Abstract
Visual deprivation such as dark rearing (DR) prolongs the critical period for ocular dominance plasticity and retards the maturation of γ-aminobutyric acid (GABA)ergic inhibition in visual cortex. The molecular signals that mediate the effects of DR on the development of visual cortex are not well defined. To test the role of brain-derived neurotrophic factor (BDNF), we examined the effects of DR in transgenic mice in which BDNF expression in visual cortex was uncoupled from visual experience and remained elevated during DR. In dark-reared transgenic mice, visual acuity, receptive field size of visual cortical neurons, critical period for ocular dominance plasticity, and intracortical inhibition were indistinguishable from those observed in light-reared mice. Therefore, BDNF overexpression is sufficient for the development of aspects of visual cortex in the absence of visual experience. These results suggest that reduced BDNF expression contributes to retarded maturation of GABAergic inhibition and delayed development of visual cortex during visual deprivation.
The mammalian primary visual cortex is largely immature at the time of eye opening (1–5). For example, visual acuity is much less than that of the adult level. Selectivity of visual cortical neurons for orientation and movement direction of visual stimuli is poor. Ocular dominance (OD) and binocular vision are rudimentary (2, 5). The gradual development of these functional properties during the subsequent postnatal period critically depends on appropriate visual experience. Visual deprivation such as dark rearing (DR) from birth delays the normal development of the visual cortex (5, 6) and can impair visual function permanently. For example, in dark-reared adult animals, visual acuity remains low and the receptive field (RF) sizes of visual cortical neurons remain large (2, 7, 8). In addition, in dark-reared animals, OD of visual cortex remains sensitive to monocular deprivation (MD) beyond the critical period defined in light-reared animals (9, 10). Therefore, DR seems to delay the normal maturation and maintains visual cortex in an immature state.
The cellular correlates of the experience-dependent development of visual cortex and the effects of DR are not well defined. Recent results suggest that the development of γ-aminobutyric acid (GABA)ergic inhibition within the cortex is an important component of critical period plasticity. For example, postnatal maturation of GABAergic inhibition in visual cortex is well correlated with the time course of the critical period of OD plasticity (11, 12). In addition, mice deficient in an isoform of the GABA synthetic enzyme GAD65 show a complete absence of OD plasticity (13). Furthermore, enhancement of GABAergic inhibition by pharmacological (14) or genetic (15, 16) manipulations accelerates the time course of the critical period. Interestingly, several studies have shown that the maturation of the GABAergic inhibitory circuits in visual cortex is retarded in dark-reared animals. For example, dark-reared visual cortex shows significantly more spontaneous activity and prolonged responses to visual stimuli, hallmarks of deficits in intracortical inhibition (17). Indeed, the number of GABA immunoreactive neurons is reduced in dark-reared visual cortex (18). Therefore, a retarded maturation of GABAergic inhibition is an important component of and may contribute to the delayed development of visual cortex under DR conditions.
The molecular signals that mediate the effects of visual experience and visual deprivation on visual cortex have been studied (19, 20). Among these, brain-derived neurotrophic factor (BDNF) is one of the most attractive candidates. The expression of BDNF in visual cortex increases after eye opening and during the critical period (21, 22). In transgenic mice in which the postnatal rise of BDNF is accelerated, there is a precocious development of visual acuity and a critical period of OD plasticity, which are correlated with an accelerated maturation of cortical GABAergic inhibition (15, 16). On the other hand, DR dramatically down-regulates the expression of BDNF mRNA (22) and reduces the phosphorylation of trkB receptors in visual cortex (23). DR also may alter BDNF trafficking and reduce the availability of BDNF to target neurons (24). Taken together, these results suggest the possibility that the down-regulation of BDNF in visual cortex by DR results in a retarded maturation of GABAergic inhibition and delayed development of visual cortex. However, DR also results in altered expression of a variety of other genes (19, 20, 25) and changes in neuronal activity (17, 26) and morphology (27). To establish a key role for BDNF in mediating the effects of DR on the development of the visual cortex, we examined whether an overexpression of BDNF in visual cortex is sufficient to rescue visual function and maturation of GABAergic inhibition from the effects of DR.
Materials and Methods
BDNF Transgenic Mice and DR. The generation and genotyping of transgenic mice has been described in detail (15). The resulting offspring were screened for the transgene by PCR. The transgene-negative littermates were taken as wild-type controls. All experiments were performed blind to genotypes. Dark-reared mice were kept in ventilated, completely light-tight shells from postnatal day (P) 5 to P40–P45. Light-reared control mice were reared from birth with normal visual experience (12-h light/12-h dark cycle). For MD experiments, dark-reared and light-reared mice eyelid margins were trimmed and sutured with 7-0 silk under anesthesia [avertin (tribromoethanol in amylene hydrate) 20 μl/g, i.p.]. Mice were allowed to recover from anesthesia and returned to their cages in a normal light/dark cycle for ≈4–5 days.
In Situ Hybridization. Mice were killed by cervical dislocation, and the brains were dissected and rapidly frozen in mounting medium. Cryostat sections (20-μm) were taken, postfixed for 10 min in 4% paraformaldehyde in PBS (pH 7.3), dehydrated, and stored frozen at -70°C until use. The slices were hybridized to a BDNF oligonucleotide probe (5′-CAGTTGGCCTTTTGATACCGGGACTTTCTCCAGGACTGTGACCGTCCC-3′) that detects both the endogenous and the transgenic BDNF mRNA. The probes were labeled by 3′-poly(A) tailing by using [α-35S]thio-dATP and terminal transferase to a specific activity of 0.5 × 108 to 1 × 108 cpm/μg. Hybridization was performed overnight at 42°C in a solution containing 50% formamide, 10% dextran sulfate, 25 mM Hepes (pH 7.0), 600 mM NaCl, 100 mM DTT, 1× Denhardt's solution, 200 μg/ml denatured salmon sperm DNA, 200 μg/ml poly(dA), and 107 cpm/ml oligonucleotide probe. Slides were washed two times for 10 min each in 2× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) at room temperature and two times for 60 min each in 0.2× SSC at 65°C, dried, and exposed to film for 2 weeks.
In Vivo Recordings. Single-cell activity and visual evoked potentials (VEPs) were recorded from the binocular portion of the mouse visual cortex. Mice were anesthetized in urethane (0.4 ml/hg, 20% solution in saline, i.p.; Sigma) and placed in a stereotaxic frame. Body temperature was monitored continuously and maintained at 37°C during the experiments. ECGs and electroencephalograms were also monitored continuously. A large portion of the skull (4 × 4 mm) overlying the visual cortex was carefully drilled and removed, leaving the dura intact. After exposure of the brain surface, a micropipette (2 MΩ) filled with NaCl (3 M) was inserted into the cortex perpendicularly to the stereotaxic plane 3.2–3.5 mm lateral to lambda. In the same penetration either single-cell or VEP recordings were performed. The position of the RFs of single units were mapped by using a hand-held stimulator. Only cells with a RF within 20° of the vertical meridian were included in our sample.
Single-Unit Recordings. Cell responsiveness and RF size were determined from peristimulus time histograms recorded in response to a computer-generated bar of optimal velocity drifting on a display (28 × 22 cm, 15 cd/m2; Daewoo, Seoul, South Korea), averaged over at least 10 stimulus presentations. The stimulus bar (contrast, 90%; thickness, 3°; speed, 28°/sec) was presented in either the vertical or horizontal orientation to determine orientation selectivity. OD was evaluated according to the classification of Hubel and Wiesel (28): neurons in classes 1 and 7 are monocular, exclusively driven by the stimulation of the contralateral or ipsilateral eye, respectively; neurons in class 4 are binocular, equally driven by both eyes; and neurons in classes 2, 3, 5, and 6 are binocular and preferentially driven by the contralateral and ipsilateral eye, respectively. For each mouse, the bias of the OD distribution (ODD) toward the contralateral eye, the CBI (contralateral bias index), was calculated according to the formula CBI = {[N(1) - N(7)] + 1/2[N(2/3) - N(5/6)] + Ntot}/2Ntot, where N(i) is the number of cells in class i and Ntot is the total number of recorded cells. The binocularity was evaluated by calculating the binocular index (IB) with the formula IB = [1/2N(2/3) + N(4) + 1/2N(5/6)]/Ntot. To determine RF size we assumed as visual response the signal above a value equal to mean spontaneous discharge +2 SD (2).
VEPs. VEP recording in mice was performed as described by Porciatti et al. (29). Microelectrodes were positioned 400 μm within the cortex where VEPs have their maximal amplitude. Transient VEPs were recorded in response to abrupt reversal of horizontal square wave gratings computer-generated on a display (28 × 22, 15 cd/m2; Daewoo), which was positioned in front of the mouse's eyes to include the binocular visual field. The signal was amplified, bandpass-filtered (0.3–100 Hz), and fed to a computer for analysis as described (29). At least 128 events were averaged in synchrony with the stimulus contrast reversal. Transient VEPs were evaluated in the time domain by measuring the peak latency of the major negative component. The spatial resolution of the mouse, namely visual acuity, was determined by extrapolation to 0 V of the linear regression through the four to six points closest to noise level in a curve where VEP amplitude is plotted against log spatial frequency (29). Different spatial frequencies were tested for each experimental animal because we took care to increase the sampling around threshold. Depending on the number of sampling points, we performed linear regression to 0 V of the four to six amplitudes immediately above noise level.
Electrophysiology in Visual Cortical Slices. Brain slices from visual cortex were prepared as described (30). The slices were maintained in an interface storage chamber containing artificial cerebrospinal fluid at 30°C for at least 1 h before recording. The artificial cerebrospinal fluid was saturated with 95% O2/5% CO2 and contained 124 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, 26 mM NaHCO3, and 10 mM dextrose. Voltage-clamp measurements were performed on submerged slices and continuously perfused at a rate of 2 ml/min with 30°C ASCF saturated with 95% O2/5% CO2. Stimulation was applied at a site in the middle of the cortical thickness, confirmed histologically to correspond to layer IV and upper layer V. Whole-cell patch-clamp recordings were made from layer II/III pyramidal neurons visualized by IR differential interference contrast microscopy videomicroscopy (Axioskop, Zeiss) with a Warner PC-505A amplifier (Warner Instruments, Hamden, CT). Patch electrodes (2–6 MΩ) were filled with internal solution containing 130 mM Cs-gluconate, 2 mM MgCl2, 2 mM CaCl2, 10 mM EGTA, 10 mM Hepes, 2 mM Na-ATP, 10 mM QX-314; pH was adjusted to 7.25 with 275–285 milliosmoles CsOH. For analysis we considered only those cases in which the initial access resistance was <15 MΩ and the input resistance was >250 MΩ. Data were discarded if during the experiment the access resistance increased to a value >20 MΩ or if the input resistance of the cell decreased to <100 MΩ. These experiments were done in double-blind fashion: genotyping of the mice was performed after the physiological recordings were finished and the data were analyzed. Data are expressed as mean ± SE.
Results
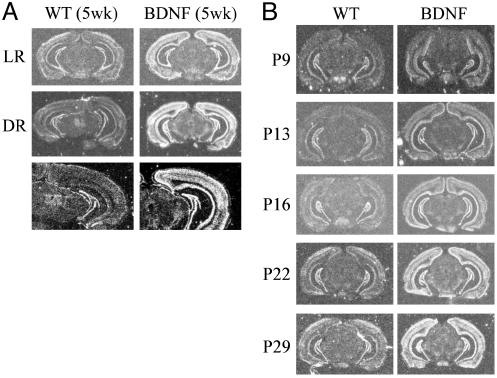
BDNF Overexpression in Visual Cortex Persists in Dark-Reared Transgenic Mice. We previously generated transgenic mice overexpressing BDNF by using the promoter of α-calcium/calmodulin-dependent protein kinase II (15). In the heterozygous A9 transgenic line, the postnatal rise of BDNF mRNA in cerebral cortex is accelerated such that by 3 weeks of age, BDNF mRNA levels in neocortex of A9 mice were already significantly higher than those in neocortex of 5-week-old wild types. At 5 weeks of age, total BDNF mRNA levels were ≈3-fold higher in the neocortex of A9 mice compared with those in wild-type litter-mates (15). To examine whether the levels of BDNF mRNA in visual cortex of A9 mice were down-regulated by DR, we compared BDNF expression in light- and dark-reared wild-type (LRWT and DRWT) and transgenic littermates by using in situ hybridization. Although DR from birth to 5 weeks of age dramatically reduced BDNF levels in visual cortex of wild-type mice, it had almost no effect on BDNF levels in identically treated transgenic mice (Fig. 1A). Therefore, BDNF overexpression in visual cortex of A9 mice was uncoupled from visual deprivation. In addition, we also studied the developmental expression of BDNF in visual cortex. Previous estimation of BDNF overexpression in A9 mice was based on Northern blotting by using tissues from visual and adjacent cortical areas. Here we confirmed, using in situ hybridization in visual cortex, that BDNF overexpression in A9 mice started as early as P9 (Fig. 1B). By P22, BDNF expression in visual cortex of A9 mice was higher than that in 5-week-old wild-type mice, and BDNF overexpression persisted to adult ages (Fig. 1B).
Fig. 1.
(A) BDNF expression in visual cortex is down-regulated in wild-type but not in BDNF transgenic mice during DR. Coronal brain sections from 5-week-old wild-type (WT) and BDNF transgenic mice under light-rearing (LR) or DR conditions were hybridized with a BDNF oligonucleotide probe that detects both the endogenous and transgenic BDNF mRNA. (Bottom) Higher magnifications of DR cases. (B) Postnatal expression of BDNF in visual cortex of wild-type and BDNF transgenic mice.
Normal Development of Visual Acuity and RF Sizes in Dark-Reared (DR) BDNF Transgenic Mice. It has been shown in rodents that, at the time of eye opening in the second postnatal week, visual acuity is less than half of that of the adult (2, 15). In addition, RFs of single units in primary visual cortex are much larger than those at adult ages. In subsequent weeks, visual acuity increases while RF size decreases, both parameters reaching adult levels by 4 weeks of age (2). DR prevents the normal increase of visual acuity and the decrease of RF size of visual cortical neurons in rats (2).
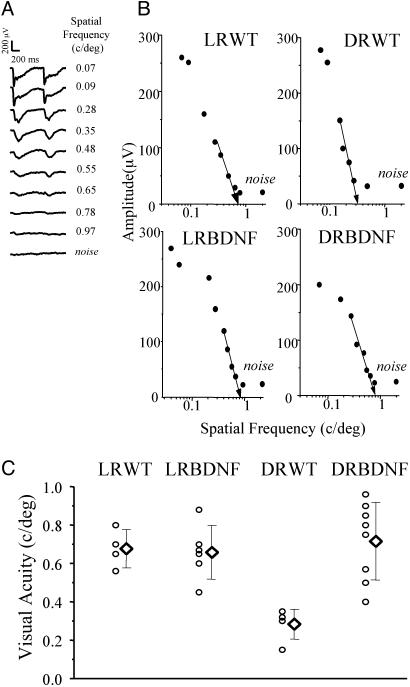
To examine the effects of DR on the development of visual acuity in our BDNF transgenic mice, we compared visual acuity in DRWT, LRWT, and BDNF transgenic littermates. Visual acuity in adult LRWT and transgenic mice are similar (15). Here we found that visual acuity in DRWT mice dark-reared from birth to 5 weeks of age was reduced to less than half with respect to the light-reared controls (LRWT mice; Fig. 2). Mean visual acuity for DRWT mice is 0.28 cycles per degree (SD = 0.08, five mice) and for LRWT mice is 0.68 cycles per degree (SD = 0.10, four mice; one-way ANOVA, P < 0.001; post hoc Tukey's test, P < 0.05). In dark-reared transgenic mice (DRBDNF mice), however, visual acuity was at the same level as that of light-reared controls (LRBDNF mice). Mean visual acuity for DRBDNF mice is 0.72 cycles per degree (SD = 0.20, eight mice) and for LRBDNF mice is 0.66 cycles per degree (SD = 0.14, six mice; post-ANOVA Tukey's test, P > 0.05). Therefore, visual acuity reached normal adult values in BDNF transgenic mice independent of visual experience.
Fig. 2.
Effects of DR on visual acuity of wild-type and BDNF transgenic mice. (A) Representative examples of VEPs in response to alternating gratings of different spatial frequencies in a wild-type mouse. VEP amplitudes decrease with increasing spatial frequency of stimulus. At spatial frequency of 0.65 cycles per degree, VEP amplitude is barely distinguishable from response to a blank field (noise). (B) Representative examples of VEP-extrapolated visual acuity for LRWT, light-reared (LR) BDNF, DRWT, and DRBDNF mice. Visual acuity is calculated by linear extrapolation (semilog coordinates) to 0 V of the set of data points close to the noise level. Maximal VEP amplitude was not significantly different among the mouse groups (one-way ANOVA, P = 0.13). (C) Mean visual acuity (diamonds) in DRWT mice (0.28 cycles per degree, SD = 0.08, five mice) is significantly reduced in comparison with that in LRWT mice (0.68 cycles per degree, SD = 0.10, four mice). On the other hand, mean visual acuity in DRBDNF mice (0.72 cycles per degree, SD = 0.20, eight mice) is not different from that in LRBDNF (0.66 cycles per degree, SD = 0.14, six mice) and LRWT mice. Error bars indicate SD.
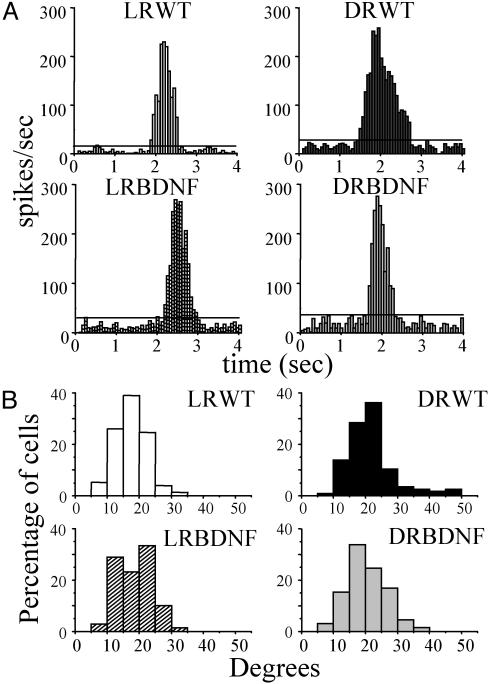
We also compared RF sizes in 5-week-old DRWT, LRWT, and BDNF transgenic littermates. Examples of RF size measured by peristimulus time histograms are shown in Fig. 3A. The distribution of RF size in DRWT mice was shifted toward larger values (Fig. 3B), with a median (20.29°) significantly larger than that obtained in LRWT mice (16.80°; Kruskal–Wallis ANOVA, P < 0.05; post hoc Dunn's test, P < 0.05). On the other hand, the RF size distribution in DRBDNF mice was not affected by DR. Median RF size in DRBDNF mice was identical to that obtained in LRBDNF mice (18.50° and 18.44°, respectively; Kruskal–Wallis ANOVA, P < 0.05; post hoc Dunn's test, P > 0.05). Therefore, the RF size of visual cortical neurons also reached normal adult values in DRBDNF transgenic mice.
Fig. 3.
Effects of DR on RF size in wild-type and BDNF transgenic mice. (A) Examples of peristimulus time histograms for cells recorded in primary visual cortex of LRWT, DRWT, and BDNF transgenic mice. Vertical stimulus bar, contrast 90%; velocity 28°/sec. Calculated RF sizes are: LRWT, 16.5°; DRWT, 32.5°; LRBDNF, 18.3°; DRBDNF, 14.75°. (B) RF size distribution for each group of mice. RFs were divided into 10 classes of 5° each, from 0° to 50°, and plotted as percentage of cells. Median RF size in DRWT (20.29°, 116 cells, eight mice) is significantly larger than that obtained in LRWT (16.80°, 77 cells, five mice; Kruskal–Wallis ANOVA, P < 0.05; post hoc Dunn's test, P < 0.05). On the other hand, median RF size in DRBDNF (18.50°, 65 cells, seven mice) is identical to that obtained in LRBDNF mice (18.44°, 69 cells, five mice).
It is also known that the dark-reared visual cortex shows significantly more spontaneous activity than the normal adult cortex (17). Although there were no differences among groups in the cell-peak responses (ANOVA, P > 0.05), we found that spontaneous discharge was increased in DRWT mice (median, 11.3 spikes per sec; interquartile ranges, 5–16.6) with respect to light-reared controls (LRWT: median, 5.9 spikes per sec; inter-quartile ranges, 3.1–10.5; Kruskal–Wallis ANOVA, P < 0.05; post hoc Dunn's test, P < 0.05). However, no differences were observed between DRBDNF (median, 6.8 spikes per sec; inter-quartile ranges, 3.1–8.3) and LRBDNF (median, 6.2 spikes per sec; interquartile ranges, 2.8–11; Kruskal–Wallis ANOVA, P < 0.05; post hoc Dunn's test, P > 0.05) mice. Thus, DR substantially increased the spontaneous discharge of cortical neurons in wild-type mice but not in BDNF transgenics.
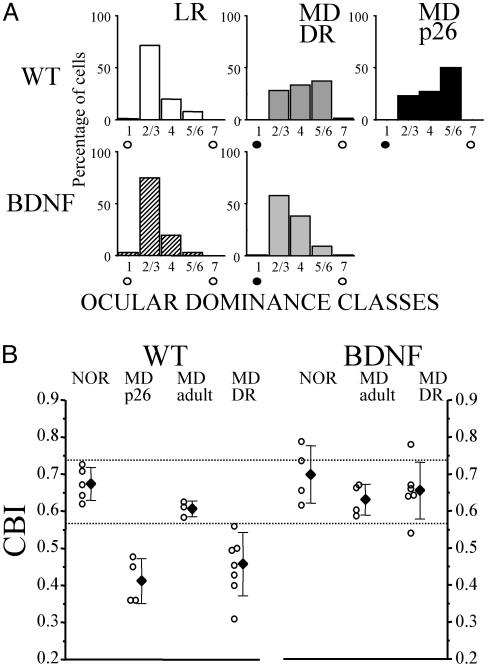
Normal Development of the Critical Period for OD Plasticity in DRBDNF Transgenic Mice. The critical period for OD plasticity in response to MD in mice and rats has been measured by using single-unit recording and VEPs. Sensitivity of OD to MD begins at ≈3 weeks of age, peaks at 4 weeks of age, and quickly diminishes after 5 weeks of age (15, 31–33). In higher mammals, DR renders the OD of visual cortex sensitive to MD even at adult ages and therefore prolongs the critical period (6, 9). Similarly, here we found that in wild-type mice, DR also prolonged the critical period for OD plasticity. A brief period (4 days) of MD in LRWT mice induced significant OD shift when performed at P26 but not at P40. In DRWT mice, however, 4-day MD at P40 still induced significant OD shift (Fig. 4A). CBI (Fig. 4B) in P40 DRWT mice was reduced greatly as compared with P40 LRWT mice (Fig. 4B; one-way ANOVA, post hoc Tukey's test, P < 0.01) and was similar to that in P26 LRWT mice (P > 0.05). In LRBDNF transgenic mice, ODD is similar to that in wild-type mice. The critical period for OD plasticity in BDNF mice begins and ends earlier than that in wild-type mice (15, 16). Here we found that DR did not delay the progression of critical period in BDNF transgenic mice. In contrast to DRWT mice, 4-day MD at P40 did not induce OD shift in DRBDNF mice. Indeed, CBI in DRBDNF mice was the same as in P40 LRBDNF and P40 LRWT mice (one-way ANOVA; post hoc Tukey's test, P > 0.05 for both comparisons).
Fig. 4.
Effects of DR on OD plasticity of wild-type (WT) and transgenic mice. DR prolongs the critical period of MD in wild-type but not in BDNF mice. (A) ODD was measured by dividing single units into five classes following the classification of Hubel and Wiesel (28). (Upper) In adult (P40) LRWT mice, ODD was strongly biased toward the contralateral eye (91 cells, five mice). MD during the peak of critical period (at P26, MDp26, 74 cells, four mice) but not in adult (88 cells, three mice) shifted ODD toward the open/ipsilateral eye. In DRWT mice, MD at adult age (P40) still shifted ODD toward the open/ipsilateral eye (MDDRWT, 153 cells, seven mice), indicating a delayed critical period by DR. (Lower) In adult (P40) LRBDNF mice, as in LRWT, ODD is also strongly biased toward the contralateral eye (67 cells, five mice). MD at adult age did not induce OD plasticity in LRBDNF mice (77 cells, four mice). In DRBDNF mice, however, MD at adult age (P40) still did not induce OD shift toward the open/ipsilateral eye (MDDRBDNF, 153 cells, seven mice), indicating normal closure of the critical period. (B) Results in A are quantified by calculating the CBI (see Materials and Methods). In LRWT mice, binocular visual cortex is dominated by contralateral eye (meanCBI = 0.67, SD = 0.04); MD at P26 induced OD shift (meanCBI = 0.41, SD = 0.06); MD at adult age had no effect on ODD (meanCBI = 0.61, SD = 0.03). In DRWT, MD even at adult age induced OD shift (meanCBI = 0.45, SD = 0.08). In LRBDNF mice, contralateral eye input is also dominant (meanCBI = 0.67, SD = 0.04); MD at adult age had no effect on ODD (meanCBI = 0.63, SD = 0.04). In DRBDNF mice, however, MD at adult age remains ineffective in inducing OD shift (meanCBI = 0.66, SD = 0.02).
It has been reported that the number of binocular cells is increased in dark-reared animals (2, 9). In our experiments, DR does not affect the binocularity in mice: no significant differences could be found between DRWT and LRWT mice and transgenic mice. The binocular indexes (IB) (see Materials and Methods) were 0.59 (SD = 0.09, five mice) for LRWT mice, 0.52 (SD = 0.04, nine mice) for DRWT mice, 0.50 (SD = 0.09, four mice) for LRBDNF mice, and 0.54 (SD = 0.15, five mice) for DRBDNF mice. Values in the four groups did not differ significantly from each other (one-way ANOVA, P > 0.05).
Normal Development of Intracortical Inhibition in DRBDNF Transgenic Mice. The postnatal maturation of GABAergic inhibition in visual cortex proceeds slowly and gradually in comparison with excitation (12, 34). For example, GABAergic synapses in rodent visual cortex do not appear in large number until the third postnatal week and continue to increase until at least the fifth week (35, 36). The expression level and synaptic localization of the GABA synthetic enzymes increase and mature significantly during the critical period (11, 15). Physiological studies indicate that GABAergic inhibitory responses can be evoked reliably only after the third week (12, 34) and reach adult level after the fifth week (37). In dark-reared animals, the number of GABA immunoreactive neurons is significantly reduced in visual cortex (18).
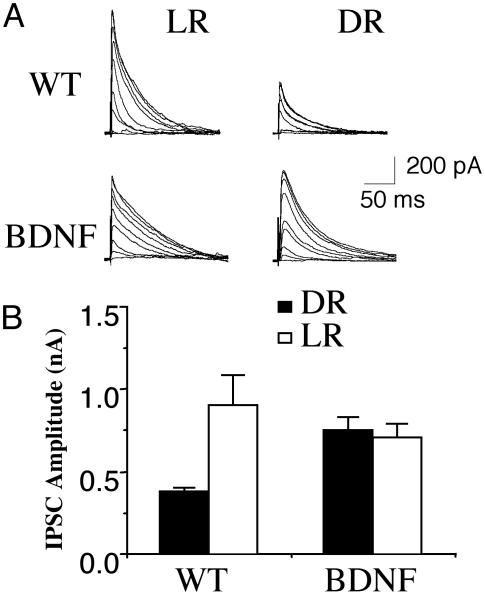
To characterize further the functional effects of DR on GABAergic inhibition, we determined the amplitude of the maximal inhibitory postsynaptic currents (IPSCs) in layer III pyramidal neurons evoked by layer IV stimulation. Maximal IPSC has been used to quantify GABAergic inputs converging onto pyramidal cells (15, 37, 38). Monosynaptic IPSCs were recorded at 0 mV in the presence of 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione and 100 μM 2-amino-5-phosphonovaleric acid to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-d-aspartate receptors, respectively. The stimulus intensity to recruit a saturating IPSC was similar in slices from LRWT (mean ± SE, 74 ± 11 μA, n = 5) and DRWT (80 ± 11 μA, n = 11) mice (Fig. 5). However, the amplitude of this maximal IPSC was significantly smaller in DRWT (373 ± 29 pA, n = 11) than that in LRWT (900 ± 186 pA, n = 6; P < 0.01) mice (Fig. 5). In contrast, there was no difference (P = 0.27) in the maximal IPSC amplitudes in DRBDNF (773 ± 38 pA, n = 10) and LRBDNF (721 ± 71 pA, n = 6) transgenic mice (Fig. 5). These results suggest that BDNF overexpression allows the normal maturation of GABAergic inhibition in the visual cortex under DR conditions. They also confirm that the maturation of GABAergic inhibition correlates with the development of visual acuity and critical period.
Fig. 5.
Normal development of intracortical inhibition in DRBDNF transgenic mice. (A) IPSCs in layer 2/3 pyramidal neurons evoked by a layer 4 stimulus series with increasing intensities of 5, 7.5, 10, 15, 20, 30, 40, 80, and 160 μA. These responses were recorded from visual cortical slices of age-matched wild-type (WT) (Upper) and BDNF transgenic (Lower) mice reared either normally (Left, LR) or in the dark (Right, DR). (B) Average amplitude of the maximal IPSCs recorded in layer 2/3 pyramidal neurons from visual cortical slices of LRWT, DRWT, and BDNF transgenic mice. Error bars indicate SE.
Discussion
It has been reported that exogenous administration of nerve growth factor in the lateral ventricles could counteract some effects of DR (39). Here we show that overexpression of BDNF in transgenic mice essentially rescues the effects of DR on the development of several physiological properties of the visual cortex and on the critical period for MD. To our knowledge there have been no previous genetic manipulations that prevented the effects of DR on the development of visual cortex.
Different paradigms of visual deprivation perturb defined aspects of the development of the visual system. DR results in profound deficits in the development of visual acuity and RF properties of visual cortical neurons, and a prolongation of critical period for OD plasticity (2, 7–10). It is possible, in this regard, that the effects of DR are mediated by multiple and parallel molecular and cellular mechanisms. However, here we show that the overexpression of a single gene is sufficient to rescue the effects of DR on the development of visual acuity, RF properties of visual cortical neurons, and critical period for OD plasticity. Our results therefore suggest that BDNF is an essential molecular signal that mediates the effects of DR on the development of the visual cortex. It follows that a mechanism by which normal visual experience promotes the development of visual cortex might be to stimulate the production of sufficient levels of BDNF in visual cortex.
Visual experience-regulated BDNF expression may have both a permissive and an instructive role in the development of visual cortex. In the instructive-role scenario, specific patterns of visual input may stimulate BDNF production in specific ensembles of visual cortical neurons and direct the development of circuits that are best suited to detect the corresponding patterns of visual input. In the permissive-role scenario, on the other hand, the overall level of visual input drives the production of certain levels of BDNF throughout the visual cortex. BDNF then promotes the development of basic cortical circuits (e.g., GABAergic circuits) necessary for the emergence of elementary RF properties and critical period plasticity. Our results, showing that a general overexpression of BDNF throughout the visual cortex allows normal development of physiological properties in dark-reared transgenic mice, are more consistent with a permissive role of BDNF and visual experience. Our results by themselves do not address whether BDNF also plays an instructive role in the development of visual cortex (for a review see ref. 40).
DR has been reported to increase rapid habituation of cortical neurons after repeated stimuli. Because habituation was also often observed in light-reared mice, we did not consider this parameter to be reliable for comparing DRWT and DRBDNF mice. We also did not analyze orientation selectivity of visual cortical neurons in DRWT and DRBDNF mice because orientation tuning is not well developed in mice (≈40%; ref. 13) and, when present, is exceedingly broad (32, 41).
Recent studies suggest that the development of GABAergic inhibition within the cortex is an important component of the development of the visual cortex and critical period plasticity (14, 15). Several lines of evidence show that BDNF is a potent neurotrophic factor that promotes the maturation of cortical GABAergic inhibitory circuits under normal rearing conditions (15, 42). In dark-reared animals, a down-regulation of BDNF expression in visual cortex (22) is correlated with a retarded maturation of the GABAergic inhibitory circuits (18, 43). However, it is not known whether the down-regulation of BDNF by DR results in retarded maturation of the GABAergic inhibitory circuits. Our data showed that overexpression of BDNF is sufficient to allow the normal maturation of GABAergic inhibition in visual cortex during DR. These results suggest a causal relationship between DR, down-regulation of BDNF, and the delayed maturation of visual cortical GABAergic inhibition.
Taken together, a likely explanation of our results is that BDNF overexpression prevents the retardation or impairment of the development of inhibitory processes caused by DR, therefore allowing a normal development of the basic physiological properties of the visual cortex in the absence of visual experience. DR is also known to retard the maturation of excitatory processes, such as the maturation of N-methyl-d-aspartate (NMDA) responses and the switch of expression from NR2B to NR2A subunits of NMDA receptors (44). The possible effect of BDNF overexpression on the development of cortical excitatory circuitry remains to be investigated.
Acknowledgments
We thank E. Putignano, C. Orsini, G. C. Cappagli, and G. Kuhn for technical assistance, and Drs. M. Caleo, E. Ruthazer, and G. di Cristo for comments on the manuscript. This work was supported by Ministero dell'Istruzione dell'Università e della Ricerca Cofinanziamento and Targeted Project in Biotechnology Grant SP5 (to L.M.), grants from the Eppley Foundation and the Whitehall Foundation (to Z.J.H.), National Institutes of Health Grant MH58880 (to S.T.), National Eye Institute Grant R01 EY12124 01 (to A.K.), and a grant from the Sloane Foundation (to A.K.).
Abbreviations: OD, ocular dominance; ODD, OD distribution; DR, dark rearing; RF, receptive field; MD, monocular deprivation; GABA, γ-aminobutyric acid; BDNF, brain-derived neurotrophic factor; Pn, postnatal day n; VEP, visual evoked potential; CBI, contralateral bias index; DRBDNF, dark-reared BDNF; LRBDNF, light-reared BDNF; IPSC, inhibitory postsynaptic current.
References
- 1.Boothe, R. G., Dobson, V. & Teller, D. Y. (1985) Annu. Rev. Neurosci. 8, 495-545. [DOI] [PubMed] [Google Scholar]
- 2.Fagiolini, M., Pizzorusso, T., Berardi, N., Domenici, L. & Maffei, L. (1994) Vision Res. 34, 709-720. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore, C. & van Sluyters, R. C. (1975) J. Physiol. (London) 248, 663-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fregnac, Y. & Imbert, M. (1978) J. Physiol. (London) 278, 373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman, S. M. & Spear, P. D. (1982) Physiol. Rev. 62, 738-855. [DOI] [PubMed] [Google Scholar]
- 6.Timney, B. (1987) In Imprinting and Cortical Plasticity, eds. Rauscheker, J. P. & Marler, P. (Wiley, New York), pp. 3321-3345.
- 7.Regal, D. M., Boothe, R., Teller, D. Y. & Sackett, G. P. (1976) Vision Res. 16, 523-530. [DOI] [PubMed] [Google Scholar]
- 8.Teller, D. Y., Regal, D. M., Videen, T. O. & Pulos, E. (1978) Vision Res. 18, 561-566. [DOI] [PubMed] [Google Scholar]
- 9.Mower, G. D. (1991) Dev. Brain Res. 58, 151-158. [DOI] [PubMed] [Google Scholar]
- 10.Cynader, M. & Mitchell, D. E. (1980) J. Neurophysiol. 43, 1026-1039. [DOI] [PubMed] [Google Scholar]
- 11.Guo, Y., Kaplan, I. V., Cooper, N. G. & Mower, G. D. (1997) Dev. Brain Res. 103, 127-141. [DOI] [PubMed] [Google Scholar]
- 12.Luhmann, H. J. & Prince, D. A. (1991) J. Neurophysiol. 65, 247-263. [DOI] [PubMed] [Google Scholar]
- 13.Hensch, T. K., Fagiolini, M., Mataga, N., Stryker, M. P., Baekkeskov, S. & Kash, S. F. (1998) Science 282, 1504-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagiolini, M. & Hensch, T. K. (2000) Nature 404, 183-186. [DOI] [PubMed] [Google Scholar]
- 15.Huang, Z. J., Kirkwood, A., Pizzorusso, T., Porciatti, V., Morales, B., Bear, M. F., Maffei, L. & Tonegawa, S. (1999) Cell 98, 739-755. [DOI] [PubMed] [Google Scholar]
- 16.Hanover, J. L., Huang, Z. J., Tonegawa, S. & Stryker, M. P. (1999) J. Neurosci. 19, RC40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benevento, L. A., Bakkum, B. W., Port, J. D. & Cohen, R. S. (1992) Brain Res. 572, 198-207. [DOI] [PubMed] [Google Scholar]
- 18.Benevento, L. A., Bakkum, B. W. & Cohen, R. S. (1995) Brain Res. 689, 172-182. [DOI] [PubMed] [Google Scholar]
- 19.Kaminska, B., Kaczmarek, L., Zangenehpour, S. & Chaudhuri, A. (1999) Mol. Cell. Neurosci. 13, 405-414. [DOI] [PubMed] [Google Scholar]
- 20.Yamada, Y., Hada, Y., Imamura, K., Mataga, N., Watanabe, Y. & Yamamoto, M. (1999) Neuroscience 92, 473-484. [DOI] [PubMed] [Google Scholar]
- 21.Bozzi, Y., Pizzorusso, T., Cremisi, F., Rossi, F. M., Barsacchi, G. & Maffei, L. (1995) Neuroscience 69, 1133-1144. [DOI] [PubMed] [Google Scholar]
- 22.Castren, E., Zafra, F., Thoenen, H. & Lindholm, D. (1992) Proc. Natl. Acad. Sci. USA 89, 9444-9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viegi, A., Cotrufo, T., Berardi, N., Mascia, L. & Maffei, L. (2002) Eur. J. Neurosci. 16, 1925-1930. [DOI] [PubMed] [Google Scholar]
- 24.Pollock, G. S., Vernon, E., Forbes, M. E., Yan, Q., Ma, Y. T., Hsieh, T., Robichon, R., Frost, D. O. & Johnson, J. E. (2001) J. Neurosci. 21, 3923-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedivi, E. (1999) J. Neurobiol. 41, 135-147. [PMC free article] [PubMed] [Google Scholar]
- 26.Leventhal, A. G. & Hirsch, H. V. (1980) J. Neurophysiol. 43, 1111-1132. [DOI] [PubMed] [Google Scholar]
- 27.Borges, S. & Berry, M. (1978) J. Comp. Neurol. 180, 277-300. [DOI] [PubMed] [Google Scholar]
- 28.Hubel, D. H. & Wiesel, T. N. (1962) J. Physiol. (London) 160, 106-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porciatti, V., Pizzorusso, T. & Maffei, L. (1999) Vision Res. 39, 3071-3081. [DOI] [PubMed] [Google Scholar]
- 30.Kirkwood, A., Silva, A. & Bear, M. F. (1997) Proc. Natl. Acad. Sci. USA 94, 3380-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drager, U. C. (1978) J. Neurophysiol. 41, 28-42. [DOI] [PubMed] [Google Scholar]
- 32.Gordon, J. A. & Stryker, M. P. (1996) J. Neurosci. 16, 3274-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maffei, L., Berardi, N., Domenici, L., Parisi, V. & Pizzorusso, T. (1992) J. Neurosci. 12, 4651-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komatsu, Y. (1983) Dev. Brain Res. 8, 136-139. [Google Scholar]
- 35.Miller, M. W. (1986) Brain Res. 390, 271-285. [DOI] [PubMed] [Google Scholar]
- 36.Winfield, D. A. (1983) Brain Res. 285, 155-169. [DOI] [PubMed] [Google Scholar]
- 37.Morales, B., Choi, S. Y. & Kirkwood, A. (2002) J. Neurosci. 22, 8084-8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benardo, L. S. (1997) J. Neurophysiol. 77, 3134-3144. [DOI] [PubMed] [Google Scholar]
- 39.Pizzorusso, T., Porciatti, V., Tseng, J. L., Aebischer, P. & Maffei, L. (1997) Neuroscience 80, 307-311. [DOI] [PubMed] [Google Scholar]
- 40.Katz, L. C. & Shatz, C. J. (1996) Science 274, 1133-1138. [DOI] [PubMed] [Google Scholar]
- 41.Drager, U. C. (1975) J. Comp. Neurol. 160, 269-290. [DOI] [PubMed] [Google Scholar]
- 42.Sala, R., Viegi, A., Rossi, F. M., Pizzorusso, T., Bonanno, G., Raiteri, M. & Maffei, L. (1998) Eur. J. Neurosci. 10, 2185-2191. [DOI] [PubMed] [Google Scholar]
- 43.Gabbott, P. L. A. & Stewart, M. G. (1987) Exp. Brain Res. 68, 103-114. [DOI] [PubMed] [Google Scholar]
- 44.Quinlan, E. M., Olstein, D. H. & Bear, M. F. (1999) Proc. Natl. Acad. Sci. USA 96, 12876-12880. [DOI] [PMC free article] [PubMed] [Google Scholar]