Abstract
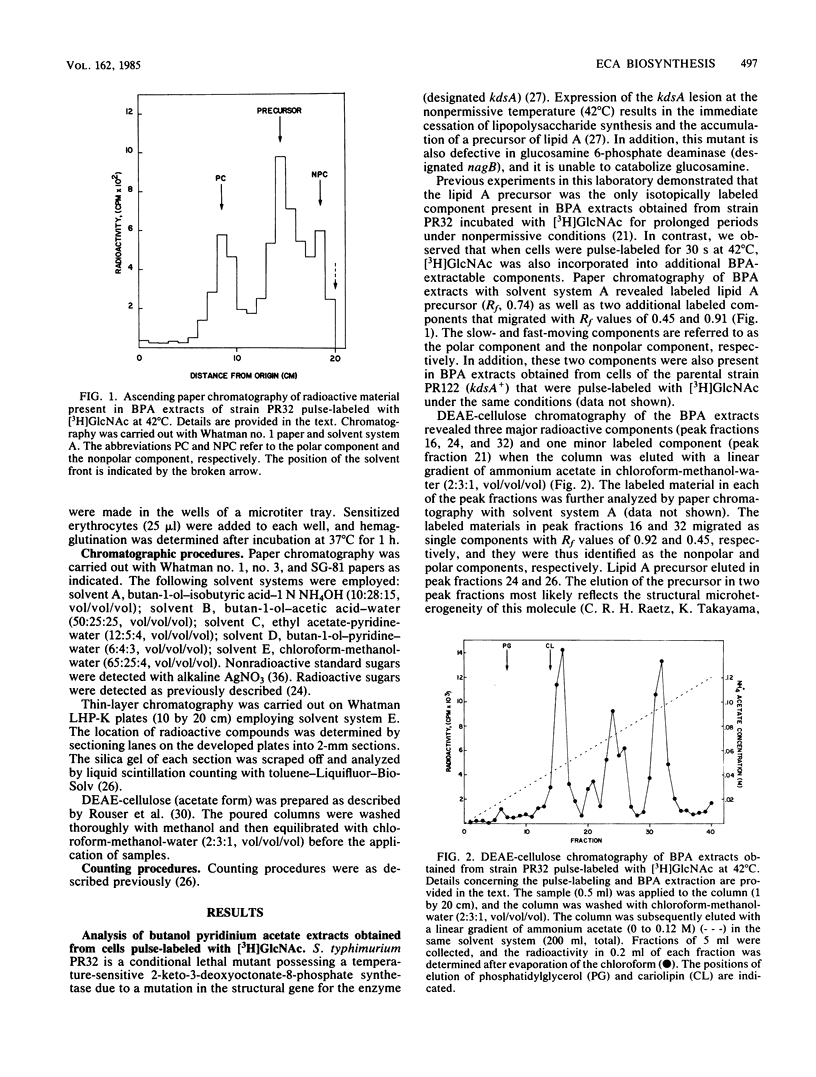
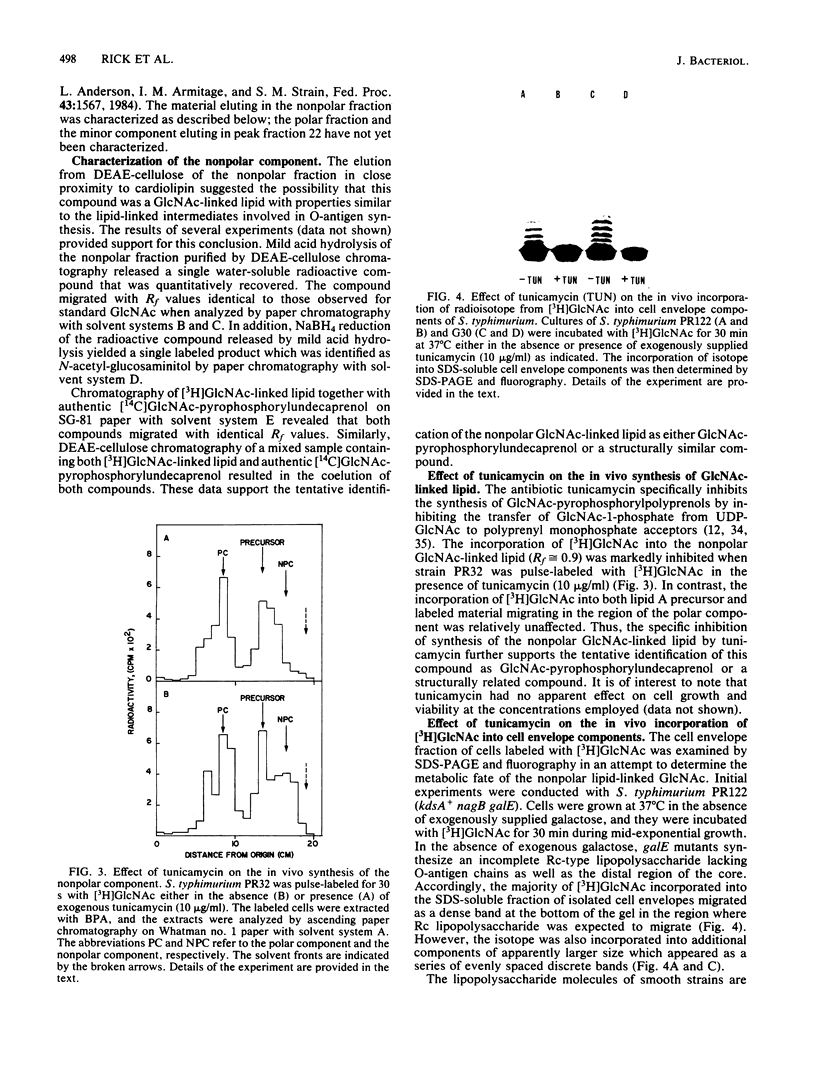
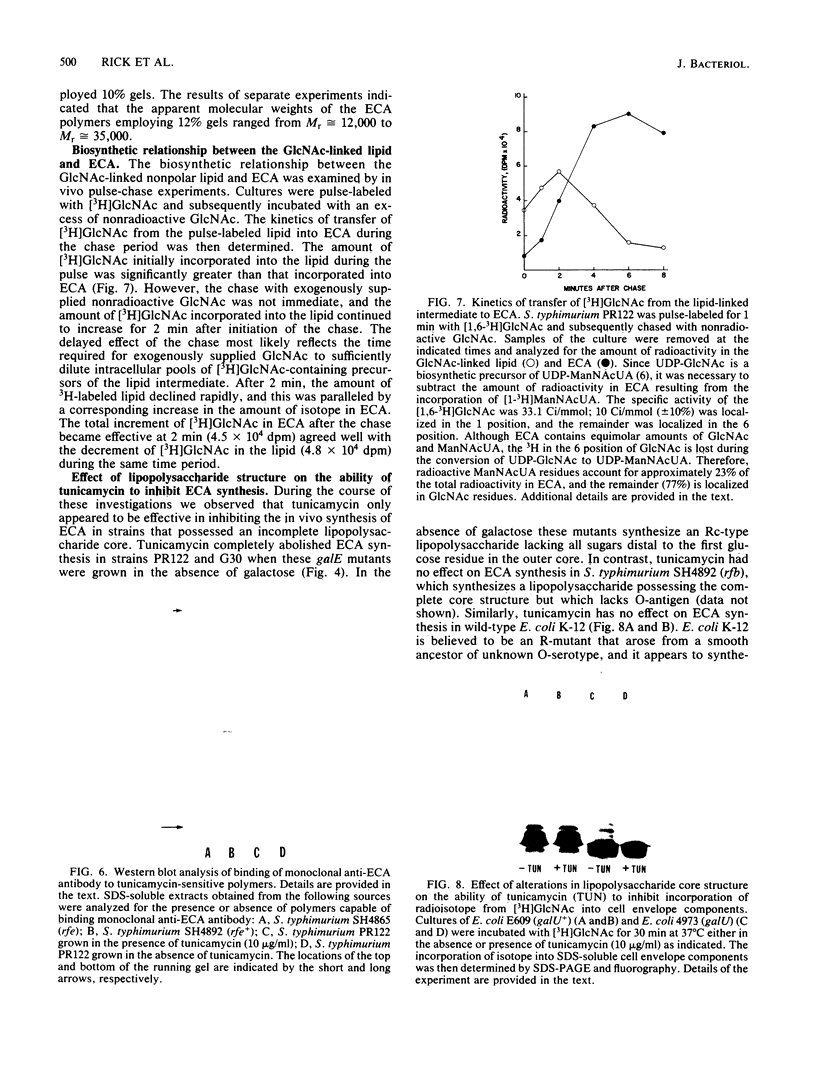
Cultures of Salmonella typhimurium pulse-labeled with N-acetyl-D-[3H]glucosamine ([3H]GlcNAc) incorporated isotope into a GlcNAc-linked lipid that was tentatively identified as GlcNAc-pyrophosphorylundecaprenol. The incorporation of [3H]GlcNAc into this compound was abolished when cells were pulse-labeled in the presence of the antibiotic tunicamycin. Tunicamycin also abolished the in vivo synthesis of the haptenic form of enterobacterial common antigen (ECA) in S. typhimurium as determined by the passive hemagglutination test. These data indicated that the synthesis of the GlcNAc-linked lipid is related to ECA synthesis. Support for this conclusion was provided by the following observations. Cultures of Escherichia coli and S. typhimurium incorporated [3H]GlcNAc into cell envelope components that migrated as a homologous series of polymers when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The [3H]GlcNAc-labeled polymers were not detected in mutants of E. coli and S. typhimurium defective in ECA synthesis due to lesions in either the rfe or rff gene clusters. These polymers were identified as ECA based on Western blot analyses employing anti-ECA monoclonal antibody. The incorporation of [3H]GlcNAc into ECA polymers was abolished by tunicamycin when the drug was added to cultures to give a minimum concentration of 3 micrograms/ml. In addition, pulse-chase experiments provided evidence for a precursor-product relationship between the GlcNAc-linked lipid and ECA. These results strongly suggest that the GlcNAc-linked lipid is involved in the biosynthesis of ECA in a manner analogous to the role of carrier lipid in the biosynthesis of O-antigen and peptidoglycan.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitter-Suermann D., Peters H., Jürs M., Nehrbass R., Montenegro M., Timmis K. N. Monoclonal antibody detection of IncF group plasmid-encoded TraT protein in clinical isolates of Escherichia coli. Infect Immun. 1984 Nov;46(2):308–313. doi: 10.1128/iai.46.2.308-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell A., Oates J., Lugowski C., Romanowska E., Kenne L., Lindberg B. The enterobacterial common-antigen, a cyclic polysaccharide. Carbohydr Res. 1984 Oct 1;133(1):95–104. doi: 10.1016/0008-6215(84)85186-1. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hayes G. R., Lucas J. J. A novel N-acetylglucosaminyl polyprene in hen oviduct. J Biol Chem. 1980 Aug 25;255(16):7536–7539. [PubMed] [Google Scholar]
- Hussey H., Baddiley J. Lipid intermediates in the biosynthesis of the wall teichoic acid in Staphylococcus lactis 13. Biochem J. 1972 Mar;127(1):39–50. doi: 10.1042/bj1270039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara N., Ishimoto N., Ito E. Enzymatic formation of uridine diphosphate N-acetyl-D-mannosaminuronic acid. FEBS Lett. 1974 Feb 1;39(1):46–48. doi: 10.1016/0014-5793(74)80013-x. [DOI] [PubMed] [Google Scholar]
- Jones R. T., Koeltzow D. E., Stocker B. A. Genetic transfer of Salmonella typhimurium and Escherichia coli lipopolysaccharide antigens to Escherichia coli K-12. J Bacteriol. 1972 Sep;111(3):758–770. doi: 10.1128/jb.111.3.758-770.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H. M., Neter E., Mayer H. Modification of the lipid moiety of the enterobacterial common antigen by the "Pseudomonas factor". Infect Immun. 1983 May;40(2):696–700. doi: 10.1128/iai.40.2.696-700.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lew H. C., Nikaido H., Mäkelä P. H. Biosynthesis of uridine diphosphate N-acetylmannosaminuronic acid in rff mutants of Salmonella tryphimurium. J Bacteriol. 1978 Oct;136(1):227–233. doi: 10.1128/jb.136.1.227-233.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Duksin D. Biological activities of the two major components of tunicamycin. J Biol Chem. 1979 Jul 25;254(14):6572–6576. [PubMed] [Google Scholar]
- Mayer H., Schmidt G. Chemistry and biology of the enterobacterial common antigen (ECA). Curr Top Microbiol Immunol. 1979;85:99–153. doi: 10.1007/978-3-642-67322-1_3. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Jahkola M., Lüderitz O. A new gene cluster rfe concerned with the biosynthesis of Salmonella lipopolysaccharide. J Gen Microbiol. 1970 Jan;60(1):91–106. doi: 10.1099/00221287-60-1-91. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H. Enterobacterial common antigen. Bacteriol Rev. 1976 Sep;40(3):591–632. doi: 10.1128/br.40.3.591-632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H., Whang H. Y., Neter E. Participation of lipopolysaccharide genes in the determination of the enterobacterial common antigen: analysis of R mutants of Salmonella minnesota. J Bacteriol. 1974 Sep;119(3):760–764. doi: 10.1128/jb.119.3.760-764.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H. Participation of lipopolysaccharide genes in the determination of the entobacterial common antigen: analysis in Salmonella groups B and C1. J Bacteriol. 1974 Sep;119(3):765–770. doi: 10.1128/jb.119.3.765-770.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Schmidt G., Mayer H., Nikaido H., Whang H. Y., Neter E. Enterobacterial common antigen in rfb deletion mutants of Salmonella typhimurium. J Bacteriol. 1976 Sep;127(3):1141–1149. doi: 10.1128/jb.127.3.1141-1149.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D., Mayer H. Isolation and chemical characterization of the enterobacterial common antigen. Eur J Biochem. 1978 May 16;86(2):361–370. doi: 10.1111/j.1432-1033.1978.tb12318.x. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- ROTHFIELD L., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. II. INCORPORATION OF GLUCOSE AND GALACTOSE CATALYZED BY PARTICULATE AND SOLUBLE ENZYMES IN SALMONELLA. J Biol Chem. 1964 Sep;239:2788–2795. [PubMed] [Google Scholar]
- Rick P. D., Fung L. W., Ho C., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Purification and characterization of a lipid A precursor produced by a mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4904–4912. [PubMed] [Google Scholar]
- Rick P. D., Neumeyer B. A., Young D. A. Effect of altered lipid A synthesis on the synthesis of the OmpA protein in Salmonella typhimurium. J Biol Chem. 1983 Jan 10;258(1):629–635. [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Characterization of a conditional lethal mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4895–4903. [PubMed] [Google Scholar]
- Rick P. D., Young D. A. Isolation and characterization of a temperature-sensitive lethal mutant of Salmonella typhimurium that is conditionally defective in 3-deoxy-D-manno-octulosonate-8-phosphate synthesis. J Bacteriol. 1982 May;150(2):447–455. doi: 10.1128/jb.150.2.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J., Kuo T. T., MacPhee D. G. The effect of defined lipopolysaccharide core defects upon antibiotic resistances of Salmonella typhimurium. J Gen Microbiol. 1977 Dec;103(2):223–234. doi: 10.1099/00221287-103-2-223. [DOI] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 4. Typisierung von S. minnesota-R-Mutanten mittels Antibiotica. Zentralbl Bakteriol Orig. 1970 Apr;213(3):356–380. [PubMed] [Google Scholar]
- Schmidt G., Mayer H., Mäkelä P. H. Presence of rfe genes in Escherichia coli: their participation in biosynthesis of O antigen and enterobacterial common antigen. J Bacteriol. 1976 Aug;127(2):755–762. doi: 10.1128/jb.127.2.755-762.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Yamamori S., Murazumi N., Araki Y., Ito E. Formation and function of N-acetyloglucosamine-linked phosphoryl- and pyrophosphorylundecaprenols in membranes from Bacillus cereus. J Biol Chem. 1978 Sep 25;253(18):6516–6522. [PubMed] [Google Scholar]