, P., Peoc'h, K., Tournois, C., Callebert, J., Nebigil, C. G., Etienne, N., Drouet, L., Humbert, M., Simonneau, G., et al. (2002) Nat. Med. 8, 1129–1135. [DOI] [PubMed] [Google Scholar]
- 23.MacLean, M. R., Herve, P., Eddahibi, S. & Adnot, S. (2000) Br. J. Pharmacol. 131, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keegan, A., Morecroft, I., Smillie, D., Hicks, M. N. & MacLean, M. R. (2001) Circ. Res. 89, 1231–1239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.