Abstract
Overexpression of the HER2/Neu protooncogene has been linked to the progression of breast cancer. Here we demonstrate that the growth of prostate cancer LNCaP cells can also be increased by the stable transfection of HER2/Neu. Using AG879, a HER2/Neu inhibitor, and PD98059, a MAP kinase inhibitor, as well as MAP kinase phosphatase-1 (MPK-1), in the transfection assay, we found that HER2/Neu could induce prostate-specific antigen (PSA), a marker for the progression of prostate cancer, through the MAP kinase pathway at a low androgen level. Reporter assays and mammalian two-hybrid assays further suggest this HER2/Neu-induced androgen receptor (AR) transactivation may function through the promotion of interaction between AR and AR coactivators, such as ARA70. Furthermore, we found this HER2/Neu → MAP kinase → AR-ARAs → PSA pathway could not be blocked completely by hydroxyflutamide, an antiandrogen used in the treatment of prostate cancer. Together, these data provide a novel pathway from HER2/Neu to AR transactivation, and they may represent one of the reasons for the PSA re-elevation and hormone resistance during androgen ablation therapy in prostate cancer patients.
Androgen and growth factors control the proliferation of prostate cells. Alterations of the receptors for these factors might disrupt the growth regulations that lead to cancer formation (1). Among growth factor receptors, the most frequently implicated in human cancer have been members of the class I receptor tyrosine kinase family, the epidermal growth factor (EGF) receptor family. This receptor family includes the EGF receptor (EGFR, erbB1), HER2/Neu (erbB2), erbB3, and erbB4 proteins (2).
The HER2/Neu (known as erbB2) gene encodes a 185,000-kDa transmembrane glycoprotein that contains intrinsic tyrosine kinase activity (3, 4). HER2/Neu is normally expressed at a low level in a number of human secretory epithelial cells. Point mutation of the Neu protooncogene, the rat homologue of erbB2, results in malignant transformation of several types of cells in culture (5, 6). However, amplification of the HER2/Neu gene was discovered in several different types of tumors. This overexpression is thought to involve the transactivation mechanism of HER2/Neu gene, but the detailed molecular mechanism remains unclear (7). Some studies indicated that the expression of HER2/Neu could be detected in prostatic interepithelial neoplasia (8, 9). However, it is not clear how HER2/Neu affects the progression of prostate cancer and androgen receptor (AR) function.
As a member of the steroid receptor superfamily, the AR functions primarily as a ligand-activated transcription factor that may play critical roles in prostate cancer growth and maintenance of libido (10, 11). AR regulates the androgen target genes by binding to androgen response elements that may involve coactivators and corepressors (12).
The AR may exist as a phosphoprotein in different cell lines; however, the importance of receptor phosphorylation in androgen-dependent or androgen-independent transactivation is still unclear. While the consensus sites for Ser-Pro-directed protein kinase in AR (Ser-81 and Ser-94) have been reported to play some roles in the AR phosphorylation, the substitution of Ala for Ser-81 or Ser-94 has no effect on AR transactivation, whereas substitution of Ala for Ser-650 caused a 30% decrease in transactivation (13).
Early studies showed that protein kinase C could induce the mouse mammary tumor virus (MMTV), an androgen target gene, 2- to 4-fold and suggested that these effects might involve reversible phosphorylation of the receptor (14). Another report also suggested that protein kinase A (PKA) activators, such as cAMP, could activate AR transactivation in a ligand-independent manner (15). The linkage of this PKA-mediated AR transactivation to prostate cancer growth remains unclear. In addition, AR could also be activated in a ligand-independent manner by EGF, insulin-like growth factor 1 (IGF-1), and keratinocyte growth factor (KGF), but the implications of these observations for prostate cancer progression remain unclear (16).
Prostate cancers typically start as androgen-sensitive lesions but frequently develop into androgen-insensitive lesions with progression to advanced stage. To date, even though prostate cancer patients respond to the androgen ablation therapy, they eventually succumb to disease. Collectively, the proposed explanations such as mutations of AR, the change of the steroid specificity of AR through AR mutations, or through AR interaction with cofactors, have been actively investigated. The induction of AR transactivation through the phosphorylation signal cascade may provide an alternative explanation as to why the activation of AR is less dependent on androgens.
Several lines of evidence indicate that the amplification of HER2/Neu gene in node-positive breast cancer, as well as ovarian cancer, is associated with decreased survival rates (17), but there is less linkage among HER2/Neu, AR function, and prostate cancer growth. Currently, the immunohistochemical studies of HER2/Neu expression in prostate cancer have provided contradictory results (18, 19), but the demonstration of elevated serum level of the extracellular portion of HER2/Neu in patients with metastatic lesions supports the concept that increased expression of HER2/Neu is one possible characteristic of prostate cancer (8). Furthermore, the immunostaining of a strong expression of HER2/Neu in prostatic intraepithelial neoplasia (18, 20) suggests that aberrant expression of HER2/Neu could represent an early event in the development of prostate cancer.
Several AR coactivators, such as ARA70, ARA55, ARA54, and Rb, have been isolated and characterized (21–24). Results from these studies suggested coactivators not only can enhance AR transactivation but also may be able to modulate the specificity of sex hormones or antiandrogens in prostate cells (25–27). It will be of great interest to know whether a phosphorylation signal cascade can crosstalk to the AR–ARAs pathway.
Here, we show HER2/Neu is an inducer for AR transactivations in two different prostate cancer cell lines, LNCaP and DU145. Furthermore, transfection of HER2/Neu in LNCaP resulted in higher expression of prostate-specific antigen (PSA) and higher proliferating rate. Finally, our data also suggested the HER2/Neu could potentiate the expression of AR target genes through the MAP kinase pathway.
MATERIALS AND METHODS
Materials.
5α-Dihydrotestosterone (DHT) was obtained from Sigma, hydroxyflutamide (HF) was from Schering, AG879 and PD98059 were from Calbiochem, and the plasmids pSG5-AR and ARA55 were constructed as previously described (21, 22). pSG5-ARA70N, with the transcriptional activation domain of ARA70 (amino acids 1–401), was used here for its better activity. pCMV-HER2/Neu was a gift from M. C. Hung (M. D. Anderson Cancer Center, Houston, TX). CL100 was from E. Nishida (Kyoto University, Japan).
Cell Culture and Transfections.
Human prostate cancer DU145 cells were maintained in Dulbecco’s minimum essential medium (DMEM) containing penicillin (25 units/ml), streptomycin (25 μg/ml), and 5% fetal calf serum (FCS). LNCaP cells were maintained in RPMI medium 1640 with 10% FCS. Transfections were performed using the calcium phosphate precipitation method, as previously described (21). Briefly, 4 × 105 cells were plated on 60-mm dishes 24 h before transfection, and the medium was changed to DMEM with 5% charcoal/dextran-stripped FCS (CS-FBS) 1 h before transfection with precipitate containing AR expression plasmid (wild type or mutated), chloramphenicol acetyltransferase (CAT) reporter gene, and ARA or HER2/Neu expression plasmid. PSA-CAT was constructed by ligating PSA promoter (nucleotides −2800 to +12) into the CAT reporter plasmid. MMTV-CAT was derived by MMTV-LTR promoter. A β-galactosidase expression plasmid, pCMV-β-gal, was used as an internal control for transfection efficiency. The total amount of DNA was adjusted to 11 μg with pSG5 in all transcriptional activation assays. After 24-h transfection, the medium was changed again, and the cells were treated with DHT, antiandrogen, or other treatment. After another 24 h, the cells were harvested for CAT assay as previously described (21). The CAT activity was visualized by PhosphorImager (Molecular Dynamics) and quantitated by imagequant software (Molecular Dynamics). At least three independent experiments were carried out in each case.
Phosphorylation Assay.
Purified recombinant, murine MAP kinase (New England Biolabs) was assayed as described by Alessi et al. (28). In brief, a 50-μl reaction mixture containing 100 μM [γ-32P]ATP (0.15 Ci/mmol; 1 Ci = 37 GBq) and 0.05 pmol of MAPK (ERK-2) was incubated with AR peptide for 15 min at 30°C.
Stable HER2/Neu Transfectant in LNCaP Cell.
The HER2/Neu gene was inserted into pCMV with neomycin resistance. The HER2/Neu-transfected LNCaP cells were selected and maintained in 400 μg/ml G418 (GIBCO).
Thiazolyl Blue (MTT) Assay.
The MTT assay is a quantitative colorimetric assay for mammalian cell survival and proliferation. The 2 × 105 LNCaP cells were grown in six-well plates. After 24 h, the medium was changed to RPMI medium 1640 with 10% normal FCS or CS-FCS for another 48 h. Then 200 μl of MTT (5 mg/ml; Sigma) was added into the each well with 1 ml of medium for 3 h at 37°C. After incubation, 2 ml of 0.04 M HCl in isopropyl alcohol was added into each well. After 5-min incubation at room temperature, the absorbance was read at a test wavelength of 595 nm.
Site-Directed Mutagenesis of AR.
pSG5-wild-type AR (wtAR) was used as the DNA mutagenesis template to anneal with the mutagenic primer: 5′-CCCTATCCCGGTCCCACTTGT-3′. The mutant strand was synthesized with T4 DNA polymerase and T4 DNA ligase by using the Gene Editor kit (Promega) and was used to transform BMH71–18 muS cells. The plasmid DNAs were isolated from the selection plates and then transformed into JM109 cells. The potential mutant plasmids were then identified by sequencing.
RESULTS
Increasing the Growth of LNCaP Cells with Stable Transfection of HER2/Neu.
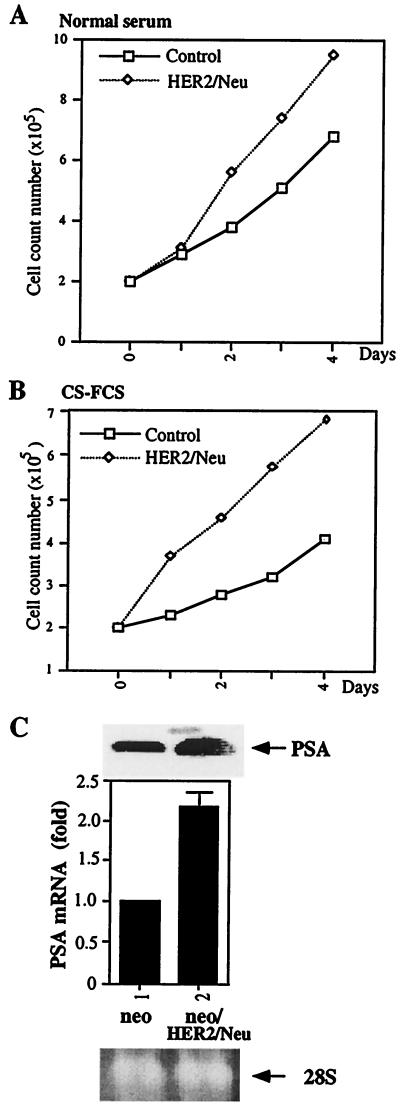
While the expression of HER2/Neu has been detected in prostatic interepithelial neoplasia (8, 9), a direct linkage between the expression of HER2/Neu and increased proliferation of prostate cancer cells is still lacking. We established a G418-resistant LNCaP cell line with a stable transfection of HER2/Neu or its vector pCMVneo. Western blotting was used to confirm the expression of HER2/Neu (data not shown). A growth assay for these two cell lines (Fig. 1 A and B) showed that the growth rate of HER2/Neu stably transfected cells was 50% and 100% greater compared with parent vector-transfected cells in FBS and CS-FBS, respectively.
Figure 1.
The growth curve and the expression of PSA in HER2/Neu stable transfected LNCaP. A HER2/Neu stable transfected LNCaP cell line was established by transfection with HER2/Neu or its parent vector under 400 μg/ml G418 selection. (A and B) Cells were seeded at 2 × 105 on a six-well plate. The cells were counted every 24 h by MTT assay. Each count represents the average of four independent experiments. (C) The blot containing 30 μg of total RNA isolated from cells on each lane was hybridized with a PSA cDNA probe. The 28S RNA shows the equal loading of RNA.
The effect of HER2/Neu transfection of LNCaP cells was confirmed by increased expression of PSA. As shown in Fig. 1C, PSA mRNA expression was increased by transfection of HER2/Neu. ELISAs for PSA protein secretion further confirm HER2/Neu could induce PSA expression in LNCaP cells (data not shown). To test whether the increased PSA is due to the increase of AR protein level, half of the cultured cells were applied for the Western blot analysis of the AR expression. Our results indicated that the AR protein level could not be changed by the transfection of HER2/Neu (data not shown). Taken together, our data suggest the growth of prostate cells and expression of PSA in prostate cells can be increased by the transfection of HER2/Neu, and transfection of HER2/Neu did not alter the expression of AR.
Induction of AR-Mediated Transactivation by HER2/Neu in Different Prostate Cancer Cells.
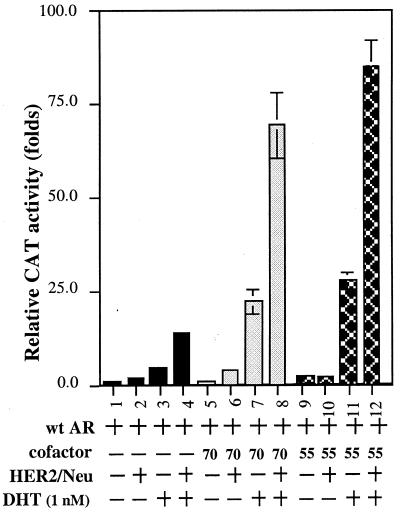
As HER2/Neu could induce PSA expression and PSA has been well characterized as an androgen target gene, we were interested in knowing whether HER2/Neu might induce PSA expression through the modulation of AR transactivation. As shown in Fig. 2, AR transactivation could be induced in DU145 cells 4- to 5-fold in the presence of 1 nM DHT (lanes 1 vs. 3). Addition of HER2/Neu further potentiated this AR-mediated transactivation (lanes 3 vs. 4). Similar induction effects also occurred when we replaced DU145 cells with LNCaP cells (data not shown). As our earlier reports (21–24) showed that AR coactivators could enhance AR-mediated transactivation, we were interested in determining the potential linkage between HER2/Neu and the ARAs. Two cofactors, ARA55 and ARA70N, were applied for this experiment. As shown in Fig. 2, the addition of HER2/Neu or ARA70N could increase AR transactivation from 5-fold to 15-fold (lanes 3 vs. 4) and from 5-fold to 24-fold (lanes 3 vs. 7), respectively. Simultaneous addition of HER2/Neu and ARA70N could increase AR transactivation synergistically from 5-fold to 70-fold (lanes 3 vs. 8). Similar synergistic induction results were also obtained when we replaced ARA70N with ARA55 (from 5-fold to 86-fold, lanes 3 vs. 12). Together, our data demonstrate that HER2/Neu could induce AR transactivation and that this signal pathway from HER2/Neu to AR might involve some AR coactivators, such as ARA70 or ARA55.
Figure 2.
Induction of AR transactivation by HER2/Neu in the presence of androgen in DU145 cells. One microgram of human AR alone or 2.5 μg of pCMVHER2/Neu and/or 2.5 μg of pSG5ARAs was transfected into DU145 cells. PSA-CAT, which contains the 2.8 kb 5′-promoter region of PSA gene, was used as the AR-target reporter gene. Cells were treated with 1 nM DHT after transfection. Cells were harvested after 24 h for CAT assay, and relative CAT activity was normalized by the β-galactosidase activity and quantitated by image- quant software. Data represent an average of three independent experiments.
Androgen-Dependent and Androgen-Independent Induction of AR Transactivation by HER2/Neu.
The popular treatment for prostate cancer is to use antiandrogens in combination with surgical or medical castration, which will decrease the serum testosterone concentration to less than 10−10 M. While the response rate to such androgen ablation therapy is high, the effect is often short-lived and the patients’ serum PSA will increase again despite the low castration level of androgen (29).
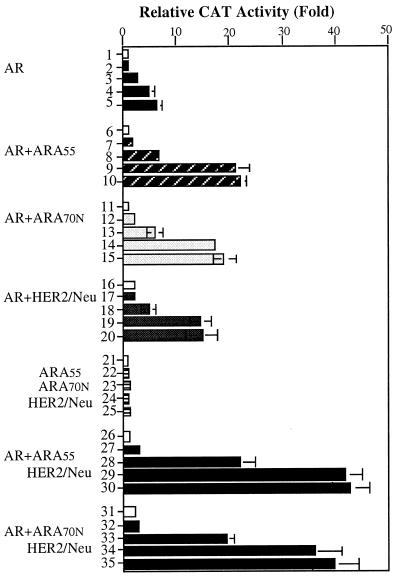
It was of great interest to learn whether HER2/Neu could increase the PSA at this low level of androgen. First, as shown in Fig. 2, HER2/Neu could activate AR transactivation 3-fold in the presence of AR and ARA70N, without addition of androgen (lanes 5 vs. 6). Second, at 10−11 to 10−10 M DHT, the very low androgen concentration of patients undergoing androgen ablation therapy, our data also showed HER2/Neu could induce AR transactivation, in the presence of ARA70N or ARA55 (Fig. 3, lanes 29 and 34 vs. 9 and 14). In the absence of AR, the cotransfection of ARA55, ARA70N, and HER2/Neu could not induce any transcriptional activity (lanes 21–25). This result indicated that AR is essential for this HER2/Neu-mediated AR activity. Together, these data provide one possible mechanism to explain why PSA may increase despite the low androgen level in the patients’ serum. The HER2/Neu-stimulated AR transactivation at this stage may explain the re-evaluation of PSA.
Figure 3.
Effects of androgen concentration on the HER2/Neu-AR-mediated transactivation. The DU145 cells were transfected with or without 1 μg of human pSG5AR, and/or 2.5 μg of pSG5ARA70 or ARA55, and/or 2.5 μg of pCMV-HER2/Neu (lanes 1–5, transfected with AR only; lanes 6–10, AR and ARA55 only; lanes 11–15, AR and ARA70N only; lanes 16–20, AR and HER2/Neu only; lanes 21–25, ARA70N, ARA55, and HER2/Neu only, without AR; lanes 26–30, AR, ARA55, and HER2/Neu; and lanes 31–35, AR, ARA70N, and HER2/Neu). PSA-CAT was applied as the AR-target reporter gene. After transfection the DU145 cells were treated with serial concentrations of DHT from 10−12 M to 10−9 M for another 24 h (mock: lanes 1, 6, 11, 16, 21, 26, and 31; 10−12 M: lanes 2, 7, 12, 17, 22, 27, and 32; 10−11 M: lanes 3, 8, 13, 18, 23, 28, and 33; 10−10 M: lanes 4, 9, 14, 19, 24, 29, and 34; 10−9 M: lanes 5, 10, 15, 20, 25, 30, and 35). Data represent an average of three independent experiments.
Roles of MAP Kinase in HER2/Neu-Mediated AR Transactivation.
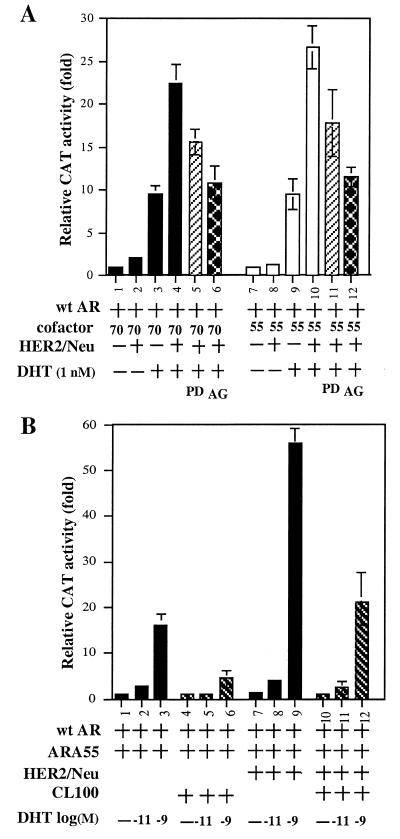
As HER2/Neu is a membrane tyrosine kinase, we were interested in knowing which downstream kinase cascade could mediate the HER2/Neu → AR-ARAs pathway. We first demonstrated that AR transactivation could be repressed by adding 10 μM AG879, an inhibitor of HER2/Neu (30), in the presence of AR and ARAs (Fig. 4A, lanes 4 vs. 6; lanes 10 vs. 12). We then demonstrated that AR transactivation could also be inhibited by adding 15 μM PD98059 (31), which suppresses the activation of MAP kinase by preventing the activation of its upstream activator MAPK kinase-1 (MKK-1) (Fig. 4A, lanes 4 vs. 5; lanes 10 vs. 11). Furthermore, our data showed that the expression of MAP kinase phosphatase 1 (MKP-1, also known as CL100) (32), could also inhibit the HER2/Neu-induced AR transactivation (Fig. 4B lanes 3 vs. 6; lanes 9 vs. 12). Taken together, results from the three experiments described in Fig. 4 provide strong evidence that HER2/Neu-induced AR transactivation is mediated through the MAP kinase pathway. To our knowledge, this is the first evidence that is able to link HER2/Neu through MAP kinase to AR-ARAs and may represent a new signal cascade in the induction of PSA expression.
Figure 4.
(A) Inhibition of the HER2/Neu-mediated AR transactivation by AG879 or PD98059. (B) Suppression of AR transactivation by CL100. (A) One microgram of human pSG5AR or 3 μg of pSG5ARAs and/or 3 μg of pCMV-HER2/Neu was transfected into DU145 cells. MMTV-CAT was used as the AR-target reporter gene. After transfection, the cells were treated with or without DHT, 10 μM AG879, or 15 μM PD98059 for another 24 h, and cells were then harvested for CAT assay. (B) One microgram of human pSG5AR or 3 μg of pSG5ARA55 and/or 3 μg of pCMV HER2/Neu plus CL100 was transfected into DU145 cells for 24 h, and the cells were mock treated or treated with 10−11 or 10−9 M DHT for 24 h and then harvested for CAT assay. Values represent the mean ± SD of at least three determinations.
Phosphorylation of AR by MAP Kinase.
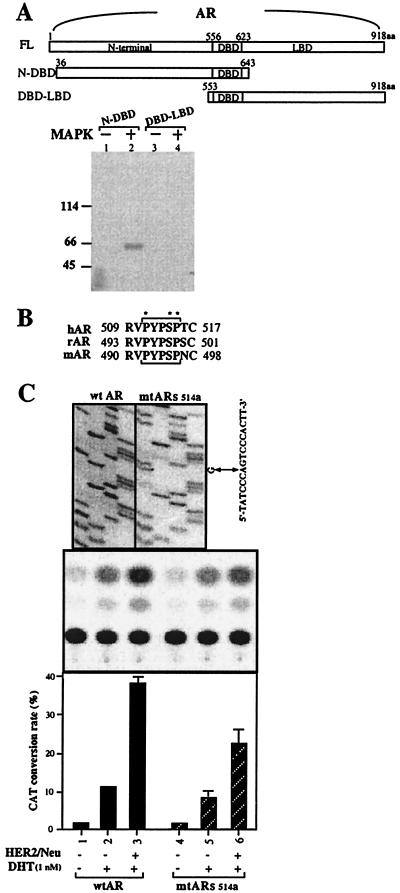
To prove AR could be phosphorylated by MAP kinase, two purified Escherichia coli expressed AR peptides that cover most of the N-terminal and DNA-binding domains (N-DBD, amino acids 36–643), as well as DNA-binding domain and ligand-binding domain (DBD-LBD, amino acids 553–918) were used as the substrates for the MAP kinase (ERK2). As shown in Fig. 5A, the N-DBD AR peptide, but not the DBD-LBD AR peptide, could be phosphorylated by the MAP kinase. Together, these data not only showed that AR could be the target for the MAP kinase but further strengthened our above results (Fig. 4) that HER2/Neu could induce AR transactivation through the MAP kinase system. They also suggested that the MAP kinase phosphorylation site on AR is located in the N-terminal segment (amino acids 36–552). The amino acid surveys further identify a consensus MAP kinase phosphorylation site [PXn(S/T)P, where X is a neutral or basic amino acid and n = 1 or 2] located at amino acids 511–515 (PYPSP) that is conserved among human, rat, and mouse ARs (Fig. 5B). Site-directed mutagenesis studies confirmed that this consensus site might play important roles in the HER2/Neu-MAP kinase-mediated AR transactivation. Compared with wtAR, the mutant AR (mtARs514a, with codon 514 changed from serine to alanine) has lower HER2/Neu-mediated AR transcriptional activity (Fig. 5C, lane 6 vs. 3).
Figure 5.
Mapping the phosphorylation site on AR. (A) The N terminus of AR, but not the DNA-binding domain–ligand-binding domain (DBD-LBD), was phosphorylated by the MAP kinase. (B) The conserved MAP kinase site on human, rat, and mouse AR. (C) Site-directed mutagenesis of the conserved MAP kinase site on AR. (A) The purified AR peptides, N-DBD (N-terminal AR from amino acid 36 to 643), and DBD-LBD (C-terminal AR from amino acid 153 to 918) were phosphorylated by 0.05 pmol of MAPK2 and subjected to SDS/10% PAGE. (B) The conserved MAP kinase site (PYPSP) on human, rat, and mouse AR (hAR, rAR, and mAR). These five amino acids, PYPSP, correspond to the consensus phosphorylation site [PXn(S or T)P, where X is a neutral or basic amino acid and n = 1 or 2] for MAP kinase. (C) The nucleotide sequence of wtAR and mtARs514a is shown in Top. DU145 cells were cotransfected with MMTV-CAT with wtAR or mtARs514a in the absence of or in the presence of HER2/Neu. Middle is a chromatogram of CAT reaction products. The results are quantified in Bottom, in which each bar represents the mean of three independent experiments.
Promotion of the Interaction of AR and ARAs by HER2/Neu.
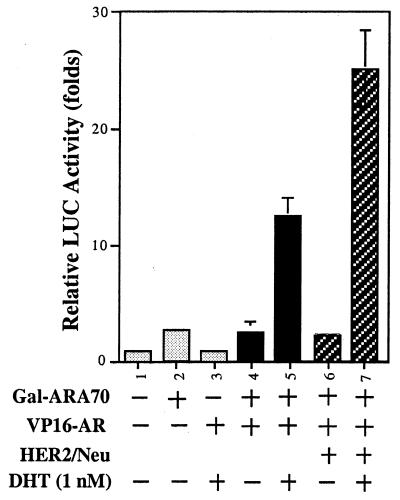
To further study the molecular mechanism of how HER2/Neu can induce AR transactivation, we used a mammalian two-hybrid system to study the potential effects of HER2/Neu on the interaction of AR and ARA70. Gal4DBD fused to ARA70 amino acids 176–401 (Gal4-ARA70aa176–401) and VP16-AR fusion were transfected into DU145 cells in the presence or the absence of HER2/Neu. As shown in Fig. 6, transient transfection of AR or ARA70 alone showed basal activity (lanes 2 and 3 vs. 1). However, the CAT activity could be induced by cotransfection of AR and ARA70 in the presence of 1 nM DHT. Interestingly, addition of HER2/Neu further promoted the interaction of AR and ARA70 (lanes 7 vs. 6). Together, these data indicate the induction of AR transactivation by HER2/Neu may involve the promotion of interaction between AR and ARAs.
Figure 6.
Promotion of the interaction of AR and ARAs by HER2/Neu. The DU145 cells were transiently cotransfected with 2.5 μg of reporter plasmids, pG5-LUC, 2.5 μg of Gal4DBD fused to ARA70 amino acids 176–401 (Gal4-ARA70), and 2.5 μg of VP16-AR fusion in the presence or the absence of HER2/Neu. DHT was then added at 1 nM for 24 h before cells were harvested for luciferase (LUC) assay.
Effects of Antiandrogen on the HER2/Neu-Induced AR Transactivation.
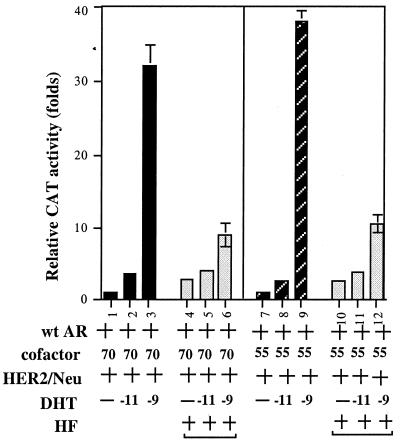
We were able to demonstrate that HER2/Neu could increase PSA at a very low androgen concentration, a condition very similar to the prostate cancer patients undergoing androgen ablation therapy. Thus, we were interested in knowing whether HF, a popular antiandrogen used in androgen ablation therapy, could have any inhibiting effects on this newly discovered HER2/Neu-induced AR transactivation. Unexpectedly, as shown in Fig. 7, HF could partially inhibit AR transactivation in the presence of ARAs and HER2/Neu at 10−9 M DHT (lane 3 vs. 6; lane 9 vs. 12). However, we found HF had some androgenic activity and failed to block AR transactivation in the presence of ARAs and HER2/Neu at 10−11 M DHT (lane 2 vs. 5; lane 8 vs. 11). These findings may therefore provide one possible mechanism to explain the failure of androgen ablation therapy and why PSA eventually increases again in prostate cancer patients.
Figure 7.
Antiandrogen cannot completely block the HER2/Neu-induced AR transactivation. PSA-CAT activity was determined in DU145 cells cotransfected with the expression plasmids of pSG5AR (1 μg) with or without pSG5ARA55 or pSG5ARA70N (3 μg). Cells were then incubated with ethanol (mock) or DHT in the absence or presence of 2 μM HF for 24 h before cells were harvested for CAT assay. The mock treatment was set as 1-fold. Values are the mean (±SD) of at least three determinations.
DISCUSSION
Overexpression of HER2/Neu has been found in some human breast and ovarian cancers and used as an indicator for the resistance to antiestrogens such as tamoxifen (17). However, there is still no strong evidence to link the overexpression of HER2/Neu to prostate cancer growth or increase of AR transactivation. It is also unclear whether elevated HER2/Neu is a cause of or results from prostate cancer progression.
A recent report from Qiu et al. (33) has identified IL-6 as one of the upstream inducers for HER2/Neu; the linkage from HER2/Neu to AR function, however, remains unknown. Here, we demonstrated that HER2/Neu could increase growth rate, PSA level, and AR transactivation in prostate cancer cells via the HER2/Neu → MAP kinase → AR–ARAs pathway.
Early reports also suggested that other growth factors, such as insulin-like growth factor 1, EGF, and keratinocyte growth factor, were able to induce AR-mediated reporter genes in DU145 cells (16). The issue whether growth factors can induce AR target genes in the absence of androgens remains unclear. Even our data (Fig. 2, lane 5 vs. 6) showed that HER2/Neu could activate AR transactivation without addition of exogenous androgens. The contamination of androgens in experiments, either from charcoal-treated serum or from other media and reagents, remains unclear. It is also very difficult to define a condition as an “absence of androgen” in the human body. Prostate cancer patients undergoing maximal androgen ablation (either surgical or medical castration, plus HF or casodex) still have low androgen levels (less than 10−10 M) in their serum. The importance of induction of AR transactivation at this castrated and low androgen level, as compared with “absence of androgen” should, therefore, have much more physiological and clinical relevance.
In the current report, as both PD98059 and CL100 could not completely block the HER2/Neu-stimulated AR–ARA activity (Fig. 4 A and B), it is possible that HER2/Neu may also function through some unknown signal cascade other than MAP kinase to induce the AR transactivation.
Our conclusion that HER2/Neu could induce AR transactivation at a low androgen level not only provides one possible explanation of the increase of PSA in hormone-refractory prostate cancer patients and the failure of androgen ablation therapy but also may provide the following two potential future developments. First, overexpression of some components from the HER2/Neu to AR signal pathway, such as HER2/Neu, MAP kinase, or ARAs, could be developed as markers to monitor the progression of prostate cancer and used as an early indication for the beginning of failure of androgen ablation therapy. Second, some therapeutic drugs that can suppress HER2/Neu, MAP kinase, or ARAs could be developed to battle prostate cancer.
In summary, the novel signal pathway from HER2/Neu → MAP kinase → AR–ARAs → PSA described here may have direct clinical linkage for the progression of prostate cancer.
Acknowledgments
This work was supported by National Institutes of Health Grants CA55639, CA68568, and CA75732.
ABBREVIATIONS
- EGF
epidermal growth factor
- AR
androgen receptor
- DHT
5α-dihydrotestosterone
- PSA
prostate-specific antigen
- HF
hydroxyflutamide
- mtAR
mutant androgen receptor
- ARA
androgen receptor coactivator
- MMTV
mouse mammary tumor virus
- CAT
chloramphenicol acetyltransferase
- MTT
thiazolyl blue
References
- 1.Harris A L, Nicholson S, Sainsbury R, Wright C, Farndon J. J Natl Cancer Inst Monogr. 1992;11:181–187. [PubMed] [Google Scholar]
- 2.Kwong K Y, Hung M C. Mol Carcinog. 1998;23:62–68. doi: 10.1002/(sici)1098-2744(199810)23:2<62::aid-mc2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Gullick W J, Berger M S, Bennett P L, Rothbard J B, Waterfield M D. Int J Cancer. 1987;40:246–254. doi: 10.1002/ijc.2910400221. [DOI] [PubMed] [Google Scholar]
- 4.Press M F, Cordon-Cardo C, Slamon D J. Oncogene. 1990;5:953–962. [PubMed] [Google Scholar]
- 5.Muller W J, Sinn E, Pattengaale P K, Leader P. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 6.Sikes R A, Chung L W. Cancer Res. 1992;52:3174–3181. [PubMed] [Google Scholar]
- 7.Miller S J, Hung M C. Oncol Rep. 1995;2:497–503. doi: 10.3892/or.2.4.497. [DOI] [PubMed] [Google Scholar]
- 8.Myers R B, Brown D, Oelschlager D K, Waterbor J W, Marshall M E, Srivastava S, Stockard C R, Urban D A, Grizzle W E. Int J Cancer. 1996;69:398–402. doi: 10.1002/(SICI)1097-0215(19961021)69:5<398::AID-IJC8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Robinson D, He F, Pretlow T, Kung H J. Proc Natl Acad Sci USA. 1996;93:5958–5962. doi: 10.1073/pnas.93.12.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C S, Kokontis J, Liao S T. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 11.Trachtenberg J. Eur Urol. 1997;32, Suppl. 3:78–80. [PubMed] [Google Scholar]
- 12.Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee H J, Wang C, Mizokami A. Crit Rev Eukaryot Gene Expr. 1995;5:97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z X, Kemppainen J A, Wilson E M. Mol Endocrinol. 1995;9:605–615. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]
- 14.Ikonen T, Palvimo J J, Kallio P J, Reinikainen P, Janne O A. Endocrinology. 1994;135:1359–1366. doi: 10.1210/endo.135.4.7925097. [DOI] [PubMed] [Google Scholar]
- 15.Nazareth L V, Weigel N L. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 16.Culig Z, Hobisch A, Cronauer M V, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 17.Leitzel K, Teramoto Y, Konrad K, Chinchilli V M, Volas G, Grossberg H, Harvey H, Demers L, Lipton A. J Clin Oncol. 1995;13:1129–1135. doi: 10.1200/JCO.1995.13.5.1129. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn E J, Kurnot R A, Sesterhenn I A, Chang E H, Moul J W. J Urol. 1993;150:1427–1433. doi: 10.1016/s0022-5347(17)35799-3. [DOI] [PubMed] [Google Scholar]
- 19.Myers R B, Srivastava S, Oelschlager D K, Grizzle W E. J Natl Cancer Inst. 1994;86:1140–1145. doi: 10.1093/jnci/86.15.1140. [DOI] [PubMed] [Google Scholar]
- 20.Ware J L, Maygarden S J, Koontz W W, Jr, Strom S C. Hum Pathol. 1991;22:254–258. doi: 10.1016/0046-8177(91)90159-m. [DOI] [PubMed] [Google Scholar]
- 21.Yeh S, Chang C. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimoto N, Yeh S, Kang H Y, Inui S, Chang H C, Mizokami A, Chang C. J Biol Chem. 1999;274:8316–8321. doi: 10.1074/jbc.274.12.8316. [DOI] [PubMed] [Google Scholar]
- 23.Kang H Y, Yeh S, Fujimoto N, Chang C. J Biol Chem. 1999;274:8570–8576. doi: 10.1074/jbc.274.13.8570. [DOI] [PubMed] [Google Scholar]
- 24.Yeh S, Miyamoto H, Nishimura K, Ludlow J, Wang C, Hsiao P, Su C, Chang C. Biochem Biophys Res Commun. 1998;248:361–367. doi: 10.1006/bbrc.1998.8974. [DOI] [PubMed] [Google Scholar]
- 25.Yeh S, Miyamoto H, Chang C. Lancet. 1997;348:852–853. doi: 10.1016/S0140-6736(05)61756-4. [DOI] [PubMed] [Google Scholar]
- 26.Yeh S, Miyamoto H, Shima H, Chang C. Proc Natl Acad Sci USA. 1998;95:5524–5532. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyamoto H, Yeh S, Lardy H, Messing E, Chang C. Proc Natl Acad Sci USA. 1998;95:11083–11088. doi: 10.1073/pnas.95.19.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alessi D R, Cohen P, Ashworth A, Cowley S, Leevers S J, Marshall C J. Methods Enzymol. 1995;255:279–290. doi: 10.1016/s0076-6879(95)55031-3. [DOI] [PubMed] [Google Scholar]
- 29.Crawford E D, Eisenberger M A, McLeod D G, Spaulding J-T, Benson R, Dorr F A, Blumenstein B A, Davis M A, Goodman P J. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 30.Levitzki A, Gazit A. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 31.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 32.Alessi D R, Smythe C, Keyse S M. Oncogene. 1993;8:2015–2020. [PubMed] [Google Scholar]
- 33.Qie Y, Ravi L, Kung H-J. Nature (London) 1998;393:83–85. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]