Abstract
Mutations in the parkin gene are linked to autosomal-recessive juvenile parkinsonism (AR-JP). Parkin functions as a ubiquitin protein ligase in the degradation of several proteins, including the neuron-specific septin CDCrel-1. AR-JP-associated parkin mutations inhibit ubiquitination and degradation of CDCrel-1 and other parkin target proteins. Here we show that recombinant adeno-associated virus-mediated CDCrel-1 gene transfer to the substantia nigra of rats results in a rapid onset (6-10 days) of nigral and striatal CDCrel-1 expression that is followed by a progressive loss of nigral dopaminergic neurons and a decline of the striatal dopamine levels. In contrast, neurons of the globus pallidus are spared from CDCrel-1 toxicity. Furthermore, CDCrel-1 inhibits the release of dopamine from stably-transfected PC12 cells, and pharmacological inhibition of tyrosine hydroxylase and dopamine synthesis in rats prevents CDCrel-1-induced nigral neurodegeneration. These results show that CDCrel-1 overexpression exerts dopamine-dependent neurotoxicity and suggest that inhibition of dopamine secretion by CDCrel-1 may contribute to the development of AR-JP.
Parkinson's disease (PD) is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc), declining levels of dopamine in the striatum (STR), and motor impairments, including bradykinesia, rigidity, and tremor (1, 2). Several genetic loci have been implicated in the pathogenesis of familial forms of PD (3, 4). Two missense mutations in the α-synuclein gene are linked to a rare, dominant form of PD (5, 6). In addition, a variety of mutations in the parkin gene are responsible for autosomal-recessive juvenile parkinsonism (AR-JP) (7, 8), and a dominant mutation in the ubiquitin C-terminal hydrolase gene L1 (UCH-L1) has been linked to PD in one pedigree (9). Most recently, mutations in the DJ-1 gene (10), and the NR4A2 gene (11) encoding the nuclear receptor and transcription factor Nurr-1, have been reported to segregate with familial PD.
Parkin functions as a ubiquitin protein ligase to promote the degradation of specific target proteins (12, 13). The first parkin target to be identified was CDCrel-1 (13), a protein of the septin gene family that is predominantly expressed in the brain and localized mainly to presynaptic axon terminals of inhibitory neurons (14-16). CDCrel-1 associates with membranes including synaptic vesicles (15) and directly binds to the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein syntaxin-1 (17). Subsequently, several other parkin substrates were identified, including a G protein-coupled receptor termed Pael-R (18), an unusual O-glycosylated 22-kDa form of α-synuclein (19), synphilin-1 (20), as well as α- and β-tubulin (21). AR-JP-linked parkin mutants fail to ubiquitinate and degrade the parkin targets, suggesting that AR-JP is caused by the accumulation of proteins that are ultimately toxic to dopamine neurons (13, 19, 20). Interestingly, parkin is able to protect neurons from diverse cellular insults, suggesting a central role for parkin in maintaining dopamine neuron integrity (22).
We have shown earlier that recombinant adeno-associated viruses (rAAV) carrying the neuron-specific platelet-derived growth factor β-chain (PDGF-β) promoter confer efficient and long-lasting transgene expression throughout the rat dopamine system without causing toxicity or inflammation (23, 24). Here, we exploited rAAV-mediated gene transfer to investigate whether CDCrel-1 may be detrimental to dopamine neurons. For this purpose, we expressed human CDCrel-1 or enhanced GFP (EGFP, control) unilaterally in the nigrostriatal system of rats by stereotactic nigral delivery of rAAV vectors and studied the effects of the two proteins on the survival of dopamine neurons and on the levels of dopamine and its metabolites in the STR. Our results show that CDCrel-1 expression induces selective dopamine neurodegeneration that can be blocked by inhibition of tyrosine hydroxylase (TH) and dopamine biosynthesis. We also find that CDCrel-1 inhibits dopamine release from PC12 cells, suggesting that a block in dopamine secretion may contribute to the development of AR-JP.
Materials and Methods
Plasmid Constructions. The human CDCrel-1 ORF (GenBank accession no. U74628) was amplified by RT-PCR with primers 5′-CGACGCGTGCCACCATGAGCACAGGCCTG-3′ and 5′-CGACGCGTTCACTGGTCCTGCATCTGCTG-3′, and inserted into plasmid psubPDGF-WPRE (24) to generate psub-PDGF-CDCrel-1-WPR E. psubPDGF-EGFP-WPR E was generated by insertion of the EGFP fragment derived from pEGFP-N1 (Clontech) into psubPDGF-WPRE. The CDCrel-1 coding sequence was fused to the EGFP ORF within pEGFP-N1 (Clontech), and the fragment encoding the fusion protein was subsequently transferred to psubPDGF-WPRE.
Generation and Purification of rAAV. High-titer, helper virus-free rAAV-2 were generated with plasmid pDG (25) and purified as described (25, 26). The rAAV stocks were sterile-filtered (0.2 μm) and titrated by slot blot hybridization (56). Titers (genomes per ml) were 7.1 × 1011 (rAAV-PDGF-CDCrel-1), 8.3 × 1011 (rAAVPDGF-EGFP), and 3.6 × 1011 (rAAV-PDGF-CDCrel-1/EGFP).
Animals, Stereotactic Surgery, and α-Methyl-dl-Tyrosine (AMPT) Treatment. All animals were housed and treated according to institutional and national guidelines. Male Wistar rats (285 ± 22 g) were stereotactically injected with 2 μl of rAAV dorsal to the right SNc at two coordinates anterio-posterior (AP) -5.3 mm, medio-lateral (ML) + 1.8 mm, dorso-ventral (DV) -7.7 mm and AP -5.3 mm, ML + 2.5 mm, DV -7.3 mm, relative to bregma). In the globus pallidus (GP), 2 μl of virus solution was injected at each of the following two coordinates: AP -0.90 mm/-1.30 mm, ML + 2.8 mm/+3.2 mm, DV -6.3 mm/-6.6 mm. To inhibit TH and dopamine synthesis, animals were injected i.p. twice per day with 175 mg/kg AMPT-HCl (Fluka) dissolved in 0.9% NaCl (saline).
Tissue Preparation and Determination of Dopamine and Dopamine Metabolites. Rats were killed by decapitation at the indicated time points after rAAV injections. Brains were rapidly removed and placed on an ice-cold plate for dissection of the neostriatum. Tissues were processed, and dopamine and its metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were analyzed by using reverse-phase ion-pair chromatography combined with electrochemical detection under isocratic conditions, as described (27).
Immunohistochemistry. Coronal cryosections (20 μm thick) containing the SNc were prepared from 4% paraformaldehyde-fixed brains. Primary Abs included CDCrel-1-specific hybridoma supernatant SP18 (1:50) (16), mouse anti-rat TH (1:1,000) (Pharmingen), rabbit anti-dopamine transporter (1:100) (Chemicon), rabbit anti-glutamic acid decarboxylase (1:1,000) (Affinity, Exeter, U.K.), and mouse anti-NeuN (1:200) (Chemicon). Secondary Cy3-(1:500), FITC-(1:400), and biotin-conjugated (1:1,000) immunoglobulins were from Milan Analytica, La Roche, Switzerland. Biotin-conjugated Abs were used in combination with diaminobenzidine staining (ABC vector kit, Vector Laboratories). Expression of EGFP and the CDCrel-1/EGFP fusion protein in the substantia nigra were detected by native fluorescence microscopy (without Abs).
Western Blot Analysis. Virus-injected striatal hemispheres were dissected out and homogenized in 0.3 ml of 4% Triton X-100, 150 mM NaCl, and 10 mM Tris·HCl (pH 8.0) containing protease inhibitor mixture (Sigma), and the homogenates were clarified by centrifugation for 15 min at 1,000 × g. Total protein (75 μg) was separated by SDS/PAGE and analyzed by Western blot with the SP18 mAb against CDCrel-1 (1:400) to detect endogenous CDCrel-1, or with 1:500 diluted anti-GFP mAb (Sigma, clone GFP-20) to detect rAAV-derived CDCrel-1/EGFP, followed by incubation with 1:15,000 diluted horseradish peroxidaseconjugated goat anti-mouse IgG (Roche Diagnostics). Bands were visualized by enhanced chemiluminescence (Pierce).
Quantification of Neurons and TH Fiber Density. Diaminobenzidinestained, TH-positive neurons in SNc were counted in 20-μm-thick coronal sections containing the medial terminal nucleus of the accessory optical tract to distinguish between dopamine neurons of the SNc and the ventral tegmental area. Counting was carried out at ×40 magnification by using NEUROLUCIDA software (MicroBrightField, Colchester, VT). Quantification of NeuN-positive neurons in the GP was carried out by the same method in coronal sections containing a visible needle tract (from the virus injection). Optical densities of the TH-immunoreactive fibers in the STR were measured by using IMAGE software (National Institutes of Health). For each animal, the optical density was measured in four different striatal sections. Optical density readings were corrected by subtraction of nonspecific background density, determined in adjacent sections that were incubated without the primary TH Ab.
Generation and Characterization of Stably Transfected PC12 Populations. PC12 cells were electroporated (280 V, 960 μF) with pcDNA3 expression vectors (Invitrogen) encoding EGFP or CDCrel-1. Stably-transfected PC12 cell populations (pools of G418-resistant colonies) were selected with 0.3 mg/ml G418. CDCrel-1 expression was detected with the SP18 Ab as described above. EGFP expression was detected by fluorescence microscopy in the FITC channel.
Determination of Intracellular and Secreted Dopamine in Stably Transfected PC12 Cells. KCl-evoked dopamine release was measured as described (28, 29). To measure intracellular dopamine, the cells were homogenized by sonication in cold 0.3 M HClO4 and incubated on ice for 15 min, and the homogenates were centrifuged at 16,000 × g for 15 min at 4†C. Supernatants were used for HPLC analysis of dopamine content. Pellets (precipitated proteins) were washed once in PBS and solubilized in 0.1 ml 1% Triton X-100, 150 mM NaCl, and 10 mM Tris·HCl (pH 8.0) containing protease inhibitor mixture (Sigma) for protein determination (BCA kit, Pierce).
Results
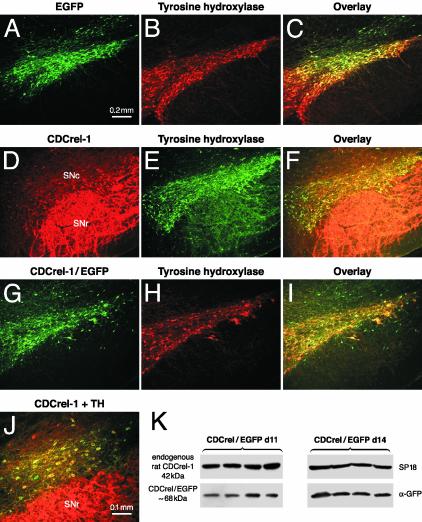
AAV-Mediated Expression of EGFP and CDCrel-1 in the Nigrostriatal System of Rats. High-titer rAAV expressing CDCrel-1, CDCrel-1/EGFP fusion protein, or EGFP (control) from the neuron-specific PDGF-β promoter were injected stereotactically dorsal to the SNc of the right hemisphere of rats. Expression of all transgenes could be detected in TH-positive neurons as early as 6 days after rAAV injection. At 10 days, the majority of dopamine neurons expressed CDCrel-1 (Fig. 1 D-F) and CDCrel-1/EGFP (Fig. 1 G-I). The presence of CDCrel-1 in cell bodies suggests that the protein was overexpressed because CDCrel-1 is not normally detected in nigral dopamine neurons but it is present at low levels in fibers of the STR (14). At 10 days, the transgene-expressing neurons appeared healthy and had normal morphology (Fig. 1J). At 15 days, and more evidently at 20 and 26 days, the number of CDCrel-1-expressing neurons and TH-positive neurons were substantially reduced in the injected hemisphere (data not shown). These results show that the rAAV vectors confer early transgene expression and suggest that a progressive loss of dopamine neurons expressing CDCrel-1 or the CDCrel-1/EGFP fusion protein began between 10 and 20 days after rAAV delivery.
Fig. 1.
AAV-mediated expression of EGFP, CDCrel-1, and CDCrel-1/EGFP in the nigrostriatal system of rats. Transgene products were detected by native (EGFP) fluorescence (A and G) or immunohistochemistry with an Ab against CDCrel-1 (D). Dopamine neurons in the same sections were identified with a mAb against rat TH (B, E, and H). Dopamine neurons expressing EGFP (C), CDCrel-1 (F), or CDCrel-1/EGFP (I) are shown. (J) A higher magnification of CDCrel-1-transduced dopamine neurons. Expression is shown at 10 days (D-J) or 42 days (A-C) after rAAV injection. No transgene expression is found in dopamine neuron bodies of the uninjected SNc (data not shown), but endogenous CDCrel-1 is strongly expressed in fibers of the SNr in both uninjected (data not shown) and injected (D and J) hemispheres. Striatal expression of rAAV-derived CDCrel-1/EGFP at 11 and 14 days (n = 4 rats/time) after transduction of the ipsilateral SNc (K). No transgene expression was found in the uninjected striatal hemispheres of the same animals (data not shown). (Bar = 0.2 mm in A-I and 0.1 mm in J.)
Because CDCrel-1 is a synaptic vesicle-associated protein, we carried out Western blots to determine whether nigral injection of the rAAV vectors resulted in protein expression in the STR, the target area of dopamine neurons. Because CDCrel-1 is expressed in the STR (14, 15), we analyzed the striatal expression of CDCrel-1/EGFP by using an Ab against EGFP to specifically detect rAAV-derived protein. The same samples were also analyzed with the SP18 Ab to reveal endogenous CDCrel-1 expression (internal standard). Striatal CDCrel-1/EGFP was detected at both 11 and 14 days after virus injection (Fig. 1K), with higher levels at 14 days, consistent with the time-dependent increase in rAAV-mediated gene expression. Thus, CDCrel-1 is expressed in striatal axons before the onset of nigral dopamine neuron loss.
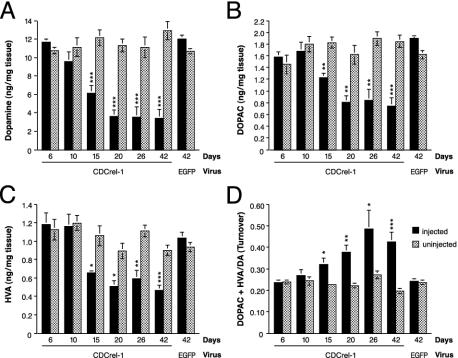
CDCrel-1 Induces Dopaminergic Neurodegeneration and a Reduction of Dopamine and Its Metabolites in the STR. To study the consequences of CDCrel-1 expression on the rat nigrostriatal system, a time course analysis (days 6-42) of the levels of striatal dopamine and its metabolites in virus-injected and uninjected hemispheres was carried out (Fig. 2). Significantly reduced levels of dopamine (Fig. 2 A), DOPAC (Fig. 2B), and HVA (Fig. 2C) were observed between days 15 and 42 after rAAV delivery. Moreover, dopamine turnover was significantly increased at all these time points (Fig. 2D). Fifteen days after injection of the rAAV-CDCrel-1 virus, the striatal levels of dopamine were reduced by 53.5% in the injected hemisphere relative to the untreated brain side. Likewise, the levels of DOPAC and HVA were reduced by 32.9% and 38.2%, and dopamine turnover was increased by a factor of 1.4 (40% increase). The levels of the various measured catecholamines were further reduced at 20 days after rAAV delivery (Fig. 2 A-C), but the relative reductions of the individual catecholamines remained similar until day 42. At this time point, the levels of dopamine, DOPAC, and HVA were reduced by 73.9%, 59.2%, and 47.8%, respectively, in the rAAV-CDCrel-1 treated hemispheres, and the dopamine turnover was increased by a factor of 2.2 (Fig. 2). Injection of a 5-fold diluted rAAV-CDCrel-1 virus stock was less toxic but still caused significant reductions in striatal catecholamines (dopamine: -46.4%; DOPAC: -34.9%; HVA: -31.0%).
Fig. 2.
CDCrel-1 induces a progressive striatal depletion of dopamine and its metabolites. Concentrations (mean ± SEM) of dopamine (A), DOPAC (B), and HVA (C) in injected (black bars) and uninjected (dotted bars) striatal hemispheres were determined between 6 and 42 days after supranigral injection of the rAAV-CDCrel-1 vector. Rats injected with the rAAV-EGFP virus were analyzed at 42 days. (D) Dopamine turnover. CDCrel-1: n = 4 rats at 6, 10, 15, 20, and 26 days, and n = 11 rats at 42 days. EGFP: n = 12 rats. Data were evaluated statistically by ANOVA and Fisher's probable least-squares difference test (***, P < 0.001; **, P < 0.01; *, P < 0.05, compared to the uninjected hemisphere).
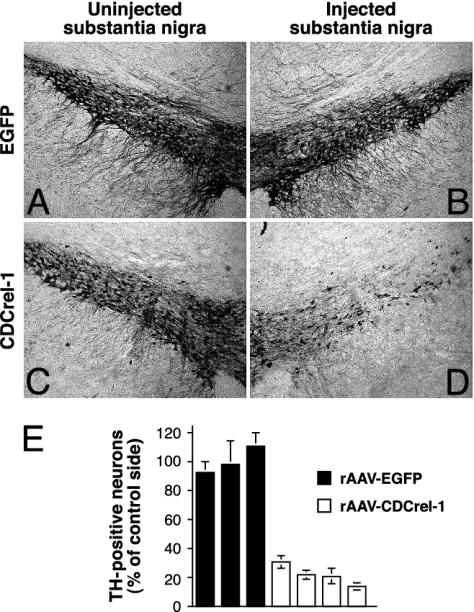
Quantitation of dopamine neurons at 42 days revealed an average loss of 78.5 ± 6.9% of the TH-positive neurons in the virus-injected hemisphere (Fig. 3 D and E), which correlated well with the decline of the dopamine levels (73.9%, Fig. 2 A). Immunohistochemistry with an Ab against the dopamine transporter, and the pan-neuronal nuclear marker NeuN yielded similar results, indicating that actual neuron loss and not simply down-regulation of TH expression had occurred (data not shown). In contrast, EGFP had no adverse effect on either dopamine neuron survival (Fig. 3 B and E) or the amounts of the analyzed catecholamines in the STR (Fig. 2), despite efficient and sustained EGFP expression in the majority of dopamine neurons (Fig. 1 A-C). These results, taken together with the expression data presented above (Fig. 1), show that an early but transient phase of CDCrel-1 expression in dopamine neurons is followed by a dramatic decline of striatal dopamine levels that is accompanied by the loss of dopamine neurons in the injected SNc.
Fig. 3.
TH-positive neurons in the substantia nigra of rats. Dopamine neurons in normal (A and C) and virus-injected (B and D) hemispheres of the SNc 42 days after injection of rAAV-EGFP (A and B) and rAAV-CDCrel-1 (C and D). (E) Survival of TH-positive neurons in rAAV-injected hemispheres, relative to uninjected control side (see Materials and Methods). Data are expressed as mean ± SD of three to four sections per rat. rAAV-EGFP: n = 3 rats. rAAVCDCrel-1: n = 4 rats.
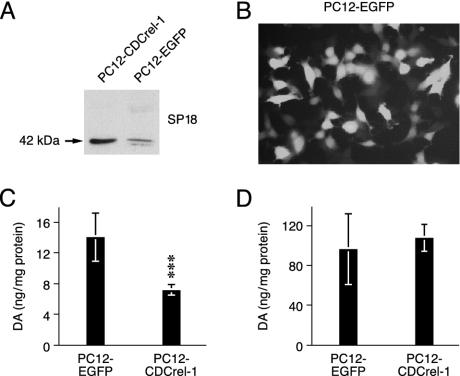
CDCrel-1 Inhibits Dopamine Release from PC12 Cells. CDCrel-1 binds to syntaxin-1 and inhibits the secretion of (transfected) growth hormone in the insulinoma cell line HIT-T15 (17). This has led to the proposal that an increased abundance of CDCrel-1 due to the impairment of parkin may inhibit dopamine release and contribute to a Parkinsonian state (13, 30). However, this hypothesis has not been addressed experimentally in cells that naturally produce and secrete dopamine. Therefore, we studied the effect of CDCrel-1 overexpression on the secretion of dopamine by rat PC12 neuroendocrine cells. These cells synthesize and store dopamine in secretory vesicles and can be depolarized to release dopamine into the medium (28, 31). We generated PC12 cell populations stably expressing CDCrel-1 or EGFP and confirmed protein expression by Western blot and fluorescence microscopy (Fig. 4 A and B). Potassium chloride (KCl)-evoked secretion of dopamine was assessed by quantitating the dopamine levels in the extracellular medium of PC12 cells by HPLC (28, 31). The extracellular medium of PC12 cells expressing CDCrel-1 contained significantly less dopamine than medium from control PC12 cells expressing EGFP (Fig. 4C). Quantitation of intracellular dopamine levels revealed that both CDCrel-1 and EGFP-expressing PC12 cells contained comparable amounts of dopamine (Fig. 4D). Therefore, the reduced extracellular dopamine levels reflect a decrease in dopamine secretion by PC12 cells expressing CDCrel-1.
Fig. 4.
CDCrel-1 inhibits dopamine secretion. Shown is expression of CDCrel-1 (A) and EGFP (B) in stably transfected PC12 cell populations (pools of G418-resistant colonies). (C) Secreted dopamine was measured after incubation of the cells in medium containing 80 mM KCl (n = 12 wells for each population) as described (28, 31). (D) Intracellular dopamine (n = 8 wells for each populations). Data are normalized to protein content and expressed as mean ± SD. ***, P < 0.001. The experiments were repeated several times with similar results.
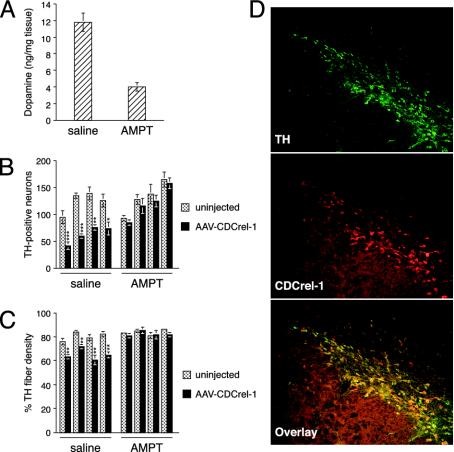
Inhibition of TH Prevents CDCrel-1-Induced Neurodegeneration. In vivo, inhibition of dopamine secretion may ultimately cause neuronal loss via dopamine-dependent toxicity (32). To address whether CDCrel-1 induced neurodegeneration depends on dopamine, we pharmacologically inhibited dopamine synthesis in rats with AMPT, a highly specific inhibitor of TH. Initial experiments showed that repeated injections of AMPT stably reduced striatal dopamine levels to ≈30% of normal amounts over the course of 4 treatment days (data not shown). Based on these findings, 16 rats were injected unilaterally with the rAAVCDCrel-1 virus above the SNc. Beginning at day 9 after virus injection, eight rats were treated with AMPT and eight rats were injected with saline as a control. At day 12, four rats of each group were killed for HPLC analysis of striatal dopamine to confirm that AMPT reduced dopamine synthesis (Fig. 5A). For the remaining four rats of each group, AMPT or saline injections were continued up to day 14. At day 15, all rats were killed to determine nigral dopamine neuron survival and striatal TH fiber density. Both neuron survival and TH fiber density were significantly reduced in the rAAV-CDCrel-1 injected hemisphere of saline-treated rats (Fig. 5 B and C). In contrast, in AMPT-treated rats TH neuron numbers and TH fiber density were comparable in virus-injected and uninjected hemispheres (Fig. 5 B and C). To rule out the possibility that AMPT interfered with the expression of CDCrel-1, nigral sections from the same animals were analyzed for expression of CDCrel-1. CDCrel-1 was indeed expressed in nigral dopamine neurons at day 15 of AMPT-treated rats (Fig. 5D). Furthermore, AMPT treatment did not result in up-regulation of TH expression, because the preservation of neuron survival was also observed by NeuN staining (data not shown). Collectively, these results show that CDCrel-1 toxicity depends on dopamine.
Fig. 5.
Inhibition of dopamine synthesis prevents CDCrel-1-induced neurodegeneration. Rats were treated with the TH inhibitor AMPT or saline (as a control) beginning 9 days after rAAV-CDCrel-1 injection. To confirm the potency of AMPT, dopamine levels were compared by HPLC in the uninjected hemispheres of saline- and AMPT-treated rats at 12 days after rAAV injection (n = 4 rats per group) (A). Shown are TH-positive neurons (B) and percentage of TH fiber density (C) at day 15 in rAAV-CDCrel-1-injected and -uninjected brain hemispheres of saline- and AMPT-treated rats (neuron counts: n = 4 rats per group, four to six sections per rat; TH fiber density: n = 4 rats per group, four sections per rat). (D) Expression of CDCrel-1 in nigral dopamine neurons of AMPT-treated rats at day 15. Data were evaluated statistically by ANOVA and Fisher's probable least-squares difference test (***, P < 0.001; **, P < 0.01; *, P < 0.05, compared to the uninjected hemisphere).
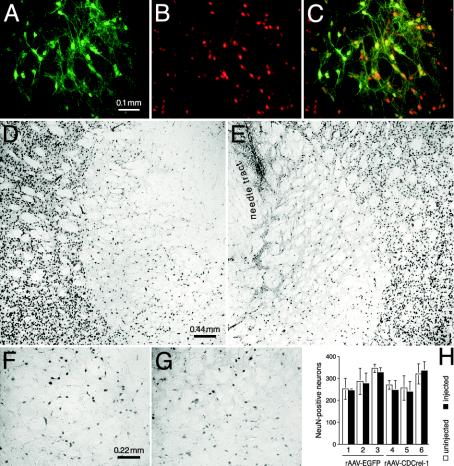
Nondopaminergic Neurons Are Unaffected by CDCrel-1 Overexpression. To further test the requirement of dopamine for CDCrel-1 toxicity, we unilaterally injected an other brain area, namely the GP, with the rAAV-EGFP, rAAV-CDCrel-1/EGFP, and rAAV-CDCrel-1 vectors (n = 3 rats per vector type). CDCrel-1/EGFP (Fig. 6A) and EGFP (not shown) were readily detected in cells of the injected GP. Colocalization with the neuronal nuclear marker NeuN (Fig. 6B) revealed that GP neurons were efficiently transduced (Fig. 6C). However, in contrast to the SNc, CDCrel-1 did not cause a loss of neurons in the GP, as revealed by immunohistochemistry for NeuN-positive cells (Fig. 6 D-G). This result was further confirmed by the quantitation of NeuN-positive neurons in injected and uninjected GP of three rats each injected with either the rAAV-CDCrel-1 or rAAV-EGFP control vector (Fig. 6H).
Fig. 6.
Neurons in the GP are unaffected by CDCrel-1 expression. Expression of CDCrel-1/EGFP (A) in NeuN-positive neurons (B) of the rAAV-injected GP 20 days after virus delivery is shown. (C) Overlay. (D-G) Normal (D and F) and rAAV-CDCrel-1-injected (E and G, needle tract indicated in E) GP, showing that the overall number of NeuN-positive neurons is comparable between normal and injected hemispheres. (H) Quantitation of NeuN-positive neurons (mean ± SD) in normal and virus-injected GP. No difference in the neuronal numbers was observed between injected and normal sides (n = 3 rats per group; three sections per rat).
In addition, in the injected SNc, the loss of TH immunoreactivity was paralleled by a loss of transgene-expressing neuronal cell bodies in rAAV-CDCrel-1- and rAAV-CDCrel-1/EGFP-injected rats, whereas neurons located dorsally (mesencephalic tegmentum) and ventrally (substantia nigra pars reticulata, SNr) to the SNc continued to express the transgene products in the same animals. Finally, although glutamic acid decarboxylase-positive neurons were also transduced with CDCrel-1, their number was comparable in rAAV-injected and uninjected hemispheres (but on both sides the number of glutamic acid decarboxylase-positive neuronal bodies was low because the Ab detects glutamic acid decarboxylase mainly in fibers) (data not shown). Taken together, these results indicate that CDCrel-1 selectively kills dopamine neurons of the SNc and support an important role of dopamine for CDCrel-1 toxicity.
Discussion
There is growing evidence that failure of the ubiquitinproteasome system is critically involved in the development of both familial and sporadic PD (33-35). Several of the AR-JP-associated mutations have been shown to abolish the ubiquitin protein ligase activity of parkin and thus the degradation of parkin target proteins. These data suggest that the accumulation of certain parkin substrates may ultimately cause dopamine neuron death and the development of AR-JP. To test this hypothesis, we have investigated the consequences of virus-mediated expression of human CDCrel-1, the first parkin target identified, on the survival of dopamine neurons in rats. We find that rAAV-mediated expression of CDCrel-1 in the rat SNc induces a massive degeneration of the dopamine neurons and a corresponding decline of dopamine and its metabolites in the STR. Importantly, CDCrel-1 toxicity depends on dopamine because pharmacological reduction of dopamine synthesis with a specific inhibitor of TH prevented CDCrel-1 induced dopamine neurodegeneration, and nondopamine neurons of the GP were spared from CDCrel-1 toxicity.
CDCrel-1 is a member of the phylogenetically conserved septin gene family (36-39) and is expressed predominantly in the brain (15, 40). In mammals, at least 10 septins have been identified to date. Several of them are associated with the sec6/8 (exocyst) complex purified from rat brain (41), suggesting a role for these proteins in the recruitment or docking of vesicles to specific sites on the plasma membrane. Indeed, it has recently been shown that septin organization is controlled by Cdc42 effector proteins termed Borgs, linking the septins with proteins that regulate cell polarity, cytoskeletal remodeling, and vesicle transport (42). How might the toxicity of CDCrel-1 for dopamine neurons come about? For neurotransmitter release, synaptic vesicles must dock to and fuse with the presynaptic membrane. These processes depend on the formation of the SNARE complex, which consists of the presynaptic membrane proteins syntaxin-1 and SNAP-25 and the synaptic vesicle proteins synaptobrevin and synaptotagmin. Because CDCrel-1 can directly bind to syntaxin-1, it may interfere with the formation of a functional SNARE complex and thus inhibit neurotransmitter release. This hypothesis is consistent with the fact that botulinum toxin C1 (which cleaves syntaxin-1) and another syntaxin-1-interacting protein, Munc-18 (43), inhibit exocytosis. Indeed, CDCrel-1 has been shown to reduce secretion in a pancreatic cell line (17), and exocytosis in platelets from CDCrel-1null mice can be triggered by subthreshold levels of collagen (44), suggesting that CDCrel-1 exerts an inhibitory effect on exocytosis. However, the relevance of these findings with respect to the role of CDCrel-1 for the development of PD has been unclear. PC12 cells have been widely used as a catecholaminergic model system, because they synthesize and store dopamine in secretory vesicles, and neurotransmitter release can be evoked by depolarization (28, 31). Our finding that CDCrel-1 decreases dopamine exocytosis from PC12 cells indicates that CDCrel-1 may be a negative regulator of dopamine neurotransmission and provides a mechanism by which CDCrel-1 may contribute to the development of PD. Chronic inhibition of dopamine release may ultimately cause dopamine-mediated neuron death (32). An important role of dopamine for CDCrel-1 toxicity is supported by our results that inhibition of dopamine synthesis by AMPT prevented dopamine neuron loss, and that GP neurons (lacking dopamine) tolerated CDCrel-1 expression in the absence of toxicity. Damage by dopamine may be further enhanced by endogenously expressed (rat) α-synuclein, as it has recently been shown that dopamine toxicity is potentiated by α-synuclein in primary midbrain dopamine neurons (45). Finally, reduced dopamine secretion may result in increased oxidative ligation of dopamine to α-synuclein and thus in the stabilization of protofibrillar (nonaggregated) α-synuclein intermediates (46), which have been suggested to be neurotoxic by their ability to permeabilize synaptic vesicles (46-49).§
Although PC12 cells expressing CDCrel-1 survived, a rapid dopamine neuron loss was observed in rAAV-CDCrel-1-injected rats. We believe that this difference is due to the efficient tropism of AAV vectors for the transduction of dopamine neurons in vivo, which leads to high-level protein expression throughout the nigrostriatal system of rats (23, 24). This notion is supported by a recent study demonstrating that rAAV-mediated targeted overexpression of α-synuclein also causes dopamine neuron loss (50), whereas several lines of α-synuclein transgenic mice failed to develop nigral TH neuron pathology (51-55).
In summary, our findings show that CDCrel-1 overexpression causes dopamine-dependent neurotoxicity and suggest that inhibition of dopamine secretion by CDCrel-1 may contribute to the development of AR-JP. However, further studies will be necessary to elucidate the detailed molecular mechanism(s) responsible for reduced dopamine secretion and to substantiate a causative pathological role of CDCrel-1 in humans affected with AR-JP.
Acknowledgments
We thank Andreas Leng for assistance with HPLC analysis and William G. Honer for the SP18 hybridoma supernatant. We are grateful to Fritz Ochsenbein for preparing the figures. This work was supported by grants from the Swiss National Science Foundation to H.B. and by the National Center for Competence in Research (NCCR) on Neural Plasticity and Repair.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PD, Parkinson's disease; AR-JP, autosomal-recessive juvenile parkinsonism; PDGF, platelet-derived growth factor; SNc, substantia nigra pars compacta; STR, striatum; rAAV, recombinant adeno-associated viruses; TH, tyrosine hydroxylase; EGFP, enhanced GFP; DOPAC, dihydroxyphenylacetic acid; HVA, homovanillic acid; GP, globus pallidus; AMPT, α-methyl-DL-tyrosine; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
Footnotes
Lashuel, H. A., Hartley, D., Petre, B. M., Walz, T. & Lansbury, P. T., Jr. (2002) Nature 418, 291 (abstr.).
References
- 1.Jenner, P. & Olanow, C. W. (1998) Ann. Neurol. 44, S72-S84. [DOI] [PubMed] [Google Scholar]
- 2.Nussbaum, R. L. & Ellis, C. E. (2003) N. Engl. J. Med. 348, 1356-1364. [DOI] [PubMed] [Google Scholar]
- 3.Dawson, T. M. & Dawson, V. L. (2003) J. Clin. Invest. 111, 145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cookson, M. R. (2003) Neuron 37, 7-10. [DOI] [PubMed] [Google Scholar]
- 5.Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L. & Riess, O. (1998) Nat. Genet. 18, 106-108. [DOI] [PubMed] [Google Scholar]
- 6.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. (1997) Science 276, 2045-2047. [DOI] [PubMed] [Google Scholar]
- 7.Abbas, N., Lucking, C. B., Ricard, S., Durr, A., Bonifati, V., De Michele, G., Bouley, S., Vaughan, J. R., Gasser, T., Marconi, R., et al. (1999) Hum. Mol. Genet. 8, 567-574. [DOI] [PubMed] [Google Scholar]
- 8.Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., Yokochi, M., Mizuno, Y. & Shimizu, N. (1998) Nature 392, 605-608. [DOI] [PubMed] [Google Scholar]
- 9.Leroy, E., Boyer, R., Auburger, G., Leube, B., Ulm, G., Mezey, E., Harta, G., Brownstein, M. J., Jonnalagada, S., Chernova, T., et al. (1998) Nature 395, 451-452. [DOI] [PubMed] [Google Scholar]
- 10.Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O., Breedveld, G. J., Krieger, E., Dekker, M. C., Squitieri, F., Ibanez, P., Joosse, M., et al. (2003) Science 299, 256-259. [DOI] [PubMed] [Google Scholar]
- 11.Le, W. D., Xu, P., Jankovic, J., Jiang, H., Appel, S. H., Smith, R. G. & Vassilatis, D. K. (2003) Nat. Genet. 33, 85-89. [DOI] [PubMed] [Google Scholar]
- 12.Shimura, H., Hattori, N., Kubo, S., Mizuno, Y., Asakawa, S., Minoshima, S., Shimizu, N., Iwai, K., Chiba, T., Tanaka, K. & Suzuki, T. (2000) Nat. Genet. 25, 302-305. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, Y., Gao, J., Chung, K. K., Huang, H., Dawson, V. L. & Dawson, T. M. (2000) Proc. Natl. Acad. Sci. USA 97, 13354-13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita, A., Noda, M. & Kinoshita, M. (2000) J. Comp. Neurol. 428, 223-239. [DOI] [PubMed] [Google Scholar]
- 15.Caltagarone, J., Rhodes, J., Honer, W. G. & Bowser, R. (1998) NeuroReport 9, 2907-2912. [DOI] [PubMed] [Google Scholar]
- 16.Honer, W. G., Hu, L. & Davies, P. (1993) Brain Res. 609, 9-20. [DOI] [PubMed] [Google Scholar]
- 17.Beites, C. L., Xie, H., Bowser, R. & Trimble, W. S. (1999) Nat. Neurosci. 2, 434-439. [DOI] [PubMed] [Google Scholar]
- 18.Imai, Y., Soda, M., Inoue, H., Hattori, N., Mizuno, Y. & Takahashi, R. (2001) Cell 105, 891-902. [DOI] [PubMed] [Google Scholar]
- 19.Shimura, H., Schlossmacher, M. G., Hattori, N., Frosch, M. P., Trockenbacher, A., Schneider, R., Mizuno, Y., Kosik, K. S. & Selkoe, D. J. (2001) Science 293, 263-269. [DOI] [PubMed] [Google Scholar]
- 20.Chung, K. K., Zhang, Y., Lim, K. L., Tanaka, Y., Huang, H., Gao, J., Ross, C. A., Dawson, V. L. & Dawson, T. M. (2001) Nat. Med. 7, 1144-1150. [DOI] [PubMed] [Google Scholar]
- 21.Ren, Y., Zhao, J. & Feng, J. (2003) J. Neurosci. 23, 3316-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feany, M. B. & Pallanck, L. J. (2003) Neuron 38, 13-16. [DOI] [PubMed] [Google Scholar]
- 23.Furler, S., Paterna, J. C., Weibel, M. & Bueler, H. (2001) Gene Ther. 8, 864-873. [DOI] [PubMed] [Google Scholar]
- 24.Paterna, J. C., Moccetti, T., Mura, A., Feldon, J. & Bueler, H. (2000) Gene Ther. 7, 1304-1311. [DOI] [PubMed] [Google Scholar]
- 25.Grimm, D., Kern, A., Rittner, K. & Kleinschmidt, J. A. (1998) Hum. Gene Ther. 9, 2745-2760. [DOI] [PubMed] [Google Scholar]
- 26.Zolotukhin, S., Byrne, B. J., Mason, E., Zolotukhin, I., Potter, M., Chesnut, K., Summerford, C., Samulski, R. J. & Muzyczka, N. (1999) Gene Ther. 6, 973-985. [DOI] [PubMed] [Google Scholar]
- 27.Dong, Z., Ferger, B., Feldon, J. & Bueler, H. (2002) J. Neurobiol. 53, 1-10. [DOI] [PubMed] [Google Scholar]
- 28.Pothos, E. N., Larsen, K. E., Krantz, D. E., Liu, Y., Haycock, J. W., Setlik, W., Gershon, M. D., Edwards, R. H. & Sulzer, D. (2000) J. Neurosci. 20, 7297-7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanis, L., Larsen, K. E., Rideout, H. J., Sulzer, D. & Greene, L. A. (2001) J. Neurosci. 21, 9549-9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giasson, B. I. & Lee, V. M. (2001) Neuron 31, 885-888. [DOI] [PubMed] [Google Scholar]
- 31.Schweitzer, E. S., Sanderson, M. J. & Wasterlain, C. G. (1995) J. Cell Sci. 108, 2619-2628. [DOI] [PubMed] [Google Scholar]
- 32.Junn, E. & Mouradian, M. M. (2001) J. Neurochem. 78, 374-383. [DOI] [PubMed] [Google Scholar]
- 33.McNaught, K. S., Belizaire, R., Isacson, O., Jenner, P. & Olanow, C. W. (2003) Exp. Neurol. 179, 38-46. [DOI] [PubMed] [Google Scholar]
- 34.McNaught, K. S. & Olanow, C. W. (2003) Ann. Neurol. 53, S73-S86. [DOI] [PubMed] [Google Scholar]
- 35.Chung, K. K., Dawson, V. L. & Dawson, T. M. (2001) Trends Neurosci. 24, S7-S14. [DOI] [PubMed] [Google Scholar]
- 36.Longtine, M. S., DeMarini, D. J., Valencik, M. L., Al-Awar, O. S., Fares, H., De Virgilio, C. & Pringle, J. R. (1996) Curr. Opin. Cell Biol. 8, 106-119. [DOI] [PubMed] [Google Scholar]
- 37.Field, C. M. & Kellogg, D. (1999) Trends Cell Biol. 9, 387-394. [DOI] [PubMed] [Google Scholar]
- 38.Trimble, W. S. (1999) J. Membr. Biol. 169, 75-81. [DOI] [PubMed] [Google Scholar]
- 39.Kartmann, B. & Roth, D. (2001) J. Cell Sci. 114, 839-844. [DOI] [PubMed] [Google Scholar]
- 40.Beites, C. L., Peng, X. R. & Trimble, W. S. (2001) Methods Enzymol. 329, 499-510. [DOI] [PubMed] [Google Scholar]
- 41.Hsu, S. C., Hazuka, C. D., Roth, R., Foletti, D. L., Heuser, J. & Scheller, R. H. (1998) Neuron 20, 1111-1122. [DOI] [PubMed] [Google Scholar]
- 42.Joberty, G., Perlungher, R. R., Sheffield, P. J., Kinoshita, M., Noda, M., Haystead, T. & Macara, I. G. (2001) Nat. Cell Biol. 3, 861-866. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, W., Efanov, A., Yang, S. N., Fried, G., Kolare, S., Brown, H., Zaitsev, S., Berggren, P. O. & Meister, B. (2000) J. Biol. Chem. 275, 41521-41527. [DOI] [PubMed] [Google Scholar]
- 44.Dent, J., Kato, K., Peng, X. R., Martinez, C., Cattaneo, M., Poujol, C., Nurden, P., Nurden, A., Trimble, W. S. & Ware, J. (2002) Proc. Natl. Acad. Sci. USA 99, 3064-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, J., Kao, S. Y., Lee, F. J., Song, W., Jin, L. W. & Yankner, B. A. (2002) Nat. Med. 8, 600-606. [DOI] [PubMed] [Google Scholar]
- 46.Conway, K. A., Rochet, J. C., Bieganski, R. M. & Lansbury, P. T., Jr. (2001) Science 294, 1346-1349. [DOI] [PubMed] [Google Scholar]
- 47.Volles, M. J., Lee, S. J., Rochet, J. C., Shtilerman, M. D., Ding, T. T., Kessler, J. C. & Lansbury, P. T., Jr. (2001) Biochemistry 40, 7812-7819. [DOI] [PubMed] [Google Scholar]
- 48.Volles, M. J. & Lansbury, P. T., Jr. (2002) Biochemistry 41, 4595-4602. [DOI] [PubMed] [Google Scholar]
- 49.Lotharius, J. & Brundin, P. (2002) Nat. Rev. Neurosci. 3, 932-942. [DOI] [PubMed] [Google Scholar]
- 50.Kirik, D., Rosenblad, C., Burger, C., Lundberg, C., Johansen, T. E., Muzyczka, N., Mandel, R. J. & Bjorklund, A. (2002) J. Neurosci. 22, 2780-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahle, P. J., Neumann, M., Ozmen, L., Muller, V., Odoy, S., Okamoto, N., Jacobsen, H., Iwatsubo, T., Trojanowski, J. Q., Takahashi, H., et al. (2001) Am. J. Pathol. 159, 2215-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masliah, E., Rockenstein, E., Veinbergs, I., Mallory, M., Hashimoto, M., Takeda, A., Sagara, Y., Sisk, A. & Mucke, L. (2000) Science 287, 1265-1269. [DOI] [PubMed] [Google Scholar]
- 53.Matsuoka, Y., Vila, M., Lincoln, S., McCormack, A., Picciano, M., LaFrancois, J., Yu, X., Dickson, D., Langston, W. J., McGowan, E., et al. (2001) Neurobiol. Dis. 8, 535-539. [DOI] [PubMed] [Google Scholar]
- 54.van der Putten, H., Wiederhold, K. H., Probst, A., Barbieri, S., Mistl, C., Danner, S., Kauffmann, S., Hofele, K., Spooren, W. P., Ruegg, M. A., et al. (2000) J. Neurosci. 20, 6021-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giasson, B. I., Duda, J. E., Quinn, S. M., Zhang, B., Trojanowski, J. Q. & Lee, V. M. (2002) Neuron 34, 521-533. [DOI] [PubMed] [Google Scholar]
- 56.Dracopoli, N. C., Haines, J. L., Korf, B. R., Moir, D. T., Morton, C. C., Seidman, C. E., Seidman, J. G. & Smith, D. R., eds. (1997) Current Protocols in Human Genetics (Wiley, New York), pp. 12.1.1-12.1.20.