Abstract
We earlier reported that the soluble form of the CD40 ligand (sCD40L), is involved in thrombosis by stabilizing platelet thrombi. In this article, we have determined the mechanism by which this protein affects platelet biology. Addition of sCD40L to washed platelets was found to activate the receptor function of αIIbβ3 as measured by the induction of fibrinogen binding and the formation of platelet microparticles. Mutation in the KGD sequence (D117E) of sCD40L, the αIIbβ3-binding domain in the N terminus of the protein resulted in a loss of the platelet-stimulatory activity of this protein. Integrilin, a αIIbβ3 antagonist, but not an antibody to CD40 that blocked the ligand-binding activity, inhibited these platelet-stimulatory events. CD40-/- platelets bound fibrinogen and formed microparticles similar to WT platelets, again indicating that CD40 is not involved in sCD40L-induced platelet activation. Exposure of platelets to sCD40L, but not D117E-sCD40L-coated surfaces, induced platelet thrombi formation under arterial shear rate. sCD40L-induced platelet stimulation resulted in the phosphorylation of tyrosine-759 in the cytoplasmic domain of β3. Platelets from the diYF mouse strain, expressing β3 in which both cytoplasmic tyrosines are mutated to phenylalanine, were defective in sCD40L-induced platelet stimulation. These data indicate that sCD40L is a primary platelet agonist and that platelet stimulation is induced by the binding of the KGD domain of sCD40L to αIIbβ3, triggering outside-in signaling by tyrosine phosphorylation of β3.
Platelet aggregation is now recognized as a primary reaction in arterial thrombosis and, accordingly, is responsible for the ischemic complications of acute myocardial infarction and stroke. αIIbβ3, the most abundant integrin on platelets, has complex roles in this reaction. On unstimulated platelets, αIIbβ3 has low affinity for soluble fibrinogen and von Willebrand factor (vWF), and is only capable of recognizing fibrinogen immobilized on surfaces (1). However, in response to platelet stimulation, induced by agents such as collagen, ADP, or thrombin, acting on distinct receptors, inside-out signaling causes the activation of the receptor function of αIIbβ3, allowing it to bind soluble fibrinogen and vWF. The polyvalent structures of these proteins allow them to crosslink the surfaces of activated platelets to mediate platelet aggregation (2). vWF binding to αIIbβ3 occurs through the RGD recognition motif found in this ligand. Fibrinogen binding occurs by the AGDV sequence found in the γ-chain of this protein (3).
Whereas αIIbβ3 activation and ligand binding are critical for initiating platelet aggregation, the stability of the aggregate appears to depend on αIIbβ3 signaling events induced by platelet-platelet contacts occurring during aggregation. One signaling event includes the phosphorylation of tyrosine residues on the cytoplasmic domain of β3 (4). Platelets from mice harboring β3, in which both cytoplasmic tyrosines have been mutated to phenylalanine, produce unstable platelet aggregates. Other secondary proteins are also involved, including Gas6 (5), Ephrin (6), and CD40L, a protein we recently found to be important in aggregate stability. CD40L, a tumor necrosis factor (TNF) family member is mainly expressed on activated T cells (7) and platelets (8). It is cryptic in unstimulated platelets, but rapidly becomes exposed on the platelet surface after stimulation where it is subsequently cleaved, producing a soluble hydrolytic product (9) Using an in vivo thrombosis model, we found that CD40L-/- mice have a platelet thrombosis defect, with a delayed occlusion time due to frequent embolization of the thrombi, despite the fact that these mice have normal hemastatic function, which also appears to be true for hyper-IgM patients (9, 10). The stability of thrombi was restored when the soluble form of the CD40 ligand (sCD40L) protein was infused into CD40L-/- mice. sCD40L was also found to be a αIIbβ3 ligand. These activities of sCD40L were shown to depend on its KGD sequence, a known αIIbβ3 binding motif, because both the αIIbβ3 binding and the thrombotic activities could be disrupted by a D117E mutation in the KGD. Although the thrombosis function of CD40L has been linked to αIIbβ3, the platelet integrin, and not to CD40, the mechanism by which CD40L participates in thrombosis is not known.
We initially envisioned that stabilization of platelet thrombi by sCD40L could occur by two mechanisms. First, because sCD40L is a trimer, this protein could crosslink platelets through interactions with αIIbβ3 on adjacent platelets. Second, sCD40L could bind αIIbβ3 and induce platelet stimulation directly. The studies reported herein demonstrate that sCD40L is a platelet agonist that activates platelets through αIIbβ3-dependent outside-in signaling.
Materials and Methods
Mice. Breeding pairs of CD40-/- mice obtained from The Jackson Laboratory, and C57BL/6 diYF mouse (mice expressing β3 integrin carrying Y747F and Y759F mutations) colonies were maintained, according to the Institutional Animal Care and Use Committees regulations at Millennium Pharmaceuticals.
Preparation of Washed Platelets. Human and mouse washed platelets were prepared as described (11-13).
Flow Cytometry Analysis. For fibrinogen binding, washed human and mouse platelets (1 × 107 per ml) were incubated for 60 min at 37°C with 2 μM sCD40L or (D117E)-sCD40L in the presence or absence of 2.5 μM Integrilin or 10 μg/ml purified and detoxified anti-human CD40 antibody (ATCC-9110, G28-5). All incubations had 150 nM Alexa-fibrinogen (Molecular Probes). Samples were diluted 10-fold with stain buffer (Pharmingen) after incubation and immediately analyzed by flow cytometry (with a FACSort fluorescence-activated cell sorter, Becton Dickinson). P-selectin expression on the washed platelets were measured with a monoclonal antibody (Becton Dickinson) after stimulating platelets with purified 2 μM sCD40L (10) or 5 μM thrombin receptor activating peptide (TRAP) (Bachem). Dense granule secretion was measured by activating mepacrine-loaded washed platelets (14) with 2 μM sCD40L or 5 μM TRAP.
Tyrosine Phosphorylation of β3 in Spread Platelets, and in Thrombi Formed on sCD40L Surface. Coverslips were coated with 10 μg/ml sCD40L for 2 h at 37°C and blocked with 5% crystalline BSA (Sigma). One-hundred microliters of washed platelets at 1 × 107 per ml were allowed to spread for 1 h at 37°C. Nonadhered platelets were washed with PBS (GIBCO/BRL). Adhered platelets were fixed with 4% paraformaldehyde (PFA) and permeabilized with 0.5% Triton X-100 and 0.5% BSA in PBS. Permeabilized platelets were blocked with 3% goat serum and 2% BSA for 30 min, followed by incubation for 2 h at room temperature with purified β3 tyrosine-759 phosphospecific rabbit polyclonal antibody (pY759) raised against a peptide corresponding to the 748-762 amino acid region of the β3 cytoplasmic tail (Research Genetics, Huntsville, AL). The specificity of the staining was determined by incubating the phosphospecific antibody with 250 μM of phosphopeptides corresponding to the β3 cytoplasmic 748-762 amino acid sequence containing pY759, or the 740-753 amino acid sequence containing pY747 residues. After 30 min of washing with PBS containing 0.1% Triton X-100 and 0.5% BSA, the coverslips were incubated with Alexa 596 goat anti-rabbit antibody and Alexa 488 phalloidin for 40 min, followed by a brief wash, and were mounted on slides with VECTASHIELD (Vector Laboratories). Images were recorded under oil-immersion ×60 objective by using a Hamamatsu (Ichinocho, Japan) ORCA-ER digital camera attached to a Nikon eclipse E1000 f luorescence microscope (Technical Instruments, San Francisco).
Platelet thrombi formed on sCD40L-coated Petri plates were rinsed with PBS, fixed with 4% PFA for 20 min, subsequently permeabilized with 0.1% Triton X-100 for 20 min, blocked with normal goat serum for 20 min, stained with purified β3 pY759 antibody for 50 min, and finally incubated with an FITC-conjugated goat-anti rabbit antibody (1/1,000 dilution) for 30 min. All incubations were performed at 4°C. Between each step, platelet aggregates were rinsed three times with a PBS/0.5% BSA solution. Pictures were taken with an Axiovert 100 (Zeiss) inverted fluorescent microscope.
Western Blot Analysis of β3 Tyrosine Phosphorylation. Washed platelets were incubated at 3 × 108 per ml at 37°C for 40 min with sCD40L or (D117E)-sCD40L in the presence or absence of 10 μM Integrilin, 1 μM fibrinogen, and 1 mM sodium vanadate. Tyrosine phosphorylation was analyzed by Western blot analysis on SDS gels by using purified β3 pY759-specific antibody. The blots were stripped and reprobed with C3A, an anti-human αIIbβ3 monoclonal antibody to quantitate levels of β3.
Perfusion Chamber Assay. Whole blood, obtained from healthy volunteers who denied having taken aspirin or other platelet function inhibitors in the preceding week, was anticoagulated with 300 μmol/liter d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone dihydrochloride (PPACK). A silicone gasket was placed between a flat perfusion chamber (Glycotech, Rockville, MD) and a Petri dish coated with fibrinogen or sCD40L or (D117E)-CD40L. Coating of the proteins was performed by using the spray method, in which 25 μl of a 1 mg/ml protein solution is coated onto the plastic with a retouching air brush, resulting in a concentration of 25 μg/cm2 (15) Whole blood with and without Integrilin or 0.1 μg/ml aspirin or 100 μM 2-methylthio-AMP (2MesAMP) or aspirin and 2MesAMP was perfused for 7 min through flat perfusion chambers at various shear rates. Shear rates of 1,500 and 550 per s were obtained with gasket of a flow path height of 127 μm and a flow path width of 2.5 mm at 0.6 and 0.24 ml/min, respectively, and 150 per s with a 254-μm thickness, and a 5-mm-width flow path at 0.48 ml/min. Measurement of thrombi (mean thrombus area) was assessed by using SIMPLE PCI software.
Results
sCD40L-Induced Platelet Activation. We previously showed that sCD40L binds to purified αIIbβ3 and that sCD40L stabilizes platelet thrombi and promotes platelet spreading. To determine whether sCD40L has a direct effect on platelet function, we measured markers of platelet stimulation. The addition of sCD40L to washed human (Fig. 1b and Table 1) or mouse (Table 2) platelets was found to induce microparticle formation. In addition, sCD40L also allowed for increased fibrinogen binding, to both the sCD40L-stimulated platelets, and to the microparticles formed by this treatment. In contrast, we did not observe any sCD40L-induced expression of P-selectin and dense granule secretion (data not shown). These data indicate that sCD40L induced selective platelet activation.
Fig. 1.
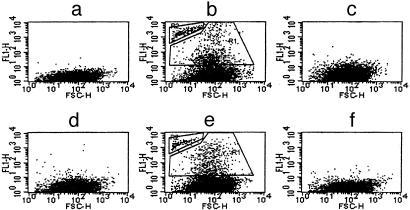
sCD40L induces fibrinogen binding to unactivated human platelets and generation of microparticles. Washed platelets were incubated for1hat 37°C with sCD40L (b) or (D117E)-sCD40L (c), sCD40L in the presence of Integrilin (d), or CD40 antibody (e). No treatment (a) and anti-CD40 antibody alone (f) served as controls. Gates R1 and R2 (b and e), respectively, represent platelets and microparticles positive for fibrinogen binding. Flow cytometry data are representative of four experiments performed with four different platelet preparations. FSC-H, forward scatter-height.
Table 1. The effect of CD40L on washed human platelets.
| Treatment | Platelets | Microparticles |
|---|---|---|
| Untreated | 0.1 ± 0.1 | 0.0 |
| sCD40L | 15 ± 5.0 | 5.0 ± 3.0 |
| (D117E)-sCD40L | 1.0 ± 0.9 | 0.1 ± 0.1 |
| sCD40L plus integrilin | 1.5 ± 1.0 | 0.2 ± 0.1 |
| sCD40L plus anti-CD40 antibody | 13.0 ± 7.0 | 4.0 ± 3.0 |
| Anti-CD40 antibody | 0.5 ± 0.2 | 0.0 |
Table 2. The effect of CD40L on washed mouse platelets.
| WT
|
CD40-/-
|
|||
|---|---|---|---|---|
| Treatment | Platelets | Microparticles | Platelets | Microparticles |
| Untreated | 0.06 ± 0.05 | 0.0 | 0.02 ± 0.05 | 0.0 |
| sCD40L | 12.8 ± 2.2 | 3.98 ± 1.2 | 13.02 ± 0.6 | 3.98 ± 1.2 |
| (D117E)-sCD40L | 0.41 ± 0.28 | 0.59 ± 0.36 | 0.41 ± 0.10 | 0.02 ± 0.01 |
sCD40L has at least two functional domains: a KGD sequence, which binds to αIIbβ3 (10), and a TNF homology domain, which binds to CD40 (16, 17). To determine whether the KGD domain is involved in platelet activation, (D117E)-sCD40L was generated and compared with the native sequence for its platelet-activating activity. The induction of fibrinogen binding and the expression of platelet microparticles was not observed when either human or mouse platelets were incubated with (D117E)-sCD40L (Fig. 1c and Tables 1 and 2), suggesting that the KGD domain of sCD40L is involved in the induction of platelet activation. To test the hypothesis that sCD40L induced platelet activation by binding to αIIbβ3, and not to CD40, the effect of specific antagonists on each receptor was examined. sCD40L-induced microparticle formation by human platelets was completely inhibited by Integrilin, a specific αIIbβ3 antagonist (Fig. 1d and Table 1), but not by a CD40 antibody that blocks the binding of CD40L (Fig. 1e and Table 1). The CD40 antibody also had no effect on sCD40L-induced fibrinogen binding (Fig. 1f). In addition, platelets from CD40-/- mice were activated similarly to those from WT mice, a reaction that was dependent on the KGD sequence of sCD40L (Table 2). These data indicate that sCD40L induces platelet activation by directly binding to αIIbβ3 through its KGD sequence.
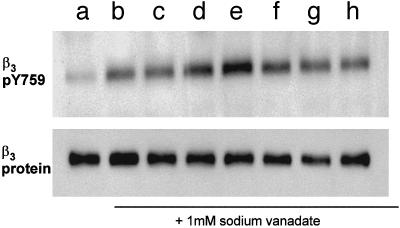
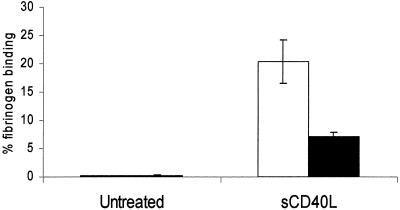
sCD40L-Induced αIIbβ3 Tyrosine Phosphorylation. We previously found that outside-in signaling through αIIbβ3 is mediated in part by the tyrosine phosphorylation of the cytoplasmic domain of β3. To determine whether tyrosine phosphorylation of β3 occurs during sCD40L-induced activation of platelets, we blotted gels of lysates with an antibody specific for β3 tyrosine-759 (pY759). We observed that addition of sCD40L to washed platelets caused a >2-fold increase in the tyrosine phosphorylation of β3 (Fig. 2, lane d). A further increase was observed when fibrinogen was included with this incubation (Fig. 2, lane e), but not when fibrinogen was added in the absence of sCD40L (Fig. 2, lane c). Integrilin inhibited sCD40L-induced β3 tyrosine phosphorylation (Fig. 2, lane h). Tyrosine phosphorylation of β3 remained at basal levels when incubated with (D117E)-sCD40L in the absence (Fig. 2, lane f) or in presence of fibrinogen (Fig. 2, lane g). To determine whether tyrosine phosphorylation of β3 is necessary for sCD40L-induced activation of platelets, we next measured the sCD40L-induced fibrinogen binding to platelets from the diYF mouse, a strain expressing β3, in which both cytoplasmic tyrosines are mutated to phenylalanine. As seen in Fig. 3, whereas 20 ± 3.9% of WT platelets bound fibrinogen when incubated with sCD40L, only 7.2 ± 0.54% of diYF platelets bound fibrinogen.
Fig. 2.
sCD40L induces β3 tyrosine-759 phosphorylation. Washed platelets were incubated with sCD40L alone (lane d), fibrinogen alone (lane c), d plus c (lane e), (D117E)-sCD40L alone (lane f), c plus f (lane g), or sCD40L with fibrinogen and Integrilin (lane h) in the presence of sodium vanadate. Unstimulated platelets (lanes a and b) served as controls with and without sodium vanadate, respectively. Platelet samples were lysed and resolved on SDS/10% PAGE and blotted with β3 pY759-specific polyclonal antibody. This blot is representative of eight separate experiments.
Fig. 3.
The diYF platelets are defective in sCD40L-induced fibrinogen binding. Washed platelets prepared from WT and diYF mice were incubated with Alexa fibrinogen in the presence or absence of sCD40L for 1 h at 37°C, fixed with formaldehyde (0.4% final), diluted, and analyzed by flow cytometry. The percentage of platelets positive for fibrinogen binding was obtained from the total number of gated events represented as a mean value with SD obtained from three separate platelet preparations.
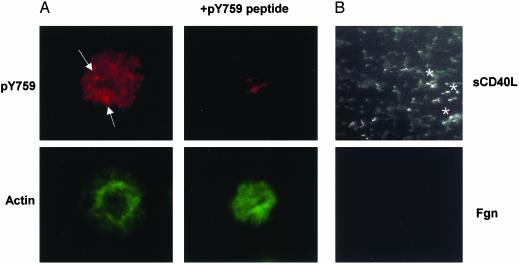
Fig. 4 shows that tyrosine phosphorylation of β3 also occurs during spreading of platelets and in platelet thrombi on immobilized sCD40L. Tyrosine phosphorylation of β3 in spread platelets was detected by staining with the β3 pY759 antibody, the staining of which appeared to be in focal contacts (Fig. 4A Left Upper). Staining with this antibody appeared to be specific, because it was blocked with 250 μM peptide containing the β3 cytoplasmic 748-762 amino acid region with pY759 (Fig. 4A Right Upper), but not by a peptide corresponding to the 740-753 amino acid region containing pY747, a site that also becomes phosphorylated on platelet aggregation (data not shown).
Fig. 4.
Platelet β3 cytoplasmic tyrosine-759 is phosphorylated when spread on the sCD40L surface under static and shear conditions. (A) Washed platelets spread on sCD40L-coated glass surface were stained with pY759 antibody. Specific staining was blocked when 250 μM Y759 phosphopeptide was incubated with the phosphoantibody before adding to spread platelets. Arrows indicate intense stained areas, and they appear to be in focal contacts. Platelets shown here represent a field of spread platelets stained positively for pY759 antibody. (B) Anticoagulated whole blood was perfused over different coated surfaces at arterial shear rate. Adhered platelets were fixed and stained with pY759 antibody. *, Platelets in the thrombi stained positive for pY759 antibody on the fluorescence image.
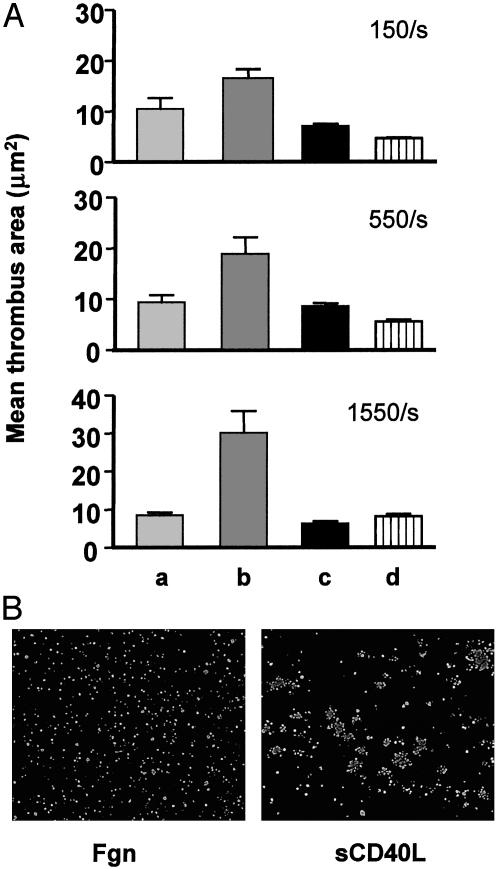
CD40L has been shown to be a prominent component of atherosclerotic lesions (18). Because ruptured lesions initiate thrombosis and sCD40L induces platelet activation, experiments were performed to determine whether immobilized sCD40L could initiate thrombosis. PPACK-anticoagulated human blood was perfused over surfaces coated with sCD40L or fibrinogen at shear rates designed to include those anticipated within the coronary circulation. Platelets are known to form a monolayer on fibrinogen surfaces at low (150 per s) to high (1,550 per s) shear rates, apparently because the adherent platelets are insufficiently activated to recruit and/or retain additional platelets (ref. 19 and Fig. 5 A and B Left). However, the sCD40L-coated surface supported thrombus formation in a shear rate-dependent manner (Fig. 5 A and B Right). Two studies indicated that the thrombogenic activity of immobilized sCD40L involved a direct interaction with αIIbβ3. First, (D117E)-sCD40L lacked thrombogenic activity. Second, thrombosis on sCD40L surfaces was completely inhibited by 2 μM Integrilin. These data indicate that immobilized sCD40L is capable not only of recruiting platelets at arterial shear rates but also of inducing sufficient platelet activation to cause the recruitment of platelets. This activity of sCD40L is caused by the KGD domain of sCD40L binding to αIIbβ3 on platelets. To determine whether platelet secretion is required for thrombus formation on the sCD40L surface, PPACK-anticoagulated whole blood was perfused in the presence or absence of aspirin and 2MesAMP, a P2Y12 antagonist. Our result demonstrated that immobilized sCD40L-mediated thrombosis is secretion independent, which is consistent with our findings in the static assays (data not shown).
Fig. 5.
The sCD40L-coated surface is prothrombotic. (A) Anticoagulated whole blood was perfused at different shear rates over the surface coated with sCD40L (lane b), or fibrinogen (lane a), or (D117E)-sCD40L (lane c). Whole blood with Integrilin was perfused over the sCD40L-coated surface (lane d). (B) Fibrinogen (Fgn)- and sCD40L-coated chambers after perfusion were fixed and stained for P-selectin expression. Whereas the sCD40L-coated surface contained platelet aggregates, the fibrinogen-coated surface had single platelets.
Thrombi formed on sCD40L surfaces at arterial shear rates also contained robust staining for the β3 pY759 antibody (Fig. 4B Upper). In contrast, platelets adherent to fibrinogen surfaces only expressed minimal staining for the tyrosine phosphorylated Integrin (Fig. 4B Lower).
Discussion
The concept of autocrine loops by secreted factors from platelets is well established. For example, ADP, which is secreted from platelet-dense bodies in response to platelet stimulation, induces additional platelet activation to promote thrombotic and hemostatic reactions by binding to its receptors, P2Y1 (20) and P2Y12 (21). Gas6, an α-granule protein, has similar activity, inducing platelet stimulation by binding to its receptors Sky, mer, and axl. The importance of these events to platelet biology has been demonstrated by the clinical antithrombotic benefit of P2Y12 antagonists (22, 23), and by the thrombosis defect in Gas6-/- mice (6). We previously showed that CD40L-/- mice have thrombosis defect, that this thrombosis defect could be corrected by sCD40L (10), and that sCD40L is a αIIbβ3 ligand. This article shows that sCD40L induces platelet stimulation, and that this process is mediated by the binding of the KGD domain of sCD40L to αIIbβ3. Biochemical and genetic data demonstrate that this outside-in integrin signaling activity of sCD40L occurs because of the tyrosine phosphorylation of β3. We conclude that sCD40L functions as a primary platelet agonist, that αIIbβ3 is a primary agonist receptor, and that sCD40L functions in autocrine loop to regulate platelet biology.
Although platelet stimulation by outside-in signaling through αIIbβ3 is well known, the activity of sCD40L is, to our knowledge, unique. Typically, the receptor function of αIIbβ3 for soluble adhesive protein ligands is expressed by inside-out signaling in response to primary platelet agonists such as collagen, thrombin, and ADP (3, 24). Outside-in αIIbβ3 signaling and subsequent platelet stimulation is mediated by adhesive protein binding to αIIbβ3 during platelet aggregation and/or during platelet adhesion, with soluble αIIbβ3 ligands having minimal platelet-stimulatory activity. We have previously established that outside-in αIIbβ3 signaling is mediated, in part, by tyrosine phosphorylation of the cytoplasmic domain of β3 (25-28). This article, however, demonstrates that sCD40L, a soluble αIIbβ3 ligand, induces platelet stimulation, as evidenced by the generation of platelet microparticles, and by the activation of the receptor function of αIIbβ3 for soluble fibrinogen. This outside-in signaling event appeared to be mediated by tyrosine phosphorylation of β3. β3 tyrosine phosphorylation occurred both on addition of sCD40L to platelets in suspension, and on the adhesion of platelets to immobilized sCD40L; platelets from the diYF mouse were defective in sCD40L platelet-stimulatory activity. Whereas this might seem a discrepancy, we have found that the sCD40L released from platelets, as well as the recombinant sCD40L, is trimeric (data not shown), as is the native sCD40L shed from human T cells (29).
sCD40L is generated in vivo in response to thrombotic events, generating concentrations approaching 5-10 ng/ml within the systemic circulation. The concentration of sCD40L required to induce platelet stimulation approaches 50-100 times these values. However, sCD40L is generated at sites of thrombosis during acute coronary syndromes or percutaneous interventions that are 1/5,000 to 1/10,000 of the volume of blood (assuming a thrombus volume of <1 ml and a blood volume of 5 liters). In addition, the interplatelet space available for the generation of sCD40L within a platelet aggregate is further restricted, concentrating the released protein. These considerations indicate that the concentrations of sCD40L developed within a growing thrombus would be sufficient to induce platelet stimulation, and sufficient to account for the thrombosis defect in the CD40L-/- mouse.
While this manuscript was in preparation, a publication by Inwald et. al (30) appeared, suggesting that sCD40L induces platelet stimulation by binding to CD40 and not to αIIbβ3. These studies, however, were performed with a sCD40L tagged at its N terminus with a isoleucine zipper motif, which is known to create oligomerization of the protein, thereby enhancing and sustaining signaling through CD40 (31). Although it is not known why the αIIbβ3-binding activity of this protein is lost, it may be that exposure of the KGD sequence was lost by the construction of the chimeric protein. Whereas the observations of Inwald et al. (30) might suggest that CD40 signaling may occur during aggregation, the absence of a thrombosis defect in CD40-/- mice indicates that such signaling, if present, is not required for platelet aggregate stability.
Patients with acute coronary syndromes, peripheral arterial occlusive disease and other thrombotic disorders, angioplasty, and postcardiopulmonary bypass, have increased concentrations of sCD40L in circulation, most of it coming from the platelet pool (13, 32-34). Plasma sCD40L is now considered as a risk factor for cardiovascular disease in women (35). Increased CD40L expression has been localized to the human atherosclerotic lesions (18), which could be contributed by platelets in the growing thrombi at the lesion, T cells, and vascular cells. Activated platelets are now known to deliver regulated on activation, normal T cell expressed and secreted (RANTES) to the endothelium in lesions (36). It is tempting to speculate that activated platelets could deliver sCD40L to these lesions as well. Although CD40L has a proven role in inflammation (9) and atherosclerotic lesion progression (37, 38), this article suggests that exposure of CD40L after plaque rupture may contribute to the initiation of thrombosis.
Patients with thrombotic disorders (39), infectious diseases like malaria, and who have undergone cardiopulmonary bypass (40), have increased microparticles in circulation (41, 42), which are procoagulant and prothrombotic. sCD40L-αIIbβ3-mediated platelet microparticle formation could potentially contribute to these pathologies.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: sCD40L, the soluble form of the CD40 ligand.
References
- 1.Lindon, J. N., McManama, G., Kushner, L., Merrill, E. W. & Salzman, E. W. (1986) Blood 68, 355-362. [PubMed] [Google Scholar]
- 2.Shattil, S. J. (1999) Thromb. Haemostasis 82, 318-325. [PubMed] [Google Scholar]
- 3.Kloczewiak, M., Timmons, S. & Hawiger, J. (1982) Biochem. Biophys. Res. Commun. 107, 181-187. [DOI] [PubMed] [Google Scholar]
- 4.Law, D. A., Nannizzi-Alaimo, L., Ministri, K., Hughes, P. E., Forsyth, J., Turner, M., Shattil, S. J., Ginsberg, M. H., Tybulewicz, V. L. & Phillips, D. R. (1999) Blood 93, 2645-2652. [PubMed] [Google Scholar]
- 5.Angelillo-Scherrer, A., de Frutos, P., Aparicio, C., Melis, E., Savi, P., Lupu, F., Arnout, J., Dewerchin, M., Hoylaerts, M., Herbert, J., et al. (2001) Nat. Med. 7, 215-221. [DOI] [PubMed] [Google Scholar]
- 6.Prevost, N., Woulfe, D., Tanaka, T. & Brass, L. F. (2002) Proc. Natl. Acad. Sci. USA 99, 9219-9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armitage, R. J., Fanslow, W. C., Strockbine, L., Sato, T. A., Clifford, K. N., Macduff, B. M., Anderson, D. M., Gimpel, S. D., Davis-Smith, T. & Maliszewski, C. R. (1992) Nature 357, 80-82. [DOI] [PubMed] [Google Scholar]
- 8.Henn, V., Slupsky, J. R., Grafe, M., Anagnostopoulos, I., Forster, R., Muller-Berghaus, G. & Kroczek, R. A. (1998) Nature 391, 591-594. [DOI] [PubMed] [Google Scholar]
- 9.Henn, V., Steinbach, S., Buchner, K., Presek, P. & Kroczek, R. A. (2001) Blood 98, 1047-1054. [DOI] [PubMed] [Google Scholar]
- 10.Andre, P., Prasad, K. S., Denis, C. V., He, M., Papalia, J. M., Hynes, R. O., Phillips, D. R. & Wagner, D. D. (2002) Nat. Med. 8, 247-252. [DOI] [PubMed] [Google Scholar]
- 11.Nannizzi-Alaimo, L., Alves, V. L. & Phillips, D. R. (2003) Circulation 107, 1123-1128. [DOI] [PubMed] [Google Scholar]
- 12.Andre, P., Denis, C. V., Ware, J., Saffaripour, S., Hynes, R. O., Ruggeri, Z. M. & Wagner, D. D. (2000) Blood 96, 3322-3328. [PubMed] [Google Scholar]
- 13.Nannizzi-Alaimo, L., Rubenstein, M. H., Alves, V. L., Leong, G. Y., Phillips, D. R. & Gold, H. K. (2002) Circulation 105, 2849-2854. [DOI] [PubMed] [Google Scholar]
- 14.Wall, J. E., Buijs-Wilts, M., Arnold, J. T., Wang, W., White, M. M., Jennings, L. K. & Jackson, C. W. (1995) Br. J. Haematol. 89, 380-385. [DOI] [PubMed] [Google Scholar]
- 15.Houdijk, W. P., Sakariassen, K. S., Nievelstein, P. F. & Sixma, J. J. (1985) J. Clin. Invest. 75, 531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graf, D., Korthauer, U., Mages, H. W., Senger, G. & Kroczek, R. A. (1992) Eur. J. Immunol. 22, 3191-3194. [DOI] [PubMed] [Google Scholar]
- 17.Karpusas, M., Hsu, Y. M., Wang, J. H., Thompson, J., Lederman, S., Chess, L. & Thomas, D. (1995) Structure (London) 3, 1031-1039. [DOI] [PubMed] [Google Scholar]
- 18.Mach, F., Schonbeck, U., Sukhova, G. K., Bourcier, T., Bonnefoy, J. Y., Pober, J. S. & Libby, P. (1997) Proc. Natl. Acad. Sci. USA 94, 1931-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaidi, T. N., McIntire, L. V., Farrell, D. H. & Thiagarajan, P. (1996) Blood 88, 2967-2972. [PubMed] [Google Scholar]
- 20.Leon, C., Hechler, B., Freund, M., Eckly, A., Vial, C., Ohlmann, P., Dierich, A., LeMeur, M., Cazenave, J. P. & Gachet, C. (1999) J. Clin. Invest. 104, 1731-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollopeter, G., Jantzen, H. M., Vincent, D., Li, G., England, L., Ramakrishnan, V., Yang, R. B., Nurden, P., Nurden, A., Julius, D. & Conley, P. B. (2001) Nature 409, 202-207. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous (1996) Lancet 348, 1329-1339.8918275 [Google Scholar]
- 23.Yusuf, S., Zhao, F., Mehta, S. R., Chrolavicius, S., Tognoni, G. & Fox, K. K. (2001) N. Engl. J. Med. 345, 494-502. [DOI] [PubMed] [Google Scholar]
- 24.Calvete, J. J. (1999) Proc. Soc. Exp. Biol. Med. 222, 29-38. [DOI] [PubMed] [Google Scholar]
- 25.Law, D. A., DeGuzman, F. R., Heiser, P., Ministri-Madrid, K., Killeen, N. & Phillips, D. R. (1999) Nature 401, 808-811. [DOI] [PubMed] [Google Scholar]
- 26.Law, D. A., Nannizzi-Alaimo, L. & Phillips, D. R. (1996) J. Biol. Chem. 271, 10811-10815. [DOI] [PubMed] [Google Scholar]
- 27.Cowan, K. J., Law, D. A. & Phillips, D. R. (2000) J. Biol. Chem. 275, 36423-36429. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins, A. L., Nannizzi-Alaimo, L., Silver, D., Sellers, J. R., Ginsberg, M. H., Law, D. A. & Phillips, D. R. (1998) J. Biol. Chem. 273, 13878-13885. [DOI] [PubMed] [Google Scholar]
- 29.Pietravalle, F., Lecoanet-Henchoz, S., Blasey, H., Aubry, J. P., Elson, G., Edgerton, M. D., Bonnefoy, J. Y. & Gauchat, J. F. (1996) J. Biol. Chem. 271, 5965-5967. [DOI] [PubMed] [Google Scholar]
- 30.Inwald, D. P., McDowall, A., Peters, M. J., Callard, R. E. & Klein, N. J. (2003) Circ. Res. 92, 1041-1048. [DOI] [PubMed] [Google Scholar]
- 31.Morris, A. E., Remmele, R. L., Jr., Klinke, R., Macduff, B. M., Fanslow, W. C. & Armitage, R. J. (1999) J. Biol. Chem. 274, 418-423. [DOI] [PubMed] [Google Scholar]
- 32.Lee, Y., Lee, W. H., Lee, S. C., Ahn, K. J., Choi, Y. H., Park, S. W., Seo, J. D. & Park, J. E. (1999) Cardiology 92, 11-16. [DOI] [PubMed] [Google Scholar]
- 33.Aukrust, P., Muller, F., Ueland, T., Berget, T., Aaser, E., Brunsvig, A., Solum, N. O., Forfang, K., Froland, S. S. & Gullestad, L. (1999) Circulation 100, 614-620. [DOI] [PubMed] [Google Scholar]
- 34.Garlichs, C. D., Eskafi, S., Raaz, D., Schmidt, A., Ludwig, J., Herrmann, M., Klinghammer, L., Daniel, W. G. & Schmeisser, A. (2001) Heart 86, 649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schonbeck, U., Varo, N., Libby, P., Buring, J. & Ridker, P. M. (2001) Circulation 104, 2266-2268. [DOI] [PubMed] [Google Scholar]
- 36.Huo, Y., Schober, A., Forlow, S. B., Smith, D. F., Hyman, M. C., Jung, S., Littman, D. R., Weber, C. & Ley, K. (2003) Nat. Med. 9, 61-67. [DOI] [PubMed] [Google Scholar]
- 37.Mach, F., Schonbeck, U., Sukhova, G. K., Atkinson, E. & Libby, P. (1998) Nature 394, 200-203. [DOI] [PubMed] [Google Scholar]
- 38.Lutgens, E., Cleutjens, K. B., Heeneman, S., Koteliansky, V. E., Burkly, L. C. & Daemen, M. J. (2000) Proc. Natl. Acad. Sci. USA 97, 7464-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nomura, S., Komiyama, Y., Miyake, T., Miyazaki, Y., Kido, H., Suzuki, M., Kagawa, H., Yanabu, M., Takahashi, H. & Fukuhara, S. (1994) Thromb. Haemostasis 72, 519-522. [PubMed] [Google Scholar]
- 40.George, J. N., Pickett, E. B., Saucerman, S., McEver, R. P., Kunicki, T. J., Kieffer, N. & Newman, P. J. (1986) J. Clin. Invest. 78, 340-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piguet, P. F., Kan, C. D. & Vesin, C. (2002) Apoptosis 7, 91-98. [DOI] [PubMed] [Google Scholar]
- 42.Piguet, P. F., Kan, C. D., Vesin, C., Rochat, A., Donati, Y. & Barazzone, C. (2001) Am. J. Pathol. 159, 733-742. [DOI] [PMC free article] [PubMed] [Google Scholar]