Abstract
The homeostasis of nitric oxide (NO) is attained through a balance between its production and consumption. Shifts in NO bioavailability have been linked to a variety of diseases. Although the regulation of NO production has been well documented, its consumption is largely thought to be unregulated. Here, we have demonstrated that under hypoxic conditions, NO accelerates its own consumption by increasing its entry into RBCs. When RBCs were exposed to NO (1:400 NO/heme ratio) under hypoxic conditions to form HbFe(II)NO, the consumption rate of NO increased significantly. This increase in NO consumption converted the bioactivity of serotonin from a vasodilator to a vasoconstrictor in isolated coronary arterioles. We identified HbFe(II)NO as a potential mediator of accelerated NO consumption. Accelerated NO consumption by HbFe(II)NO-bearing RBCs may contribute to hypoxic pulmonary vasoconstriction and the rebound effect seen on termination of NO inhalation therapy. Furthermore, accelerated NO consumption may exacerbate ischemia-mediated vasospasm and nitrate tolerance. Finally, this phenomenon may be an evolved mechanism to stabilize the vasculature in sepsis.
Nitric oxide (NO) is a signaling molecule produced by three isoforms of NO synthases for a variety of functions, including regulation of vasculature, participation in immune responses, and neuronal signaling/regulation. Regulation of NO concentration has generated considerable interest, because changes in NO bioavailability have been linked to a variety of disease states (1). The homeostasis of NO is attained through a balance between its production and consumption. In the vasculature, RBCs are the major scavenger of NO, because RBCs contain high concentrations of Hb, an effective NO scavenger. HbFe(II)O2 converts NO to nitrate, whereas HbFe(II) binds to NO to form HbFe(II)NO. Because Hb efficiently consumes NO at extremely high rates, the consumption of NO has generally been considered to be unregulated.
However, when Hb is enclosed within RBCs, its consumption of NO decreases significantly (2–5). This reduction in NO consumption has been attributed to (i) the RBC-free zone near the vessel wall (2), (ii) extracellular diffusion resistance from the bulk solution to the RBC surface (3), and (iii) the intracellular barrier associated with the RBC membrane and membrane skeleton (4). In support of the last mechanism, Huang et al. (4) have demonstrated that chemical modifications to the RBC result in the modulation of NO bioavailability by altering the rate RBCs consume NO. This result suggested that changes to the consumption of NO can potentially be an effective means to regulate NO bioavailability.
To investigate the physiological relevance of this potential regulatory mechanism, we tested various molecules that can serve as a physiological modulator of NO consumption. Surprisingly, we found that NO consumption by RBCs can be modulated by NO itself at concentrations found in vivo, but only under hypoxic conditions that allow for the formation of HbFe(II)NO. This modulation was reversible, because HbFe(II)NO was unstable under aerobic conditions. These results demonstrated that regulation of NO consumption can occur under physiological conditions and may indeed explain, in part, many unsolved puzzles, such as hypoxic pulmonary vasoconstriction (HPV), the rebound effect on termination of NO inhalation therapy, and ischemic vasospasm. In addition, increased uptake of NO may stabilize the vasculature during sepsis.
Materials and Methods
Materials. All NO donors were purchased from Cayman Chemical (Ann Arbor, MI). Glucose, potassium cyanide, and sodium hydroxide were purchased from Fisher Scientific. All other chemicals were purchased from Sigma.
Treatment of RBCs. Isolated and washed human or bovine RBCs (4) were held under hypoxic conditions with ultrahigh-purity argon (<1% PO2) until the desired intracellular HbFe(II)O2 fraction was reached. Hypoxic RBCs were washed by centrifugation at 800 × g and by aspirating the supernatant. A subset of these RBCs was treated with NO generated from proline 2,2′-(hydroxynitrosohydrazino) (NONOate) or diethylamine–NONOate at NO/Hb ratios of 1:2,000 to 1:200, on a heme basis. The suspension of RBCs and NO donor was incubated for at least two half-lives of the respective NO donor. Both NO-treated and untreated RBCs were then washed three times in 50:1 ratios with isotonic 40 mM Hepes buffer containing 5 mM glucose (pH 7.4) under hypoxic (<1% PO2) or normoxic conditions (21% PO2). The hematocrit (Hct) was adjusted to 15% for the competition assay.
Competition Assay. This procedure has been described (6, 7), and a detailed description of the procedure along with the computer program for calculating the kinetic constant is available online (www.seas.ucla.edu/~liaoj). Briefly, each test consisted of at least three solutions run simultaneously: cell-free HbFe(II)O2 with spermine/NONOate (Sp/NO) donor in buffer, cell-free HbFe(II)O2 in a suspension of untreated bovine RBCs with Sp/NO, and cell-free HbFe(II)O2 in a suspension of treated bovine RBCs with Sp/NO. Each solution (≈15 ml) was loaded into a 20-ml syringe. The syringes were placed in a rotator to keep the cells uniformly dispersed. Samples were taken every 20 min and centrifuged (20 s at 16,000 × g) to pellet the RBCs, and the supernatant was assayed for HbFe(II)O2 and methemoglobin [HbFe(III)] on a Beckman DU 640 spectrophotometer. For competition assays performed under anaerobic conditions, HbFe(III) and HbFe(II)NO were measured by electron paramagnetic resonance (EPR, described later).
Microvessel Assay. The methodologies for isolation of porcine coronary arterioles have been described (8). Briefly, pigs were anesthetized with pentobarbital sodium (20 mg/kg, i.v.) and ventilated. The heart was removed and immediately placed in iced saline. Subepicardial arterioles (1.5-mm length, 50- to 100-μm i.d., in situ) were carefully dissected and cannulated with glass micropipettes. The vessels were then pressurized to 60 cm of H2O intraluminal pressure without flow and incubated at 37°C throughout the duration of the experiment. After developing a stable basal tone, vessel i.d. changes, in response to an accumulating increase in 5-hydroxytryptamine (5-HT, serotonin) concentration, were recorded by using videomicroscopic techniques. After establishing the control 5-HT response, the vessel bath was washed with physiological salt solution, and untreated or NO-pretreated hypoxic RBCs (40% Hct) were introduced into the lumen. The diameter changes in response to 5-HT stimulation were reassessed.
Aortic Ring Assay. The preparation of rat aortic rings was similar to that described previously (9). Briefly, the thoracic aortas of six female Sprague–Dawley (Harlan–Sprague–Dawley) rats were carefully removed and cut into 5-mm transverse rings, four to five per animal). The rings were mounted in tissue baths and incubated in Krebs solution (10 ml) gassed with 95% oxygen and 5% carbon dioxide and maintained at 37°C. The rings were stretched to a resting tension of 1 g. The amount of resting tension was determined in previous experiments performed in our laboratory. After determining maximal constriction to high-potassium Krebs and confirming the presence of a functional endothelium by relaxation to acetylcholine, the vessels were preconstricted with phenylephrine to elicit 50–60% of maximal constrictions. In each experiment, two to three vessels were incubated with either untreated or NO-pretreated hypoxic RBCs (1% Hct). The aortic ring constriction was measured and reported as a percentage increase over baseline.
Measurement of HbFe(II)NO. The allosteric state of HbFe(II)NO in whole RBCs was probed with EPR, as described (10). Briefly, 200 μl of each sample was introduced into 3-mm-i.d. clear fused-quartz tubes and plunged into liquid nitrogen. The EPR spectrum of each sample was measured on a Bruker (Billerica, MA) EMX spectrometer in the X band at 77 K by using a suprasil liquid-nitrogen finger Dewar (Wilmad, Buena, NY) in a Bruker 4119HS-W1 high-sensitivity cavity. Detailed spectra of HbFe(II)NO were taken at a modulation amplitude of 4 G was used with an 81.92-ms time constant and a sweep width of 400 G centered at 3,288 G. The spectrometer was operated at 9.35 GHz, 10.1-mW microwave power, 100-kHz modulation frequency. Under these conditions, the power was not saturated. Two scans were taken. Quantification of HbFe(II)NO was done by double integration by using WINEPR SYSTEM 2.11 (Bruker) and comparison with a calibration curve generated by measuring UV-visible spectroscopy standardized HbFe(II)NO samples in the EPR instrument.
To measure HbFe(II)NO in crude preparations of membranes and cytoplasmic fractions of NO pretreated hypoxic RBCs, a method based on chemiluminescence was used (11). Because of HbFe(II)NO dilution from the preparation of the membrane fraction, EPR could not be used. Treated RBCs were lysed 1:40 with 10 mM phosphate buffer (pH = 7.4, 4°C) and washed twice at the same ratio with centrifugation at 18,000 × g (4°C). All solutions were maintained at 4°C to minimize HbFe(II)NO degradation. Cytoplasmic and membrane-rich fractions were treated with nonidet-P40 and mercuric chloride. Background nitrite was removed by filtration through a Sephadex G-25 column and treatment with acidified sulfanilamide. Each fraction was injected into a reaction chamber containing a solution of 50 mg of potassium iodide and one iodine crystal in 9 ml of 80% acetic acid. The NO evolved from the reaction chamber was carried in ultrahigh-purity helium (Airgas, Radnor, PA) to a gas wash bottle containing a 1 M NaOH solution and then to a Sievers NO Analyzer 280 (Ionics Instruments, Boulder, CO) to measure NO quantities by the chemiluminescence reaction with ozone. Data were recorded with NOANALYSIS 3.21 LIQUID software provided by Sievers and analyzed with PEAKFIT 4.11 (Systat, Richmond, CA). The concentration of NO was calculated from a mass-calibrated nitrite calibration curve, as described in the Sievers NO Analyzer 280 operation manual.
Results
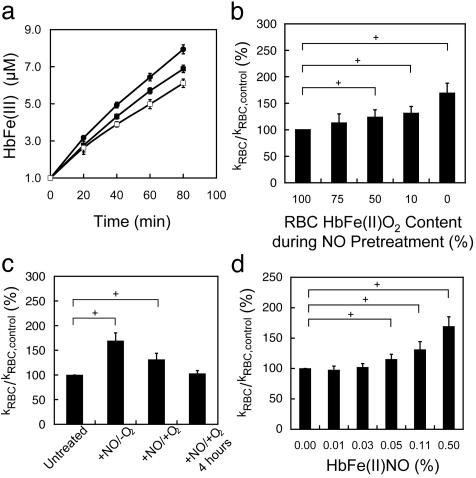
NO Pretreatment of Hypoxic RBCs Increased NO Consumption. To determine whether the rate of NO consumption by RBCs is regulated by physiological variables such as NO and O2 concentrations, we tested the effect of NO pretreatment on RBCs at Hb oxygen saturations ranging from 0% to 100%. In vivo, oxygen saturation can be as low as 10% locally (12). Total NO generated was in the low micromolar range from an NO donor (diethylamine–NONOate or proline NONOate). After NO pretreatment under various oxygen saturation conditions, the competition assay was conducted under aerobic conditions. In this assay, RBCs compete with extracellular HbFe(II)O2 for NO generated from Sp/NO, a slow-releasing NO donor. The production of extracellular HbFe(III) from the reaction of NO was measured to determine the rate of NO uptake by RBCs. The total amount of NO released from the NO donor was measured by the formation of HbFe(III) in a solution containing only the NO donor and HbFe(II)O2. In the presence of NO-pretreated hypoxic RBCs, less extracellular HbFe(III) formation was measured compared with the control sample, untreated hypoxic RBCs (Fig. 1a), suggesting that NO-pretreated hypoxic RBCs consume NO more rapidly than untreated hypoxic RBCs. Because the competition assay depends on the accurate measurement of the NO reaction with HbFe(II)O2, whether NO-pretreated RBCs released reducing or oxidizing species that may skew these results was determined. Incubation of the NO-pretreated or untreated RBCs in solutions of HbFe(II)O2 or HbFe(III) (four separate samples, n = 5) resulted in no change in the extracellular species (data not shown). Hence, both pretreated and untreated RBCs neither oxidize nor reduce extracellular Hb under these conditions. In particular, NO was not exported from NO-pretreated RBC under this condition. Because less HbFe(III) is formed in the presence of hypoxic RBCs pretreated with NO, these pretreated RBCs must consume NO more rapidly than the untreated RBCs. This result indicates that the entry of NO into the pretreated RBCs is accelerated compared with the untreated RBCs.
Fig. 1.
Pretreatment of hypoxic RBCs with NO increased the NO consumption rate as measured by the competition assay. (a) The decreased production of HbFe(III) in the extracellular space suggested that NO was consumed more rapidly by hypoxic RBCs pretreated with NO (open squares) compared with untreated RBCs (filled squares). The total amount of NO released by the NO donor in the competition assay was measured by the formation of HbFe(III) in a solution of NO donor and cell-free HbFe(II)O2 (filled circles). (b) Conversion of HbFe(III) production to rates of NO uptake demonstrated hypoxic RBCs pretreated with NO consume NO more rapidly. NO entry into hypoxic RBCs pretreated with NO was more rapid than NO entry into untreated RBCs. Pretreatment of RBCs with NO under normoxic conditions (21% PO2) results in no change in the rate of NO uptake (P < 0.05, n = 4–8). (c) When tested under anaerobic conditions (+NO/-O2), the consumption of NO by NO-pretreated hypoxic RBCs increased 69 ± 16% (P < 0.01, n = 8). Reoxygenating RBCs pretreated with NO under hypoxic conditions by washing with oxygenated buffer and performing the competition assay under normoxic conditions (21% PO2) (+NO/+O2), the rate of NO consumption remained greater than that of untreated RBCs (31 ± 13%) (P < 0.01, n = 8). Washing NO-pretreated hypoxic RBCs with oxygenated buffer and incubating for 4 h (+NO/+O2/4h) reverted the NO uptake rate back to the untreated RBC rate of NO uptake (n = 4). (d) The increase in the NO uptake rate of NO-pretreated hypoxic RBCs correlated with the formation of HbFe(II)NO (P < 0.05, n = 4 for each sample).
Interestingly, the oxygen saturation of RBCs during NO pretreatment was found to be inversely proportional to the NO consumption rate. Because the potential product, HbFe(II)NO, is unstable under aerobic conditions, we also conducted the competition assay under anaerobic conditions. In this case, the formation of extracellular HbFe(III) and HbFe(II)NO was measured by EPR. Indeed, when both pretreatment and the competition assay were performed under anaerobic conditions, the NO consumption rate increased by 69%, compared with untreated RBCs (Fig. 1b). This effect diminished as the oxygen saturation of RBCs increased and was not seen with pretreatment under normoxic conditions. The difference in NO uptake may stem from the balance of HbFe(III) and HbFe(II)NO formed under difference Hb oxygen saturations. The formation of small quantities of HbFe(III), as would form under these conditions, does not modify the NO uptake rate (3, 7). On reoxygenation (21% PO2), the NO-pretreated RBCs continued to consume NO faster (32 ± 13%) (Fig. 1c) compared with the sham controls. Thus, the increased consumption of NO by these pretreated RBCs appears to be directly related to the Hb status within RBC. Interestingly, after incubating the NO-pretreated RBCs for 4 h under aerobic conditions (21% PO2), the NO uptake rate reverted to that of the untreated RBC.
HbFe(II)NO Formed from NO Pretreatment of Hypoxic RBCs. Because the effect of NO-pretreated RBCs was seen under hypoxic conditions and was reversible on reoxygenation, HbFe(II)NO was an attractive candidate responsible for accelerated NO consumption. Indeed, the rate of NO uptake correlated with the HbFe(II)NO content (Fig. 1d) in RBC, although this did not imply a causative relationship. At HbFe(II)NO of 0.05% or higher, the NO uptake rate of RBCs pretreated with NO under hypoxic conditions was greater than untreated RBCs (Fig. 1d). At ratios <0.03%, the NO uptake rate of RBCs pretreated with NO under hypoxic conditions did not statistically differ from untreated RBCs.
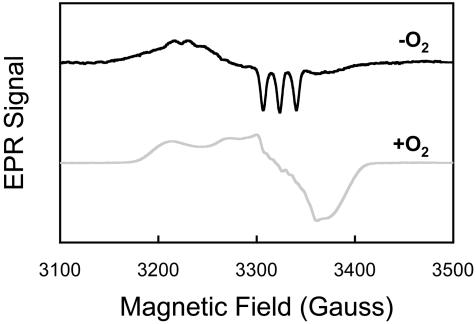
To further characterize the products of the NO reaction with hypoxic RBC, we used EPR to determine the heme state of HbFe(II)NO in RBC. The EPR spectrum (Fig. 2) showed that the NO reaction with hypoxic RBC led to the formation of the five-coordinate NO-α heme adduct, as illustrated by the characteristic hyperfine splitting. The NO-α heme adduct under hypoxic conditions has been shown to be the five-coordinated “super T” state (13). On reoxygenation, it becomes six-coordinated, and the hyperfine structure disappears (Fig. 2). This result demonstrated that NO was bound to the α hemes after its entry into hypoxic RBCs. Measurement of RBCs pretreated with NO under normoxic conditions did not yield a HbFe(II)NO signal, similar to studies with cell-free Hb (10). S-nitrosohemoglobin was not detected by using an I ozone-based chemiluminescence assay (11, 14) (data not shown). Therefore, the formation of HbFe(II)NO in RBC is most likely responsible for the accelerated NO uptake rate.
ozone-based chemiluminescence assay (11, 14) (data not shown). Therefore, the formation of HbFe(II)NO in RBC is most likely responsible for the accelerated NO uptake rate.
Fig. 2.
The formation of HbFe(II)NO in hypoxic RBCs pretreated with NO was measured by EPR. The signature three-peak hyperfine splitting demonstrated that NO was primarily bound to the α heme subunit under hypoxia and was primarily in the low-oxygen affinity T state (upper black line). On oxygenation, NO remained bound to the α heme subunit, whereas Hb converted to the high oxygen affinity R state (lower gray line).
Because NO must enter the RBC through the cytoskeletal barrier, it is expected that the ratio of HbFe(II)NO to total Hb should be higher on the membrane than in the cytoplasm fraction. In addition, evidence for the modulation of NO entry proceeding through alterations of the membrane skeleton association to the membrane has been presented previously (4). To test this hypothesis, the ratio of membrane-associated HbFe(II)NO to total Hb was measured. The ratio was found to be >85% higher in the membrane fraction than in the cytoplasmic fraction (Table 1). Hence, the observed increase in NO entry may stem from alterations due to the presence of HbFe(II)NO in the membrane fraction of the RBC.
Table 1. HbFe(II)NO preferentially binds to the membrane.
| RBC fraction | HbFe(II)NO/total Hb +/— SD, % |
|---|---|
| Cytosol | 0.49 +/— 0.08 |
| Membrane | 0.91 +/— 0.16* |
, P < 0.01, n = 5
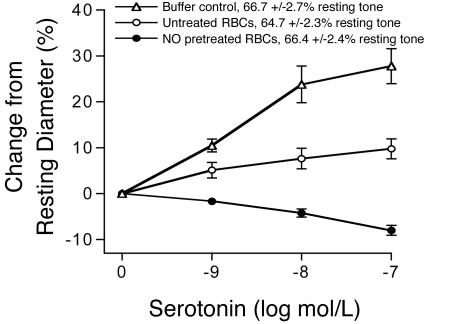
HbFe(II)NO-Containing RBCs Converted 5-HT from a Vasodilator to a Vasoconstrictor. To explore the physiological relevance of this regulation, we used isolated porcine coronary microvessels (100 μm in diameter) as a bioassay. 5-HT was used to induce NO-mediated dilation in the presence of NO-pretreated or untreated RBCs. 5-HT is a vasoactive agent that produces two opposing vasomotor responses in coronary vessels, i.e., dilation via NO release from endothelium and constriction via direct activation of 5-HT receptors on smooth muscle (15, 16). In intact isolated porcine coronary arterioles, 5-HT induced vasodilation (Fig. 3). Addition of 5-HT to arterioles containing intraluminal RBCs (40% Hct) that received sham treatment resulted in vasodilation, although attenuated compared with the buffer control. Interestingly, when RBCs were pretreated with NO under hypoxic conditions and introduced to the lumen (40% Hct), 5-HT elicited a concentration-dependent vasoconstriction response. Under this condition, the 5-HT-induced NO production was negated by the increased NO consumption from the pretreated RBCs, which allowed the 5-HT effect on smooth muscle contraction to fully express. The resting myogenic tone of the microvessels with buffer, RBCs pretreated with NO under hypoxic conditions, or untreated RBCs did not differ (Fig. 3), again indicating that NO was not exported from NO-pretreated hypoxic RBCs. Lysis of pretreated or untreated RBCs resulted in a significant increase in the resting tone, which was readily discernable.
Fig. 3.
The bioactivity of 5-HT was converted from vasodilation to vasoconstriction by RBCs treated under hypoxia with NO. Addition of 5-HT to isolated porcine arterioles containing buffer alone resulted in dose-dependent vasodilation (n = 9). The addition of untreated RBCs to the lumen of the arteriole attenuated 5-HT-induced vasodilation, suggesting that some 5-HT-induced NO is consumed by RBCs (n = 5). Addition of 5-HT to arterioles containing NO-pretreated RBCs resulted in vasoconstriction, suggesting that these RBCs consumed NO more rapidly than untreated RBCs (n = 5). The resting tone of the arterioles did not differ with the addition of untreated or NO-pretreated hypoxic RBCs to the vessel lumen, suggesting that lysis was minimal and no export of vasodilatory or vasoconstrictive species occurred.
To detect the net export of NO or other vasoregulators that may have occurred under these conditions, both NO-pretreated hypoxic and untreated RBCs were tested in an isolated rat aortic ring tissue preparation. Both types of RBCs elicited similar contraction in phenylephrine precontracted aortic rings, suggesting that no vasoregulatory bioactivity was exported by hypoxic RBCs pretreated with NO under these conditions (Table 2). This result is consistent with the constant resting tone of the isolated microvessels containing NO-treated and untreated RBCs and is in contrast to NO export from NO-pretreated RBCs under anaerobic (1% oxygen saturation) conditions (17). Taken together, these data indicate that RBCs pretreated with NO under hypoxic conditions consume NO much more rapidly than untreated RBCs. Furthermore, these pretreated RBCs exported neither vasodilatory nor vasoconstrictive bioactivity under these experimental conditions.
Table 2. Isolated rat aortic ring tissue preparations.
| Description | Increase in vessel tone +/— SE, % |
|---|---|
| Untreated RBCs | 41.5 +/— 9.1 |
| NO-treated hypoxic RBCs | 39.8 +/— 9.9 |
Discussion
The above results demonstrated that NO consumption can be regulated by the NO adduct with Hb in RBCs, and such regulation is physiologically significant because it converts 5-HT from a vasodilator to a vasoconstrictor. HbFe(II)NO was implicated as the key in this regulation. This NO-heme adduct has been detected at quantities as high as 10 μM, in vivo (18), and is increased from normal levels during NO inhalation therapy (19). The formation of HbFe(II)NO proceeds by the direct binding of NO to HbFe(II) during hypoxia, as occurs in the pulmonary circulation where deoxygenated blood enters a vascular bed in which NO is generated (20). Because HbFe(II)NO has been detected in vivo under various conditions (21–29), the increased NO entry into NO-pretreated hypoxic RBCs may have significant implications in physiological, pathological, or clinical situations where low oxygen tension and relatively high NO concentrations coexist, such as in pulmonary circulation, NO inhalation therapy, ischemia-related vasospasm, and sepsis.
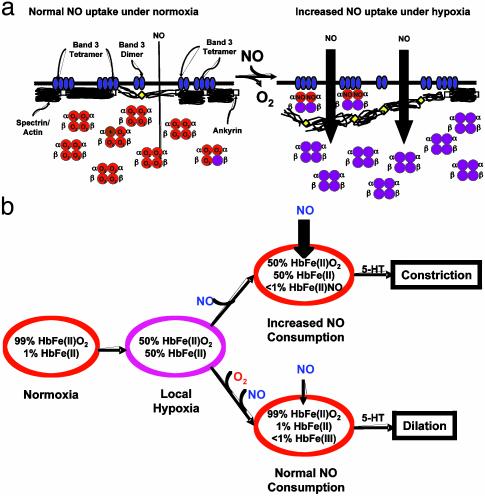
A Potential Mechanism. The potential mechanism by which NO uptake was increased may lie with the membrane skeleton, because HbFe(II)NO was more abundant in the membrane than in the cytosol (Table 1). In addition, the membrane skeleton has been previously identified as a component in the reduced entry of NO (4). Under conditions in which the NO/heme ratio is low, NO is bound primarily to the α heme moiety (α-NO, Fig. 2). This form of Hb has been characterized to exist in a “super T” allosteric state (13). Because the T state Hb binds to the cytoplasmic domain (N terminus) of Band 3 tetramers in the 2,3-bisphosphoglycerate pocket (30), it is likely that α-NO is bound to Band 3 tetramers. Because the Band 3 tetramer is also the site for ankyrin, which anchors the membrane skeleton to the membrane (31), HbFe(II)NO may displace some ankyrin to release the membrane skeleton from portions of the membrane. This “loosening” effect may be the cause of the increased NO uptake (Fig. 4a). Huang et al. (4) suggested a similar effect when shifting the population of Band 3 to dimers. Band 3 dimers have a lower affinity to ankyrin, thus reducing the association of the membrane skeleton to the membrane (31). The formation of small amounts of HbFe(II)NO in which NO is primarily bound to the α heme has also been demonstrated to enhance oxygen delivery to tissue without increasing blood flow (32). The interaction between α-NO and the membrane skeleton is likely key to this regulation.
Fig. 4.
(a) Under normal conditions, the membrane skeleton forms a barrier to NO entry into the RBC by the association to Band 3 tetramers through ankyrin. Band 3 dimers have a lower affinity for ankyrin, thus uncoupling the membrane skeleton from the membrane, which increases the entry of NO. On hypoxia and exposure to NO, HbFe(II)NO in which NO is bound to theα subunit is formed in the “super T” state and competes with ankyrin for the Band 3 cytoplasmic N terminus. The reduction in membrane skeleton/membrane interaction allows for increased NO entry. (b) In normoxia, 99% of the free heme sites on Hb in the RBC are oxygen bound [HbFe(II)O2]. In regions of low oxygen tension (hypoxia), which occurs locally, oxygen is delivered, freeing the Hb heme moiety [HbFe(II)]. NO produced in the pulmonary arteries under local hypoxia or delivered from NO inhalation/NO donors can then bind to HbFe(II) to form HbFe(II)NO. The formation of HbFe(II)NO results in the accelerated consumption of NO, and, in the presence of 5-HT, vasoconstriction occurs. If the RBC is exposed to oxygen before NO, HbFe(II)O2 is formed first. The reaction of NO with HbFe(II)O2 results in HbFe(III) formation, which does not modify the rate of NO consumption. The biological action of 5-HT reverts to vasodilation.
Implication to HPV. In the pulmonary circulation, HPV is a response in which pulmonary arteries constrict on induction of hypoxia, presumably to enhance blood oxygenation via shunting of flow to the nonhypoxic sites (33). The mechanism of this phenomenon has been extensively studied, yet a complete understanding is still lacking because of its complex and multifactorial nature (34). Previously, HPV has been demonstrated to be greatly potentiated by RBCs (35, 36). Perfusion of isolated rat lungs with RBC suspensions increased pulmonary arterial pressure to a relatively stable plateau on induction of HPV (34, 37). In contrast, perfusates void of RBCs elicited a much weaker multiphasic response (34, 37). This observation may be explained, in part, by the increased NO uptake by RBCs, because pulmonary arteries produce NO (20, 38), and the Hb oxygen saturation is sufficiently low. The formation of HbFe(II)NO would be possible in vessels where oxygen tension is locally low due to the increased availability of free heme sites. Thus, vasoconstriction may result in areas of lower oxygen tension. Blood flow would be diverted to regions in the lungs that contain more oxygen, thus enhancing blood oxygenation. Therefore, increased uptake of NO by RBCs could be an important factor contributing to HPV. In addition, Erzurum and coworkers (39) have proposed that inducible NOS in the alveoli is an oxygen sensor, which may have bearing on the phenomenon presented here.
Implication to NO Inhalation Therapy. During NO inhalation therapy, the formation of HbFe(II)NO, along with other NO metabolic species, has been identified (19, 29, 40). NO inhalation is typically used to provide relief for pulmonary hypertension (41). On termination of NO inhalation therapy, a transient but dramatic decrease in tissue oxygenation with an increase in pulmonary arterial pressure beyond the baseline pressure has been observed (42). The increased uptake of NO by RBCs bearing HbFe(II)NO may contribute to this “rebound” response to NO inhalation therapy termination. Because HbFe(II)NO formation is evident, it is likely that the entry of NO into the RBC increases during NO inhalation therapy. On termination of NO inhalation therapy, sufficient HbFe(II)NO exists in the RBC to consume endogenously produced NO more rapidly, adding to the rebound effect. Consistent with the instability of HbFe(II)NO, the rebound effect is transient (42). Evidence that NOS inhibition may also contribute to the increase in pulmonary arterial pressure exists (43–45); however, whether inhibition actually occurs is still a matter of debate (46, 47). RBCs, in conjunction with other vasoconstrictors (48, 49), may mediate the rebound effect seen on termination of NO inhalation therapy.
Implication to Ischemic Vasospasm. It is well documented that endothelial dysfunction is believed to play an important integral part in the clinical presentation of coronary artery disease (50). When the endothelium is damaged, the reduced NO and prostacyclin production at the site leads to platelet aggregation with the release of 5-HT and thromboxane A2, subsequently enhancing basal vascular tone and leading to a reduction of local blood flow and ischemia/hypoxia. Because NO production is increased by inducible NOS induction in neutrophils, macrophages, and cardiomyocytes in the inflammatory phase of myocardial ischemia and infarction (51–55), HbFe(II)NO is likely formed under these conditions. In fact, HbFe(II)NO has been detected during ischemia reperfusion (21, 24). NO consumption by RBCs bearing HbFe(II)NO is expected to increase, which would further potentiate vasoconstriction, as implicated in the present study. It has been suggested that endothelial vasodilator dysfunction plays a causative role for triggering myocardial ischemia in stable angina pectoris (56). Increased NO consumption by RBCs would aggravate the sequelae of acute ischemic syndrome and might be the primary underlying mechanism in some patients with syndrome X (56).
Implication to Sepsis. Sepsis is a complicated disease state that is the focus of much work (57). Sepsis occurs with the infiltration of bacteria, which results in the activation of the immune response. Sepsis is also known as the “systemic inflammatory response to infection,” or SIRS (58). Severe sepsis leads to hypotension and organ dysfunction. Increased production of NO, which is responsible for the hypotensive state, has been documented to lead to the formation of HbFe(II)NO (59, 60). To buffer the effects of high NO concentrations, RBCs may have evolved a mechanism by which excess NO is consumed more rapidly. The phenomenon proposed here may be such a mechanism. By consuming excess NO, HbFe(II)NO-bearing RBCs may stabilize the vasculature in sepsis.
Conclusion
In summary, we have demonstrated that NO consumption by RBCs can be regulated by HbFe(II)NO formation under hypoxic conditions. As illustrated in Fig. 4b, on exposure to local hypoxia, oxygen is released from HbFe(II)O2 to result in the formation of HbFe(II). When hypoxic RBCs are exposed to NO, HbFe(II)NO is formed, and the NO consumption rate increases. This increase in NO consumption was shown to convert the bioactivity of 5-HT to a vasoconstrictor in isolated coronary arterioles. The increase in the NO uptake rate is reversible. However, if hypoxic RBCs are first reoxygenated and then exposed to NO, the RBC uptake rate does not change, and the bioactivity of 5-HT remains vasodilatory. Interestingly, in contrast to a previous report (17), we did not find evidence of NO export from NO-pretreated RBCs under aerobic or hypoxic conditions. Finally, our results may represent a new paradigm of physiological regulation of NO by alteration of its rate of consumption and have significant implications in our understanding of the interactions of NO with RBCs in the vasculature.
Acknowledgments
This work was funded by National Institutes of Health Grants R01 HL65741 and AG15857.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HPV, hypoxic pulmonary vasoconstriction; EPR, electron paramagnetic resonance; 5-HT, 5-hydroxytryptamine (serotonin); Hct, hematocrit; NONOate, 2,2′-(hydroxynitrosohydrazino; Sp/NO, spermine/NONOate.
References
- 1.Ignarro, L. (2000) in Nitric Oxide: Biology and Pathobiology, ed. Ignarro, L. (Academic, San Diego), pp. 633-920.
- 2.Liao, J. C., Hein, T. W., Vaughn, M. W., Huang, K. T. & Kuo, L. (1999) Proc. Natl. Acad. Sci. USA 96, 8757-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu, X., Miller, M. J., Joshi, M. S., Sadowska-Krowicka, H., Clark, D. A. & Lancaster, J. R., Jr. (1998) J. Biol. Chem. 273, 18709-18713. [DOI] [PubMed] [Google Scholar]
- 4.Huang, K. T., Han, T. H., Hyduke, D. R., Vaughn, M. W., Van Herle, H., Hein, T. W., Zhang, C., Kuo, L. & Liao, J. C. (2001) Proc. Natl. Acad. Sci. USA 98, 11771-11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiter, C. D., Wang, X., Tanus-Santos, J. E., Hogg, N., Cannon, R. O., 3rd, Schechter, A. N. & Gladwin, M. T. (2002) Nat. Med. 8, 1383-1389. [DOI] [PubMed] [Google Scholar]
- 6.Vaughn, M. W., Huang, K. T., Kuo, L. & Liao, J. C. (2001) Nitric Oxide 5, 18-31. [DOI] [PubMed] [Google Scholar]
- 7.Vaughn, M. W., Huang, K. T., Kuo, L. & Liao, J. C. (2000) J. Biol. Chem. 275, 2342-2348. [DOI] [PubMed] [Google Scholar]
- 8.Kuo, L., Chilian, W. M. & Davis, M. J. (1991) Am. J. Physiol. 261, H1706-H1715. [DOI] [PubMed] [Google Scholar]
- 9.Furchgott, R. F. & Zawadzki, J. V. (1980) Nature 288, 373-376. [DOI] [PubMed] [Google Scholar]
- 10.Han, T. H., Hyduke, D. R., Vaughn, M. W., Fukuto, J. M. & Liao, J. C. (2002) Proc. Natl. Acad. Sci. USA 99, 7763-7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang, B. K., Vivas, E. X., Reiter, C. D. & Gladwin, M. T. (2003) Free Radical Res. 37, 1-10. [DOI] [PubMed] [Google Scholar]
- 12.Marcos, I. & Johnson, P. C. (1978) in Peripheral Circulation, ed. Johnson, P. C. (Wiley, New York), pp. 141-166.
- 13.Yonetani, T., Tsuneshige, A., Zhou, Y. & Chen, X. (1998) J. Biol. Chem. 273, 20323-20333. [DOI] [PubMed] [Google Scholar]
- 14.Gladwin, M. T., Wang, X., Reiter, C. D., Yang, B. K., Vivas, E. X., Bonaventura, C. & Schechter, A. N. (2002) J. Biol. Chem. 277, 27818-27828. [DOI] [PubMed] [Google Scholar]
- 15.Golino, P., Piscione, F., Willerson, J. T., Cappelli-Bigazzi, M., Focaccio, A., Villari, B., Indolfi, C., Russolillo, E., Condorelli, M. & Chiariello, M. (1991) N. Engl. J. Med. 324, 641-648. [DOI] [PubMed] [Google Scholar]
- 16.Chilian, W. M., Kuo, L., DeFily, D. V., Jones, C. J. & Davis, M. J. (1993) Eur. Heart J. 14, Suppl. I, 55-59. [PubMed] [Google Scholar]
- 17.Pawloski, J. R., Hess, D. T. & Stamler, J. S. (2001) Nature 409, 622-626. [DOI] [PubMed] [Google Scholar]
- 18.Kirima, K., Tsuchiya, K., Sei, H., Hasegawa, T., Shikishima, M., Motobayashi, Y., Morita, K., Yoshizumi, M. & Tamaki, T. (2003) Am. J. Physiol. 285, H589-H596. [DOI] [PubMed] [Google Scholar]
- 19.Cannon, R. O., III, Schechter, A. N., Panza, J. A., Ognibene, F. P., Pease-Fye, M. E., Waclawiw, M. A., Shelhamer, J. H. & Gladwin, M. T. (2001) J. Clin. Invest. 108, 279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ignarro, L. J., Byrns, R. E., Buga, G. M. & Wood, K. S. (1987) Circ. Res. 61, 866-879. [DOI] [PubMed] [Google Scholar]
- 21.Battista, S., Mengozzi, G., Bar, F., Cerutti, E., Pollet, C., Torchio, M., Biasi, F., Cavalli, G., Salizzoni, M., Poli, G. & Molino, G. (2002) Dig. Dis. Sci. 47, 528-534. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka, S., Kamiike, W., Kosaka, H., Ito, T., Kumura, E., Shiga, T. & Matsuda, H. (1996) Am. J. Physiol. 271, G405-G409. [DOI] [PubMed] [Google Scholar]
- 23.Kohno, M., Masumizu, T. & Mori, A. (1995) Free Radical Biol. Med. 18, 451-457. [DOI] [PubMed] [Google Scholar]
- 24.Kumura, E., Yoshimine, T., Tanaka, S., Hayakawa, T., Shiga, T. & Kosaka, H. (1994) Neurosci. Lett. 177, 165-167. [DOI] [PubMed] [Google Scholar]
- 25.Gladwin, M. T., Shelhamer, J. H., Ognibene, F. P., Pease-Fye, M. E., Nichols, J. S., Link, B., Patel, D. B., Jankowski, M. A., Pannell, L. K., Schechter, A. N., et al. (2002) Br. J. Haematol. 116, 436-444. [DOI] [PubMed] [Google Scholar]
- 26.Glover, R. E., Ivy, E. D., Orringer, E. P., Maeda, H. & Mason, R. P. (1999) Mol. Pharmacol. 55, 1006-1010. [DOI] [PubMed] [Google Scholar]
- 27.Kakefuda, T., Enosawa, S., Li, X. K., Tamura, A., Funeshima, N., Kanashiro, M., Amemiya, H., Kitajima, M. & Suzuki, S. (1999) Transpl. Int. 12, 307-315. [DOI] [PubMed] [Google Scholar]
- 28.Glover, R. E., Corbett, J. T., Burka, L. T. & Mason, R. P. (1999) Chem. Res. Toxicol. 12, 952-957. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi, Y., Kobayashi, H., Tanaka, N., Sato, T., Takizawa, N. & Tomita, T. (1998) Am. J. Physiol. 274, H349-H357. [DOI] [PubMed] [Google Scholar]
- 30.Schuck, P. & Schubert, D. (1991) FEBS Lett. 293, 81-84. [DOI] [PubMed] [Google Scholar]
- 31.Van Dort, H. M., Moriyama, R. & Low, P. S. (1998) J. Biol. Chem. 273, 14819-14826. [DOI] [PubMed] [Google Scholar]
- 32.Kosaka, H. & Seiyama, A. (1996) Biochem. Biophys. Res. Commun. 218, 749-752. [DOI] [PubMed] [Google Scholar]
- 33.Von Euler, U. S. & Liljestrand, G. (1946) Acta Physiol. Scand. 12, 301-320. [Google Scholar]
- 34.Ward, J. P. & Aaronson, P. I. (1999) Respir. Physiol. 115, 261-271. [DOI] [PubMed] [Google Scholar]
- 35.Deem, S., Swenson, E. R., Alberts, M. K., Hedges, R. G. & Bishop, M. J. (1998) Am. J. Respir. Crit. Care Med. 157, 1181-1186. [DOI] [PubMed] [Google Scholar]
- 36.Hakim, T. S. & Malik, A. B. (1988) Respir. Physiol. 72, 109-121. [DOI] [PubMed] [Google Scholar]
- 37.McMurtry, I. F., Hookway, B. W. & Roos, S. (1977) Chest 71, 253-256. [DOI] [PubMed] [Google Scholar]
- 38.Ignarro, L. J., Buga, G. M., Wood, K. S., Byrns, R. E. & Chaudhuri, G. (1987) Proc. Natl. Acad. Sci. USA 84, 9265-9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dweik, R. A., Laskowski, D., Abu-Soud, H. M., Kaneko, F., Hutte, R., Stuehr, D. J. & Erzurum, S. C. (1998) J. Clin. Invest. 101, 660-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gladwin, M. T., Ognibene, F. P., Pannell, L. K., Nichols, J. S., Pease-Fye, M. E., Shelhamer, J. H. & Schechter, A. N. (2000) Proc. Natl. Acad. Sci. USA 97, 9943-9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Max, M. & Rossaint, R. (1999) Curr. Opin. Cardiol. 14, 432-436. [DOI] [PubMed] [Google Scholar]
- 42.Atz, A. M., Adatia, I. & Wessel, D. L. (1996) Ann. Thorac. Surg. 62, 1759-1764. [DOI] [PubMed] [Google Scholar]
- 43.Ma, X. L., Lopez, B. L., Christopher, T. A., Birenbaum, D. S. & Vinten-Johansen, J. (1996) Am. J. Physiol. 271, H2045-H2051. [DOI] [PubMed] [Google Scholar]
- 44.Ravichandran, L. V., Johns, R. A. & Rengasamy, A. (1995) Am. J. Physiol. 268, H2216-H2223. [DOI] [PubMed] [Google Scholar]
- 45.Sheehy, A. M., Burson, M. A. & Black, S. M. (1998) Am. J. Physiol. 274, L833-L841. [DOI] [PubMed] [Google Scholar]
- 46.Frank, D. U., Horstman, D. J., Morris, G. N., Johns, R. A. & Rich, G. F. (1998) J. Appl. Physiol. 85, 1070-1078. [DOI] [PubMed] [Google Scholar]
- 47.Frank, D. U., Horstman, D. J. & Rich, G. F. (1998) Anesth. Analg. 87, 1285-1290. [DOI] [PubMed] [Google Scholar]
- 48.Chen, L., He, H., Mondejar, E. F. & Hedenstierna, G. (2003) Am. J. Physiol. 284, H290-H298. [DOI] [PubMed] [Google Scholar]
- 49.Pearl, J. M., Nelson, D. P., Raake, J. L., Manning, P. B., Schwartz, S. M., Koons, L., Shanley, T. P., Wong, H. R. & Duffy, J. Y. (2002) Crit. Care Med. 30, 89-93. [DOI] [PubMed] [Google Scholar]
- 50.Ruschitzka, F. T., Noll, G. & Luscher, T. F. (1997) Cardiology 88, Suppl. 3, 3-19. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez de Miguel, L., Arriero, M. M., Farre, J., Jimenez, P., Garcia-Mendez, A., de Frutos, T., Jimenez, A., Garcia, R., Cabestrero, F., Gomez, J., et al. (2002) J. Am. Coll. Cardiol. 39, 818-825. [DOI] [PubMed] [Google Scholar]
- 52.Feng, Q., Lu, X., Jones, D. L., Shen, J. & Arnold, J. M. (2001) Circulation 104, 700-704. [DOI] [PubMed] [Google Scholar]
- 53.Akiyama, K., Kimura, A., Suzuki, H., Takeyama, Y., Gluckman, T. L., Terhakopian, A., Katagiri, T., Suh, K. Y., Roseto, J. & Bing, R. J. (1998) J. Am. Coll. Cardiol. 32, 373-379. [DOI] [PubMed] [Google Scholar]
- 54.Akiyama, K., Suzuki, H., Grant, P. & Bing, R. J. (1997) J. Mol. Cell Cardiol. 29, 1-9. [DOI] [PubMed] [Google Scholar]
- 55.Wildhirt, S. M., Suzuki, H., Horstman, D., Weismuller, S., Dudek, R. R., Akiyama, K. & Reichart, B. (1997) Circulation 96, 1616-1623. [DOI] [PubMed] [Google Scholar]
- 56.Sztajzel, J., Mach, F. & Righetti, A. (2000) Postgrad. Med. J. 76, 16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirkeboen, K. A. & Strand, O. A. (1999) Acta Anaesthesiol. Scand. 43, 275-288. [DOI] [PubMed] [Google Scholar]
- 58.Marsh, C. B. & Wewers, M. D. (1996) Clin. Chest Med. 17, 183-197. [DOI] [PubMed] [Google Scholar]
- 59.Bateman, R. M., Jagger, J. E., Sharpe, M. D., Ellsworth, M. L., Mehta, S. & Ellis, C. G. (2001) Am. J. Physiol. 280, H2848-H2856. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Q. Z., Jacobs, J., DeLeo, J., Kruszyna, H., Kruszyna, R., Smith, R. & Wilcox, D. (1991) Life Sci. 49, PL55-PL60. [DOI] [PubMed] [Google Scholar]