Abstract
Homologous desensitization of G protein-coupled receptors is thought to occur in several steps: binding of G protein-coupled receptor kinases (GRKs) to receptors, receptor phosphorylation, kinase dissociation, and finally binding of β-arrestins to phosphorylated receptors. It generally is assumed that only the last step inhibits receptor signaling. Investigating the parathyroid hormone (PTH) receptor → inositol phosphate pathway, we report here that GRKs can inhibit receptor signaling already under nonphosphorylating conditions. GRKs phosphorylated the PTH receptor in membranes and in intact cells; the order of efficacy was GRK2>GRK3>GRK5. Transient transfection of GRKs with the PTH receptor into COS-1 cells inhibited PTH-stimulated inositol phosphate generation. Such an inhibition also was seen with the kinase-negative mutant GRK2-K220R and also for a C-terminal truncation mutant of the PTH receptor that could not be phosphorylated. Several lines of evidence indicated that this phosphorylation-independent inhibition was exerted by an interaction between GRKs and receptors: (a) this inhibition was not mimicked by proteins binding to G proteins, phosducin, and GRK2 C terminus, (b) GRKs caused an agonist-dependent inhibition (= desensitization) of receptor-stimulated G protein GTPase-activity (this effect also was seen with the kinase-inactive GRK2-mutant and the phosphorylation-deficient receptor mutant), and (c) GRKs bound directly to the PTH receptor. These data suggest that signaling by the PTH receptor already is inhibited by the first step of homologous desensitization, the binding of GRKs to the receptors.
Prolonged exposure of cells to hormones or neurotransmitters often leads to a blunting of second messenger responses, a process generally termed homologous (or agonist-specific) desensitization (1, 2). Desensitization is thought to adapt cellular responsiveness to high agonist concentrations and to prevent overstimulation of the cells in the continued presence of these agonists. For the large family of G protein-coupled receptors (3, 4) several mechanisms occurring at the receptor level are known to be involved in desensitization. These mechanisms include first phosphorylation either by second messenger-dependent kinases like protein kinase A (PKA) and protein kinase C (PKC) or by G protein-coupled receptor kinases (GRKs) (5), second sequestration of receptors from the cell surface after agonist activation (6), and third down-regulation of the total number of receptors (7).
The GRKs represent a family of kinases with six members termed GRK1–6 (8). Their unique feature is the specific phosphorylation of only agonist-activated G protein-coupled receptors, but they differ in tissue distribution, posttranslational modifications, and mechanisms of membrane targeting. GRK-mediated receptor phosphorylation facilitates binding of the arrestins, or in the case of nonvisual systems, the β-arrestins (9). The ability of GRK-phosphorylated receptors to activate G proteins is essentially normal, but the binding of β-arrestins to the phosphorylated receptors inhibits G protein activation by receptors (10, 11). Accordingly, it generally is assumed that receptor deactivation primarily is exerted by the β-arrestins, and that the GRKs only enable β-arrestin to bind and to exert its inhibitory function (2, 12, 13). However, this facilitation of β-arrestin binding may not be the only function of GRKs in this process. In fact, kinetic experiments in the rhodopsin/rhodopsin kinase system (14, 15) suggest that binding of the specific rhodopsin kinase may deactivate rhodopsin. Thus, we wondered whether GRKs might inhibit hormone receptor functions before receptor phosphorylation and binding of β-arrestins.
Our studies were done with the receptor for parathyroid hormone (PTH). In contrast to the prototypical β-adrenergic receptors, this receptor belongs to the class II of G protein-coupled receptors and stimulates the formation of both inositol-1,4,5-trisphosphate and cAMP (16). Agonist-induced desensitization of the PTH receptor is a well-established phenomenon (17–20). Based on experiments in various cell lines it is clear that the second-messenger-activated kinases PKC and PKA are involved in this process, but several findings suggest the presence of another component that might be GRK mediated (21, 22). This situation led us to study the effects of GRKs on the PTH receptor. In the course of these studies we realized that the signaling function of this receptor could be inhibited by GRKs even when no GRK-mediated phosphorylation of the receptors occurred.
MATERIALS AND METHODS
Materials.
Taq DNA polymerase and 12CA5 mAb were obtained from Boehringer Mannheim. Human (Nle8,18, Tyr34)-PTH(1–34), here always referred to as PTH, was from Bachem. Human [125I]-(Nle8,18, Tyr34)-PTH-(1–34) (3,000 Ci/mmol) was from Amersham Pharmacia; all other radiochemicals were from DuPont/NEN.
Plasmid Constructs.
A mutant of the PTH receptor truncated at position 480 (T480) was constructed by PCR with the human wild-type receptor cDNA in a pCMV-plasmid serving as template. The truncation comprised the last 113 aa of the receptor leaving only 16 of the C terminus. A hemagglutinin (HA) tag was inserted into the N terminus of the wild-type and T480 mutant PTH receptors by using PCR-based mutagenesis as described (23).
The cDNAs for GRK2 (24), GRK3 (25), and GRK5 (26) all were cloned into pcDNA expression vectors (Invitrogen). A pCMV5 vector for the kinase-inactive GRK2-K220R (27) was provided by S. Cotecchia (Université Lausanne, Switzerland). Phosducin was expressed by using the vector pBC-phd-dhfr (28). The expression vector pRK-βARK1-(495–689) for the expression of the GRK2 C terminus was provided by R.J. Lefkowitz (Duke University, Durham, NC) (29).
Generation of PTH Receptor-Expressing Cell Lines.
The human PTH receptor was stably expressed under the control of the late cytomegalovirus promoter in CHO(dhfr−) cells. Expression levels determined by saturation studies with [125I]PTH were ≈3 × 106 receptors per cell. The cells were cultured in complete MEMα with 10% FCS and 1 mg/ml of G418.
Transient Expression in COS-1 Cells.
COS-1 cells cultured in DMEM with 10% FCS were transfected by the DEAE-dextran method (30). The total amount of transfected DNA was 8 μg/100-mm dish, composed of 6 μg of receptor DNA and 2 μg of variable DNA. The expression vector pCMVβ (CLONTECH) for β-galactosidase was used as control. Twenty-four hours after transfection, cells were split, and assays were done 48–60 h after transfection. Average expression levels for wild-type, HA-tagged, and T480 receptors were 35.5 ± 0.5, 18 ± 0.2, and 28 ± 1.2 pmol/mg of protein, respectively.
Cell Membrane Preparation.
Cells were harvested, lysed in buffer A (20 mM Tris, pH 7.4/2 mM EDTA/2 μg/ml aprotinin/10 μg/ml benzamidine/100 μg/ml Pefabloc) and homogenized. After centrifugation for 20 min at 40,000 × g, the pellet was resuspended in buffer A with 20% sucrose and layered onto 40% sucrose in buffer A. After centrifugation at 126,000 × g for 1 h the 20%/40% interface was recovered, diluted 1+1 with buffer A, and centrifuged for 15 min at 126,000 × g. When membranes were prepared for phosphorylation assays, the pellet was resuspended in 5 M urea, incubated for 10 min at 20°C, and centrifuged at 126,000 × g for 15 min. After two washing steps in buffer A, the final pellet was resuspended in the same buffer and stored at −80°C.
Expression and Purification of GRKs.
GRK2, GRK3, and GRK5 were expressed in Sf9 insect cells (31, 32). The corresponding recombinant baculoviruses were generated by using the Baculogold system (PharMingen). The GRKs were purified by using successive chromatography on SP-Sepharose and heparin-Sepharose columns (33). Their specific activities were determined with rhodopsin as the substrate; using 10 nM kinase, 2.4 μM rhodopsin, and 100 μM ATP, specific activities were 0.98 ± 0.30, 0.64 ± 0.11, and 0.75 ± 0.23 mol Pi/min per mol of kinase for GRK2, GRK3, and GRK5, respectively.
Membrane Phosphorylation Assays.
Receptor-containing cell membranes (2 pmol receptor/5 μg protein) were incubated with purified kinases (100 nM) in 20 mM Tris, pH 7.4/2 mM EDTA/8 mM MgCl2 containing 100 μM [γ-32P]ATP (1–2 cpm/fmol) in a final volume of 25 μl. PTH was added as indicated, and the incubations lasted for usually 30 min at 30°C. Phosphorylation of rhodopsin in rod outer segments (31) was done under similar conditions, with [γ-32P]ATP at 0.3–0.9 cpm/fmol, 10 nM kinase, and 2.4 μM rhodopsin. Reactions were stopped by addition of 5× Laemmli buffer followed by electrophoresis on SDS-polyacrylamide gels and visualization and quantification of 32P-labeled proteins by PhosphorImager analysis.
Intact Cell Phosphorylation.
To measure phosphorylation of the HA-tagged PTH receptors in intact cells, transiently transfected COS-1 cells were labeled in 6-well plates with 100 μCi/well of [32P]orthophosphate in phosphate-free DMEM for 2 h. Labeled cells were stimulated as indicated, solubilized in 0.8 ml of RIPA buffer (1% NP-40/0.5% Na-deoxycholate/0.1% SDS/50 mM Tris, pH 7.4/100 mM NaCl/2 mM EDTA/50 mM NaF) for 30 min on ice, and the HA-tagged receptors were immunoprecipitated with 10 μg of 12CA5 antibody. Immunoprecipitated proteins were analyzed by SDS/PAGE and PhosphorImager analysis as above.
Crosslinking Experiments.
Crosslinking of GRK2 to the PTH receptor was done in intact COS-1 cells transiently cotransfected with the HA-tagged PTH receptor and GRK2. The cells were washed twice with DMEM and incubated for 10 min at 37°C in DMEM with or without PTH (100 nM). This medium was replaced by 3 ml of DMEM with 2.5 mM dithiobis(succinimidyl propionate) (Pierce) with or without PTH and incubated for 30 min at 25°C. Then, cells were solubilized in 1 ml of RIPA buffer at 4°C for 30 min, and immunoprecipitation of the HA-tagged PTH receptor was done as above. Separation of immune complexes and cleavage of the crosslinker was done for 90 min at 37°C in Laemmli buffer. Immunoprecipitated proteins were resolved by SDS/PAGE and transferred to poly (vinylidene difluoride) membranes (Immobilon-P; Millipore). GRK2 immunoreactivity on the membrane was detected with a rabbit anti-GRK2 antiserum and ECL (Amersham).
Inositol Phosphate Accumulation.
Transiently transfected COS-1 cells were labeled in 12-well plates for 16 h at 37°C with 1 μCi/ml of myo-[2-3H-(N)]-inositol in RPMI medium 1640 without inositol (supplemented as above). After labeling, cells were washed twice with Hepes buffer (137 mM NaCl/5 mM KCl/1 mM CaCl2/1 mM MgCl2/20 mM Hepes, pH 7.3) and incubated in 900 μl/well of the same buffer with 10 mM LiCl for 10 min. Addition of 100 μl of vehicle or agonist initiated the incubation at 37°C for the times indicated. Extraction and determination of total inositol phosphates were done as described (34). Basal values were subtracted to show agonist stimulation.
cAMP Accumulation.
Transiently transfected COS-1 cells were grown in 24-well plates. Forty-eight hours after transfection they were washed with Hepes buffer as above and incubated in the same buffer for 5 min at 37°C with 0.5 mM isobutyl methyl xanthine. They were stimulated (or not) with 10 nM PTH for 15 min at 37°C. The reaction was stopped by addition of boiling water, and the cellular cAMP was determined by RIA (Immunotech).
Binding Assays.
Whole-cell binding experiments with [125I]PTH (35) were done in 100 mM NaCl/5 mM KCl/2 mM CaCl2/2 mM MgCl2/50 mM Tris, pH 7.4/5% horse serum/0.5% FCS/200 μg/ml of bacitracin for 3 h at 4°C. For binding experiments with membrane preparations serum was substituted by 0.1% 3[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. Nonspecific binding was defined by using 10−6 M unlabeled ligand.
Measurement of PTH Receptor-Stimulated Activation of G Protein GTPase Activity.
Forty-eight hours after transfection COS-1 cells were washed twice with serum-free DMEM and incubated in the same medium with 1 μM PTH or vehicle for 5 min at 37°C. The cells were washed for 30 sec with ice-cold glycine-buffer (100 mM NaCl/50 mM glycine, pH 3.0) followed by ice-cold PBS. Cells were lysed in 1 ml/dish of hypotonic buffer (10 mM Tris/2 mM EDTA, pH 7.4) on ice. Membrane preparation and GTPase experiments were done as described (36). GTPase activity was expressed as the value in the presence of 1 μM PTH minus basal values.
RESULTS
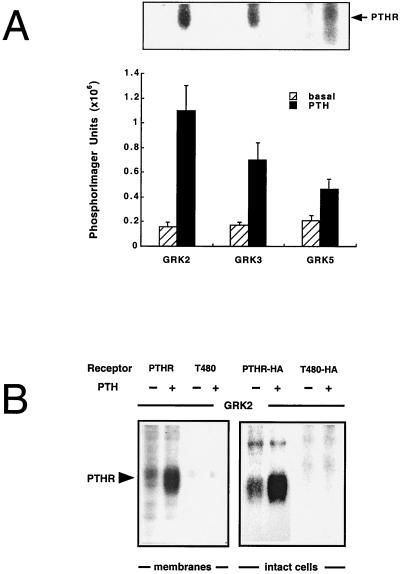
Initially we tested the ability of purified GRKs to phosphorylate the PTH receptor in membrane preparations prepared from stably transfected Chinese hamster ovary cells. The three most widely expressed GRKs (GRK2, GRK3, and GRK5) all were able to phosphorylate the receptors in an agonist-, time-, and kinase-dependent manner (Fig. 1A). Half-maximal and maximal phosphorylation was achieved after 5 and 30 min, and the EC50 value of PTH was 1 μM (not shown). The extent of phosphorylation was 1.1 ± 0.3 mol phosphate/mol receptor. The three GRK isoforms showed an order of efficacy GRK2 > GRK3>GRK5 (Fig. 1A). PTH-stimulated receptor phosphorylation by GRK2 was 1.8-fold higher than by GRK3 and 3.6-fold higher than by GRK5. A glutathione S-transferase fusion protein containing the entire intracellular C terminus (133 aa) of the PTH receptor also was phosphorylated by the GRKs (not shown), suggesting that the phosphorylation sites might be located in the receptor’s C terminus. Therefore, we constructed a receptor mutant truncated C terminally at amino acid 480 (= T480). Mutant and wild-type PTH receptors were transiently transfected into COS-1 cells, which resulted in similar expression levels for wild-type and mutant receptors. PTH-stimulated inositol phosphate production was somewhat higher in the T480 mutant (Table 1). When membranes from these cells were prepared and subjected to GRK2-mediated phosphorylation, the wild-type, but not the T480, receptor was phosphorylated by GRK2 (Fig. 1B), indicating that indeed the phosphorylation sites were located in the receptor’s C terminus. The T480 mutant receptor was, in addition, not phosphorylated by the second-messenger-activated kinases PKA and PKC (not shown).
Figure 1.
Phosphorylation of the PTH receptor (PTHR) by GRKs in cell membrane preparations and in intact cells. Membrane preparations of Chinese hamster ovary cells stably expressing the PTH receptor (A) or COS-1 cells transiently expressing the wild-type or the C terminally truncated T480 PTH receptors (B, Left) were phosphorylated with 100 nM GRK2, GRK3, or GRK5 (A) or 100 nM GRK2 (B) for 30 min without or with 10 μM PTH. The reaction products were resolved on SDS-polyacrylamide gels, and autoradiograms of these gels are shown. 32P-incorporation was quantitated by PhosphorImager analysis (A). Phosphorylation by GRK2 was 1.1 ± 0.3 mol 32P/mol receptor (as determined by radioligand binding). Data in A represent the mean ± SE of four independent experiments. Phosphorylation of HA-tagged PTH receptors in intact [32P]orthophosphate-labeled COS-1 cells (B, Right) was achieved by incubating cells with 100 nM PTH for 5 min, followed by solubilization and immunoprecipitation of the receptors with 12CA5 antibody, SDS/PAGE, and autoradiography.
Table 1.
Characteristics of wild-type and mutant PTH receptors
| Receptor |
125I-PTH binding
|
Inositol phosphate production
|
||
|---|---|---|---|---|
| KD, nM | Bmax, pmol/mg protein | EC50, nM | Max. stimulation, fold | |
| PTHR | 29 ± 3.1 | 35.5 ± 0.5 | 34.3 ± 6.4 | 4.9 ± 1.0 |
| T480 | 14 ± 1.2 | 28.0 ± 1.2 | 12.0 ± 1.3 | 7.0 ± 1.4 |
| PTHR-HA | 28 ± 3.4 | 18.1 ± 0.2 | 29.6 ± 3.0 | 2.3 ± 0.3 |
Experiments were done in transiently transfected COS-1 cells overexpressing the indicated receptor. Values are mean ± SE (n = 3–9).
For further studies we selected a COS-1 cell clone with very low endogenous GRK expression (see below). In these intact cells, GRK-mediated phosphorylation was identified by using a PTH receptor carrying a HA tag in its N terminus to allow immunoprecipitation. This HA-tagged PTH receptor had binding and signaling properties comparable to those of the wild-type receptor, but the expression levels and the maximal stimulation of inositol phosphate production were only 50% of the untagged receptor. In cells cotransfected with GRKs this receptor was phosphorylated in an agonist-dependent manner (Fig. 1B). As in membranes, the HA-tagged truncated T480 receptor showed no phosphorylation either in the absence or presence of PTH (Fig. 1B).
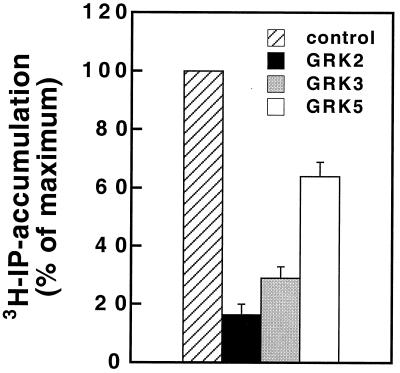
The functional consequences of GRK cotransfection then were tested by measuring PTH receptor-mediated production of inositol phosphates. All three GRKs caused a reduction in inositol phosphate production, and the order of efficacy was again GRK2>GRK3>GRK5 (Fig. 2). These data are entirely compatible with the model that GRK-mediated receptor phosphorylation triggers inhibition of receptor function.
Figure 2.
Effects of GRK cotransfection on PTH receptor-mediated [3H]inositol phosphate production. COS-1 cells (100-mm dish) were transiently cotransfected with PTH receptor cDNA (6 μg) and 2 μg of either GRK2, GRK3, or GRK5. Forty-eight hours later, [3H]inositol phosphate production was measured in response to PTH (100 nM) for 60 min at 37°C. The PTH-induced (100 nM) inositol phosphate production in the absence of exogenous GRKs was set to 100%, which corresponds to a 4.0 ± 0.6-fold stimulation. Data are mean ± SE from four independent experiments with duplicate determinations.
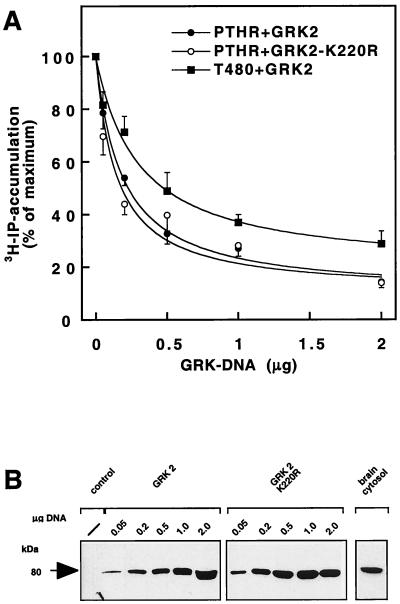
Cotransfection of the PTH receptor with increasing amounts of GRK2 cDNA resulted in a concentration-dependent inhibition in PTH-stimulated inositol phosphate production (Fig. 3A). However, the same inhibitory decrease was observed when GRK2 was replaced by a kinase-inactive GRK2 mutant, GRK2-K220R. Likewise, when the phosphorylation-deficient receptor mutant T480 was used, GRK2 cotransfection also caused a concentration-dependent reduction of inositol phosphate accumulation; compared with the experiments with the PTH receptor and either wild-type or kinase-negative GRK2, the latter curve was shifted to higher concentrations and did not reach quite the same level of inhibition (Fig. 3A).
Figure 3.
Inhibition of PTH receptor (PTHR)-mediated [3H]inositol phosphate production by wild-type and kinase-inactive GRK2. (A) Inositol phosphate experiments were done as in Fig. 2 using 0.05–2 μg cDNA of GRK2 cDNA. Wild-type (PTHR) or C terminally truncated (T480), and wild-type (GRK2) receptor and kinase-inactive (GRK2-K220R) GRK2 were investigated. Data are mean ± SE from four independent experiments with duplicate determinations. (B) Western blots of the same transfections showing expression of GRK2 in untransfected (control) or transfected (GRK2 or GRK2-K220R) COS-1 cells or in mouse brain. All lanes contain ≈200 μg of lysate protein.
An increase in the amount of GRK cDNA correlated with an increase in GRK expression as determined in Western blots (Fig. 3B). Although the endogenous GRK-level in the COS-1 cells was very low, those expressed after transfection were in the range of the GRK levels present in brain (Fig. 3B). In all of these experiments receptor expression was the same as determined by measuring specific binding (not shown).
We also investigated the effects of GRK cotransfection on the second signaling pathway of the PTH receptor, generation of cAMP. Cotransfection of the PTH receptor with GRK2 (2 μg) or of the T480 with GRK2 (2 μg) in COS-1 cells resulted in an inhibition of PTH (10 nM)-stimulated cAMP-accumulation by 22 ± 1% (PTH receptor) or 15 ± 1% (T480), respectively (data not shown).
The inhibitory effects seen in the absence of phosphorylation (i.e., with the kinase-inactive mutant GRK2-K220R or the phosphorylation-deficient T480 receptor mutant) might be exerted at the receptor itself or at the G protein level.
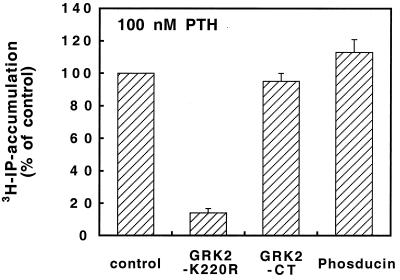
GRKs have been shown to bind with high affinity to G protein βγ subunits via their C-terminal domain (37–39). A similar binding reaction of even higher affinity occurs with the G protein regulatory protein phosducin (40, 41). Neither phosducin nor the C-terminal domain of GRK2 mimicked the inhibitory effects of wild-type or kinase-inactive GRK2 on PTH-induced inositol phosphate production (Fig. 4). These results indicate that these inhibitory effects were unlikely to be exerted at the G protein level, leaving the receptor level as the most likely site of action.
Figure 4.
Effects of GRK2, the GRK2 C terminus, and phosducin on PTH receptor-stimulated [3H]inositol phosphate accumulation. COS-1 cells were transiently transfected with the PTH receptor alone (control) or together with kinase-inactive GRK2-K220R, the G protein βγ-subunit binding GRK2 C terminus (GRK2-CT), or the high-affinity G protein βγ-subunit binding protein phosducin. [3H]inositol phosphate accumulation in response to 100 nM PTH for 1 h was measured. Data are mean ± SE (n = 4).
To verify that indeed signaling was inhibited at the receptor level we studied the stimulation of G protein GTPase activity by PTH receptors in cell membranes. To exclude phosphorylation-dependent effects, these experiments were carried out by using the kinase-inactive GRK-K220R mutant and the truncated receptor-mutant T480, because this mutant was not phosphorylated either by GRKs or by PKA or PKC (see above).
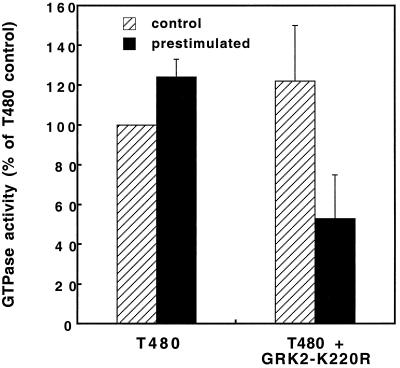
The truncated receptor alone or in combination with GRK2-K220R was transfected into COS-1 cells, the cells were prestimulated or not with 1 μM PTH for 5 min, and then membranes were prepared from these cells and the PTH-stimulated G protein GTPase activity was measured (Fig. 5). When the T480 receptor alone was present, prestimulation of the cells with PTH caused no inhibition of the GTPase response in membranes (Fig. 5). This finding is compatible with the lack of phosphorylation of this receptor mutant by any of the kinases that have been implicated in desensitization, and with the low level of GRKs in these cells. However, cotransfection of the kinase-inactive GRK2-K220R resulted in a more than 50% reduction of the GTPase response in the membranes obtained from cells pretreated with PTH. This loss of responsiveness is typical for agonist-induced or homologous desensitization and indicates that GRK2-K220R exerted its inhibitory effects at the level of the PTH receptor itself.
Figure 5.
Desensitization of the T480 receptor-stimulated GTPase activity. The T480 receptor alone or in combination with GRK2-K220R was transiently transfected into COS-1 cells. Cells were stimulated for 5 min with 1 μM PTH or not (control), PTH was washed away, and the receptor-stimulated GTPase activity was determined in membranes prepared from these cells. Experiments were performed in triplicate, and data are mean ± SE of three independent experiments where GTPase activity of the T480 receptor without prestimulation (0.39 ± 0.11 pmol Pi/min per mg of protein) was set to 100%.
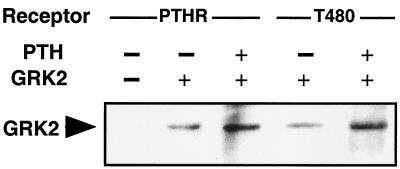
Such an inhibition presumably would be mediated by direct binding of GRK2 to the PTH receptor as well as its T480 mutant. To prove such direct binding, we attempted to crosslink GRK2 to the HA-tagged PTH receptor in intact cells, immunoprecipitate with an anti-HA antibody, and demonstrate the presence of coprecipitated GRK2 in Western blots. Fig. 6 shows that indeed GRK2 was coprecipitated from the cells with either the wild-type PTH receptor (left lanes) or the T480 mutant (right lanes). No such coprecipitation was seen without cotransfection of GRK2 (leftmost lane) or without crosslinker (not shown). Some GRK2 was coprecipitated under basal conditions, but the addition of 100 nM PTH increased the amounts of coprecipitated GRK2 both for wild-type and the T480 receptor. Similar results were obtained when purified GRK2 was crosslinked to PTH receptors in cell membranes (data not shown).
Figure 6.
Crosslinking of GRK2 to the PTH receptor (PTHR). The HA-tagged PTH receptor or the T480 mutant receptor was transfected alone (leftmost lane) or together with GRK2 into COS-1 cells. The cells were incubated for 10 min with or without 100 nM PTH and crosslinking with 2.5 mM dithiobis(succinimidyl propionate) was done as described in Materials and Methods. The receptors were solubilized and immunoprecipitated with anti-HA antibodies, and coprecipitated GRK2 was detected in Western blots.
DISCUSSION
Many G protein-coupled receptors, including the PTH receptor, undergo agonist-induced homologous desensitization, which is thought to be exerted by the sequential action of GRKs and β-arrestins. A role for GRKs in the regulation of the PTH receptors already can be inferred from experiments showing that inhibitors of PKA or PKC only partially inhibit PTH receptor phosphorylation in intact cells (21). Indeed, our studies show time- and agonist-dependent phosphorylation of the PTH receptor by GRKs in isolated membranes as well as in intact cells. The three widely distributed GRKs (2, 13, 42) showed an order of efficacy GRK2>GRK3>GRK5. In the membrane assay a stoichiometry of phosphorylation between 1 and 2 mol phosphate/mol receptor was obtained, a value that is similar to the one obtained for the prototypical β2-adrenergic receptor in a similar membrane preparation (43), even though much higher values have been reported for purified reconstituted β2-adrenergic receptors (44). This finding indicates that the PTH receptor is a good substrate for GRKs. The GRKs were also capable of reducing PTH receptor-mediated inositol phosphate signaling, and again the order of efficacy was GRK2>GRK3>GRK5. All of these data are compatible with the prevailing model of homologous desensitization triggered by GRK-mediated receptor phosphorylation and effected by β-arrestin binding to the phosphorylated receptors.
However, several observations challenge this model, because they show that GRKs could inhibit PTH receptor signaling even without receptor phosphorylation. First, signaling by the C terminally truncated T480 receptor mutant, which was not phosphorylated either by purified GRKs or second-messenger-activated kinases, also was inhibited by GRKs. Second, the kinase-negative mutant GRK2-K220R, shown to act as a dominant-negative mutant in the β2-adrenergic receptor system (45) also inhibited signaling by the PTH receptor. In both cases dampening of inositol phosphate generation correlated with the amount of kinase protein expression. And third, even the combination between phosphorylation-deficient receptor and kinase-inactive GRK2 caused agonist-induced inhibition of receptor function.
Such phosphorylation-independent inhibitory effects of GRKs might be caused by binding either to receptors or to G protein βγ subunits. Transfection with the more potent Gβγ-binding protein phosducin or with the Gβγ-binding C terminus of GRK2 did not inhibit PTH receptor-mediated inositol phosphate generation, suggesting that GRKs do not inhibit PTH receptor signaling via Gβγ binding. Therefore, the inhibition appears to be caused by the formation of a receptor-GRK complex that prevents the receptor from coupling to G proteins.
Indeed, such complex formation could be demonstrated by showing that in intact cells or in membranes GRK2 could be crosslinked to and coimmunoprecipitated with the PTH receptor, and this crosslinking was similar for the T480 truncated receptor. Formation of this complex was enhanced in the presence of agonist, but did not completely depend on receptor stimulation.
Because GRKs phosphorylate only agonist-activated receptors, they must bind to several contact points in the receptor. Multiple contact points are also likely given that some receptors are phosphorylated in their C-terminal domain while others are in the third intracellular loop, and also because the first intracellular loop appears to contain an accessory binding site (2, 42, 46). The point mutant GRK2-K220R impaired signaling of the wild-type PTH receptor with exactly the same cDNA-concentration-response relationship as did the active GRK2. In contrast, this relationship was shifted to higher concentrations when using wild-type GRK2 and the truncated T480 receptor mutant. This difference is compatible with the loss of a contact point in the receptor’s C terminus, where phosphorylation would occur.
Compared with the core of G protein-coupled receptors (<35 kDa), GRKs are large proteins (≈80 kDa). Thus, it is reasonable to assume that their binding to multiple contact points in the receptor will disturb receptor/G protein interactions. The resulting inhibition presumably was not seen in the initial reconstitution experiments with β2-adrenergic receptors and Gs (9, 11), because these were done in several steps and the GRKs were removed before Gs activation was assayed. However, we already have seen that GRK2 can modestly inhibit β-adrenergic receptor-stimulated cAMP production in cell membranes (47). Furthermore, rhodopsin function appears to be impaired by binding of rhodopsin kinase (14, 15). A direct receptor-kinase interaction also has been shown for the endothelin receptor by means of coimmunoprecipitation experiments (48). All of these data support the hypothesis that hormone and neurotransmitter receptor function already can be inhibited by binding of GRKs. Because these inhibitory effects were seen at GRK levels similar to those in brain, such phosphorylation-independent inhibition of receptor signaling may well represent a physiological mechanism.
Taken together, our results show that the PTH receptor is a good substrate for phosphorylation by different GRKs both in intact cells and in cell membrane preparations. However, contrary to the generally accepted concept, inhibition by GRKs also was seen when no receptor phosphorylation occurred. Although this finding does not invalidate the normal sequence of events during desensitization, i.e., GRK binding, receptor phosphorylation, GRK dissociation, and finally binding of β-arrestins, it suggests that inhibition of receptor function already occurs at the first step of this process and that GRK binding may by itself impair receptor signaling in a hormonal receptor system. The function of subsequent β-arrestin binding then may be to stabilize this inhibition and to initiate receptor internalization and eventually further signaling events.
Acknowledgments
We thank A. Braitmaier, M. Hoffmann, A. Straub, and J. Kriegbaum for excellent technical assistance. These studies were supported by grants from the Deutsche Forschungsgemeinschaft, Boehringer Mannheim, and the Fonds der Chemischen Industrie.
ABBREVIATIONS
- GRK
G protein-coupled receptor kinase
- HA
hemagglutinin
- PTH
parathyroid hormone
- PKA
protein kinase A
- PKC
protein kinase C
References
- 1.Hausdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 2.Lohse M J. Biochim Biophys Acta. 1993;1179:171–188. doi: 10.1016/0167-4889(93)90139-g. [DOI] [PubMed] [Google Scholar]
- 3.Strader C D, Fong T M, Graziano M P, Tota M R. FASEB J. 1995;9:745–754. [PubMed] [Google Scholar]
- 4.Baldwin J M, Schertler G F C, Unger V M. J Mol Biol. 1997;272:144–164. doi: 10.1006/jmbi.1997.1240. [DOI] [PubMed] [Google Scholar]
- 5.Pitcher J, Lohse M J, Codina J, Caron M G, Lefkowitz R J. Biochemistry. 1992;31:3193–3197. doi: 10.1021/bi00127a021. [DOI] [PubMed] [Google Scholar]
- 6.Hertel C, Müller P, Portenier M, Staehelin M. Biochem J. 1983;216:669–674. doi: 10.1042/bj2160669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark R B. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:155–209. [PubMed] [Google Scholar]
- 8.Pitcher J A, Freedman N J, Lefkowitz R J. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 9.Lohse M J, Benovic J L, Codina J, Caron M G, Lefkowitz R J. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 10.Lohse M J, Benovic J L, Caron M G, Lefkowitz R J. J Biol Chem. 1990;265:3202–3211. [PubMed] [Google Scholar]
- 11.Lohse M J, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure J P, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 12.Sterne-Marr R, Benovic J L. Vitamins Hormones. 1995;51:193–234. doi: 10.1016/s0083-6729(08)61039-0. [DOI] [PubMed] [Google Scholar]
- 13.Premont R T, Inglese J, Lefkowitz R J. FASEB J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 14.Pulvermüller A, Palczewski K, Hofmann K P. Biochemistry. 1993;32:14082–14088. doi: 10.1021/bi00214a002. [DOI] [PubMed] [Google Scholar]
- 15.Laitko U, Hofmann K P. Biophys J. 1998;74:803–815. doi: 10.1016/S0006-3495(98)74005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jüppner H, Abou-Samra A B, Freeman M, Kong X F, Schipani E, Richards J, Kolakowski L F, Jr, Hock J, Potts J T, Jr, Kronenberg H M, et al. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 17.Pun K K, Ho P W, Nissenson R A, Arnaud C D. J Bone Miner Res. 1990;5:1193–1200. doi: 10.1002/jbmr.5650051202. [DOI] [PubMed] [Google Scholar]
- 18.Fukayama S, Tashjian A H, Bringhurst F R. Endocrinology. 1992;131:1757–1769. doi: 10.1210/endo.131.4.1396321. [DOI] [PubMed] [Google Scholar]
- 19.Fujimori A, Miyauchi A, Hruska K A, Martin K J, Avioli L V, Civitelli R. Am J Physiol. 1993;264:E918–E924. doi: 10.1152/ajpendo.1993.264.6.E918. [DOI] [PubMed] [Google Scholar]
- 20.Bergwitz C, Abou-Samra A-B, Hesch R-D, Jüppner H. Biochim Biophys Acta. 1994;1222:447–456. doi: 10.1016/0167-4889(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 21.Blind E, Bambino T, Nissenson R A. Endocrinology. 1995;136:4271–4277. doi: 10.1210/endo.136.10.7664644. [DOI] [PubMed] [Google Scholar]
- 22.Blind E, Bambino T, Huang Z, Bliziotes M, Nissenson R A. J Bone Miner Res. 1996;11:578–586. doi: 10.1002/jbmr.5650110505. [DOI] [PubMed] [Google Scholar]
- 23.Lee C, Gardella T J, Abou-Samra A-B, Nussbaum S R, Segre G V, Potts J T, Jr, Kronenberg H M, Jüppner H. Endocrinology. 1994;135:1488–1495. doi: 10.1210/endo.135.4.7523099. [DOI] [PubMed] [Google Scholar]
- 24.Benovic J L, DeBlasi A, Stone W C, Caron M G, Lefkowitz R J. Science. 1989;246:235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- 25.Benovic J L, Onorato J J, Arriza J L, Stone W C, Lohse M, Jenkins N A, Gilbert D J, Copeland N G, Caron M G, Lefkowitz R J. J Biol Chem. 1991;266:14939–14946. [PubMed] [Google Scholar]
- 26.Kunapuli P, Benovic J L. Proc Natl Acad Sci USA. 1993;90:5588–5592. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diviani D, Lattion A-L, Larbi N, Kunapuli P, Pronin A, Benovic J L, Cotecchia S. J Biol Chem. 1996;271:5049–5058. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- 28.Schulz K, Danner S, Bauer P, Schröder S, Lohse M J. J Biol Chem. 1996;271:22546–22551. doi: 10.1074/jbc.271.37.22546. [DOI] [PubMed] [Google Scholar]
- 29.Koch W J, Hawes B E, Inglese J, Luttrell L M, Lefkowitz R J. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 30.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1997. [Google Scholar]
- 31.Söhlemann P, Hekman M, Buchen C, Elce J S, Lohse M J. FEBS Lett. 1993;324:59–62. doi: 10.1016/0014-5793(93)81532-5. [DOI] [PubMed] [Google Scholar]
- 32.Müller S, Straub A, Lohse M J. FEBS Lett. 1997;401:25–29. doi: 10.1016/s0014-5793(96)01424-x. [DOI] [PubMed] [Google Scholar]
- 33.Kim C M, Dion S B, Onorato J J, Benovic J L. Receptor. 1993;3:39–55. [PubMed] [Google Scholar]
- 34.Seuwen K, Lagarde A, Pouyssegur J. EMBO J. 1988;7:161–168. doi: 10.1002/j.1460-2075.1988.tb02796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iida-Klein A, Guo J, Xie L Y, Jüppner H, Potts J T, Jr, Kronenberg H M, Bringhurst F R, Abou-Samra A B, Segre G V. J Biol Chem. 1995;270:8458–8465. doi: 10.1074/jbc.270.15.8458. [DOI] [PubMed] [Google Scholar]
- 36.Gierschik P, Bouillon T, Jakobs K H. Methods Enzymol. 1994;237:13–26. doi: 10.1016/s0076-6879(94)37049-4. [DOI] [PubMed] [Google Scholar]
- 37.Pitcher J A, Inglese J, Higgins J B, Arriza J L, Casey P J, Kim C M, Benovic J L, Kwatra M M, Caron M G, Lefkowitz R J. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 38.Koch W J, Inglese J, Stone W C, Lefkowitz R J. J Biol Chem. 1993;268:8256–8260. [PubMed] [Google Scholar]
- 39.Müller S, Hekman M, Lohse M J. Proc Natl Acad Sci USA. 1993;90:10439–10443. doi: 10.1073/pnas.90.22.10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer P H, Müller S, Puzicha M, Pippig S, Obermaier B, Helmreich E J M, Lohse M J. Nature (London) 1992;358:73–76. doi: 10.1038/358073a0. [DOI] [PubMed] [Google Scholar]
- 41.Hekman M, Bauer P H, Söhlemann P, Lohse M J. FEBS Lett. 1994;343:120–124. doi: 10.1016/0014-5793(94)80302-1. [DOI] [PubMed] [Google Scholar]
- 42.Lohse M J, Krasel C, Winstel R, Mayor F., Jr Kidney Int. 1996;49:1047–1052. doi: 10.1038/ki.1996.153. [DOI] [PubMed] [Google Scholar]
- 43.Pei G, Tiberi M, Caron M G, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:3633–3636. doi: 10.1073/pnas.91.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benovic J L, Regan J W, Matsui H, Mayor F, Jr, Cotecchia S, Leeb-Lundberg L M F, Caron M G, Lefkowitz R J. J Biol Chem. 1987;262:17251–17253. [PubMed] [Google Scholar]
- 45.Kong G, Penn R, Benovic J L. J Biol Chem. 1994;269:13084–13087. [PubMed] [Google Scholar]
- 46.Palczewski K. Eur J Biochem. 1997;248:261–269. doi: 10.1111/j.1432-1033.1997.00261.x. [DOI] [PubMed] [Google Scholar]
- 47.Söhlemann P, Hekman M, Puzicha M, Buchen C, Lohse M J. Eur J Biochem. 1995;232:464–472. [PubMed] [Google Scholar]
- 48.Freedman N J, Ament A S, Oppermann M, Stoffel R H, Exum S T, Lefkowitz R J. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]