Abstract
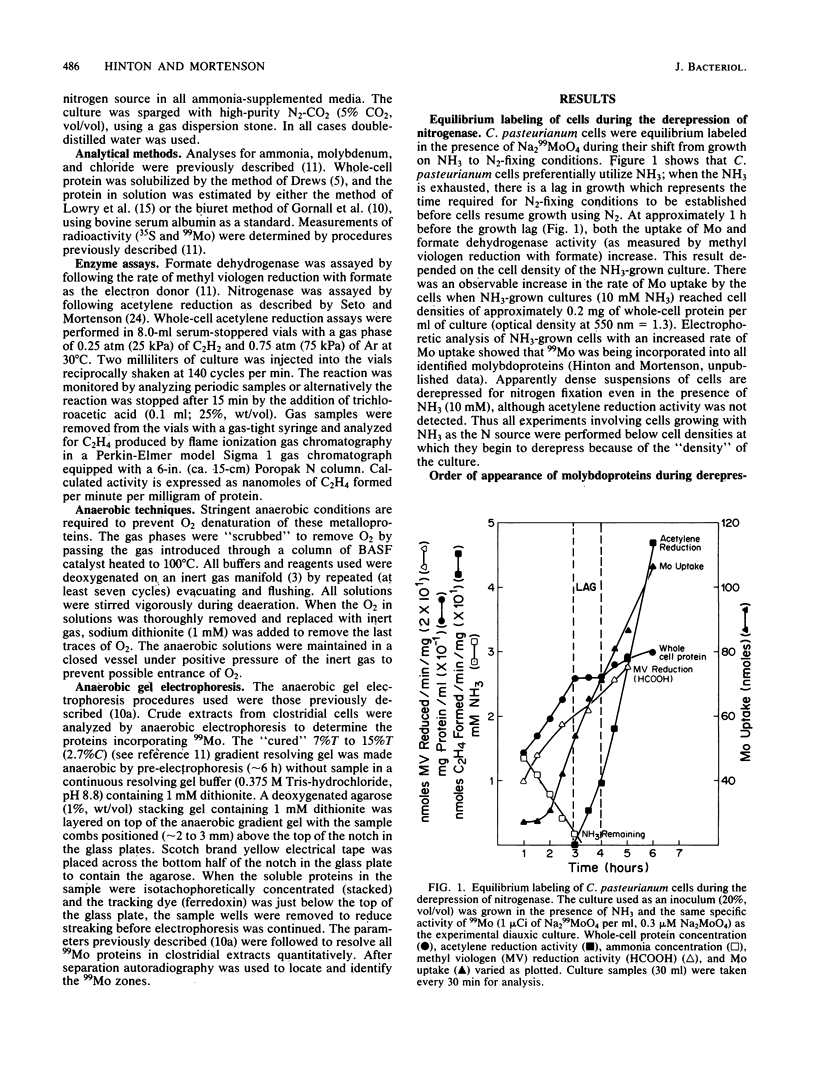
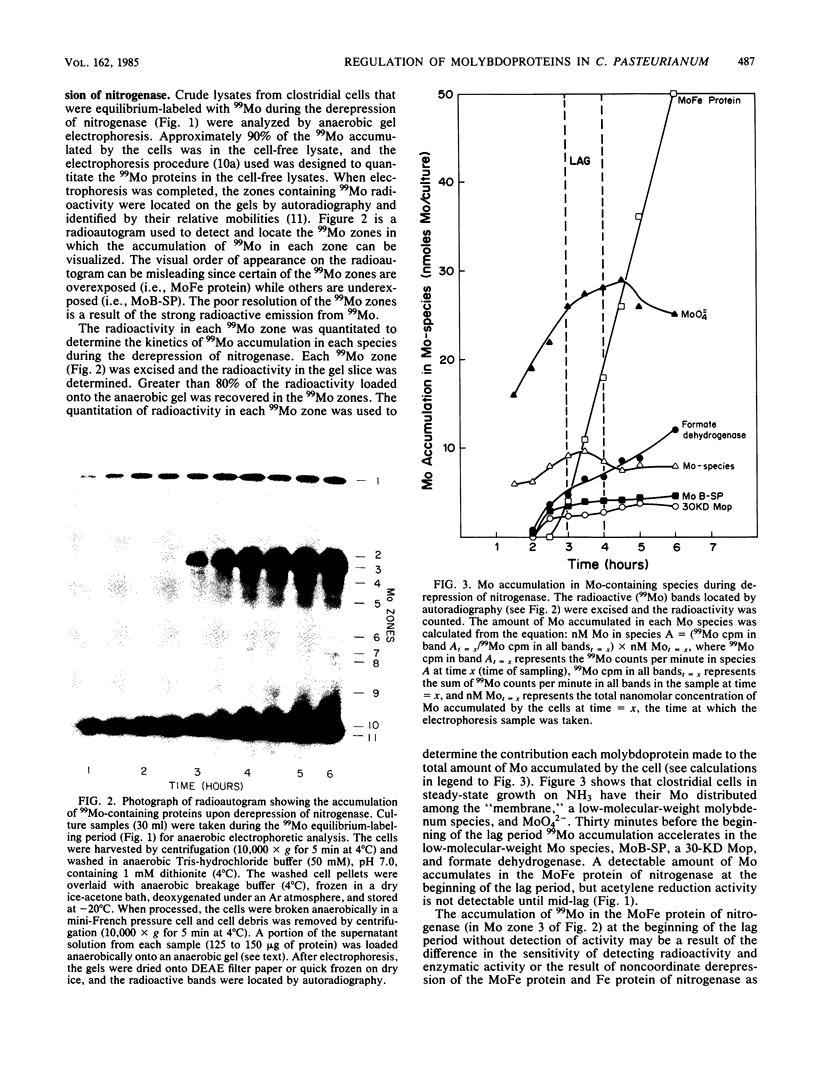
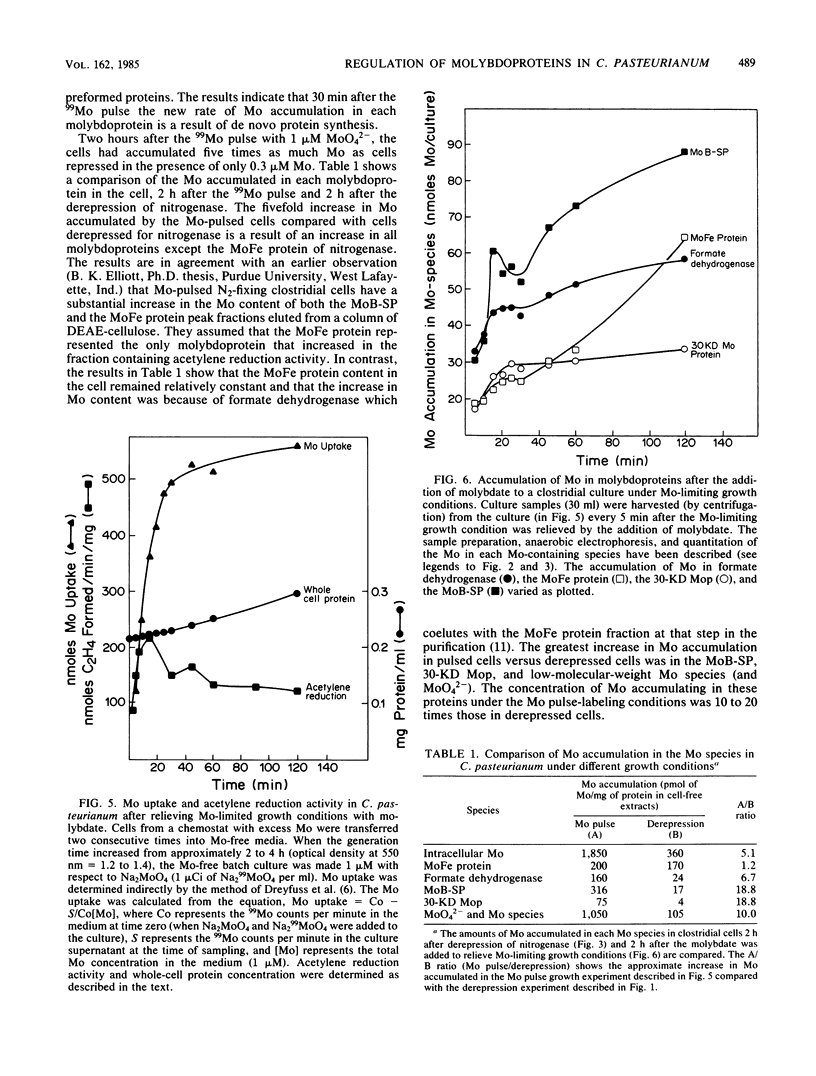
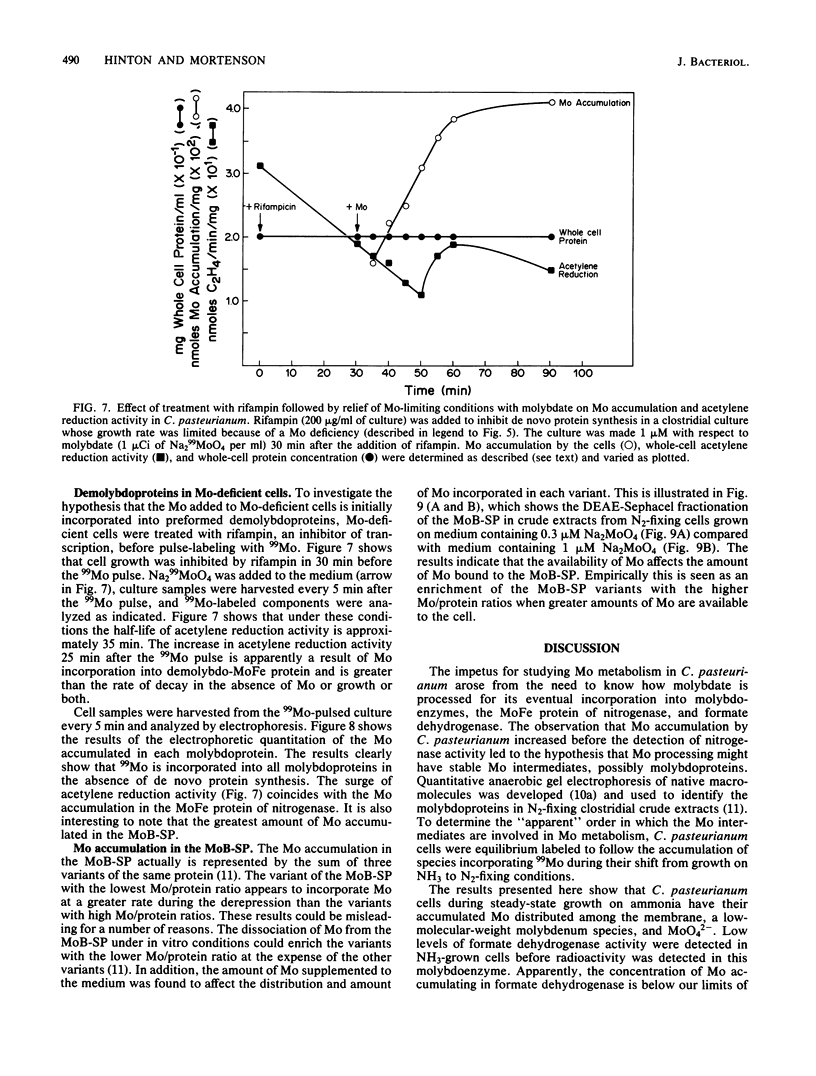
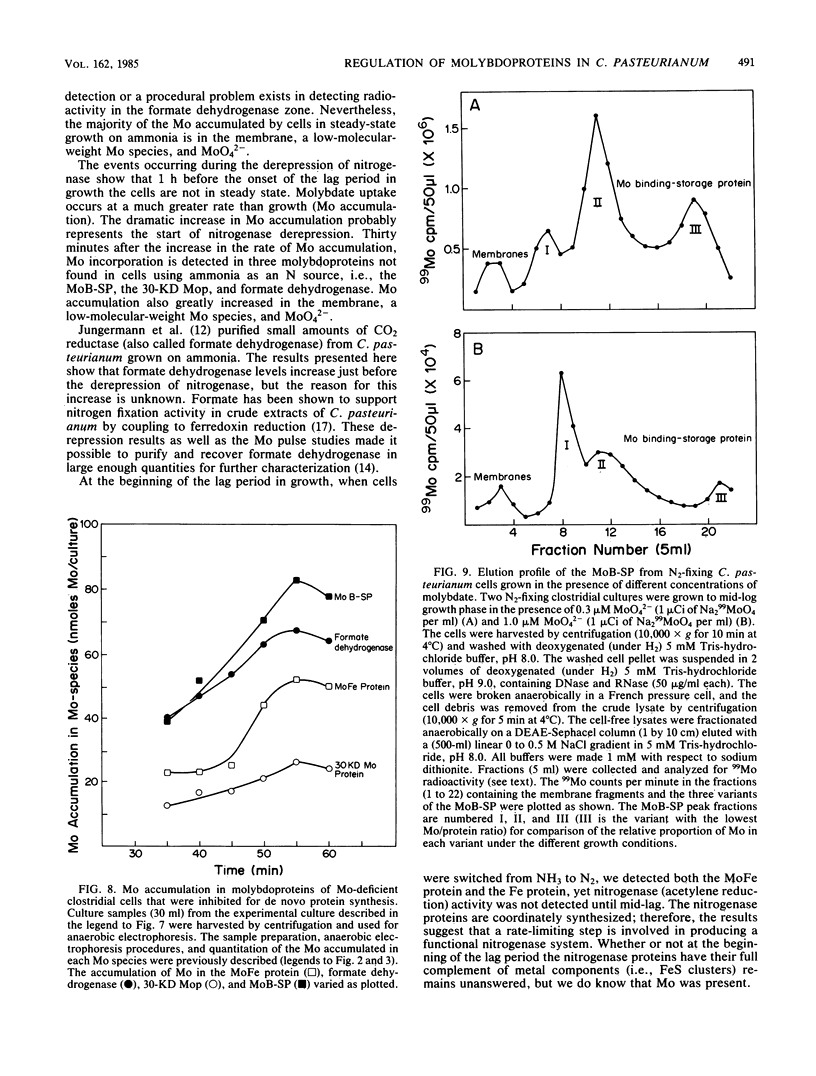
The accumulation of 99Mo (from 99MoO4(2-) into molybdenum-containing species in Clostridium pasteurianum was investigated to identify the molybdoprotein(s) involved in Mo metabolism. Mo accumulation by clostridial cells during the derepression of the nitrogenase system increased substantially beginning 1.5 h before nitrogenase activity was detected. The increase in Mo accumulation by the cells is a result of the incorporation of Mo into a high-molecular-weight molybdenum species (suspected membrane fragments), a low-molecular-weight molybdenum species, a Mo binding-storage protein, a 30-kilodalton molybdoprotein, and formate dehydrogenase. Mo incorporation into the MoFe protein was detected 1 h after the onset of metal uptake. Kinetics of Mo accumulation into the molybdoproteins during the derepression of nitrogenase suggests that Mo incorporation or uptake or both occur in the following sequence: (i) membranes and MoO4(2-), (ii) a low-molecular-weight molybdenum species, (iii) Mo binding-storage protein and a 30-kilodalton molybdoprotein, (iv) formate dehydrogenase, and (v) the MoFe protein. The intracellular level of all molybdenum components except the MoFe protein appears to be influenced by the availability of Mo. Clostridial cells grown in the presence of a limiting amount of Mo became Mo deficient as a result of growth and a MoO4(2-) supplement added to such cells rapidly accumulated within the cells to levels five times that found in steady-state nitrogen-fixing cells. The Mo accumulated by the Mo-deficient cells was rapidly incorporated into preformed demolybdoproteins in the absence of de novo protein synthesis. The increase in Mo accumulation by Mo-deficient cells was a result of an increase in all molybdoproteins except the MoFe protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battisti L., Green B. D., Thorne C. B. Mating system for transfer of plasmids among Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. J Bacteriol. 1985 May;162(2):543–550. doi: 10.1128/jb.162.2.543-550.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess B. K., Jacobs D. B., Stiefel E. I. Large-scale purification of high activity Azotobacter vinelandII nitrogenase. Biochim Biophys Acta. 1980 Jul 10;614(1):196–209. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Elliott B. B., Mortenson L. E. Regulation of molybdate transport by Clostridium pasteurianum. J Bacteriol. 1976 Aug;127(2):770–779. doi: 10.1128/jb.127.2.770-779.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B. B., Mortenson L. E. Transport of molybdate by Clostridium pasteurianum. J Bacteriol. 1975 Dec;124(3):1295–1301. doi: 10.1128/jb.124.3.1295-1301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton S. M., Mortenson L. E. Anaerobic multiphasic gel electrophoresis of the molybdoproteins in extracts of Clostridium pasteurianum. Anal Biochem. 1985 Mar;145(2):222–229. doi: 10.1016/0003-2697(85)90353-7. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Kirchniawy H., Thauer R. K. Ferredoxin dependent CO-2 reduction to formate in Clostridium pasteurianum. Biochem Biophys Res Commun. 1970 Nov 9;41(3):682–689. doi: 10.1016/0006-291x(70)90067-7. [DOI] [PubMed] [Google Scholar]
- Ketchum P. A., Cambier H. Y., Frazier W. A., 3rd, Madansky C. H., Nason A. In vitro assembly of Neurospora assimilatory nitrate reductase from protein subunits of a Neurospora mutant and the xanthine oxidizing or aldehyde oxidase systems of higher animals. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1016–1023. doi: 10.1073/pnas.66.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu C. L., Mortenson L. E. Formate dehydrogenase of Clostridium pasteurianum. J Bacteriol. 1984 Jul;159(1):375–380. doi: 10.1128/jb.159.1.375-380.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P. A., Dixon R. A. Requirement of nifV gene for production of wild-type nitrogenase enzyme in Klebsiella pneumoniae. Nature. 1981 Aug 13;292(5824):655–656. doi: 10.1038/292655a0. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E. Components of cell-free extracts of Clostridium pasteurianum required for ATP-dependent H2 evolution from dithionite and for N2 fixation. Biochim Biophys Acta. 1966 Sep 26;127(1):18–25. doi: 10.1016/0304-4165(66)90470-3. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E., Morris J. A., Jeng D. Y. Purification, metal composition and properties of molybdoferredoxin and azoferredoxin, two of the components of the nitrogen-fixing system of Clostridium pasteurianum. Biochim Biophys Acta. 1967 Aug 29;141(3):516–522. doi: 10.1016/0304-4165(67)90180-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PATEMAN J. A., COVE D. J., REVER B. M., ROBERTS D. B. A COMMON CO-FACTOR FOR NITRATE REDUCTASE AND XANTHINE DEHYDROGENASE WHICH ALSO REGULATES THE SYNTHESIS OF NITRATE REDUCTASE. Nature. 1964 Jan 4;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- Pienkos P. T., Shah V. K., Brill W. J. Molybdenum cofactors from molybdoenzymes and in vitro reconstitution of nitrogenase and nitrate reductase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5468–5471. doi: 10.1073/pnas.74.12.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto B., Mortenson L. E. In vivo kinetics of nitrogenase formation in Clostridium pasteurianum. J Bacteriol. 1974 Nov;120(2):822–830. doi: 10.1128/jb.120.2.822-830.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]