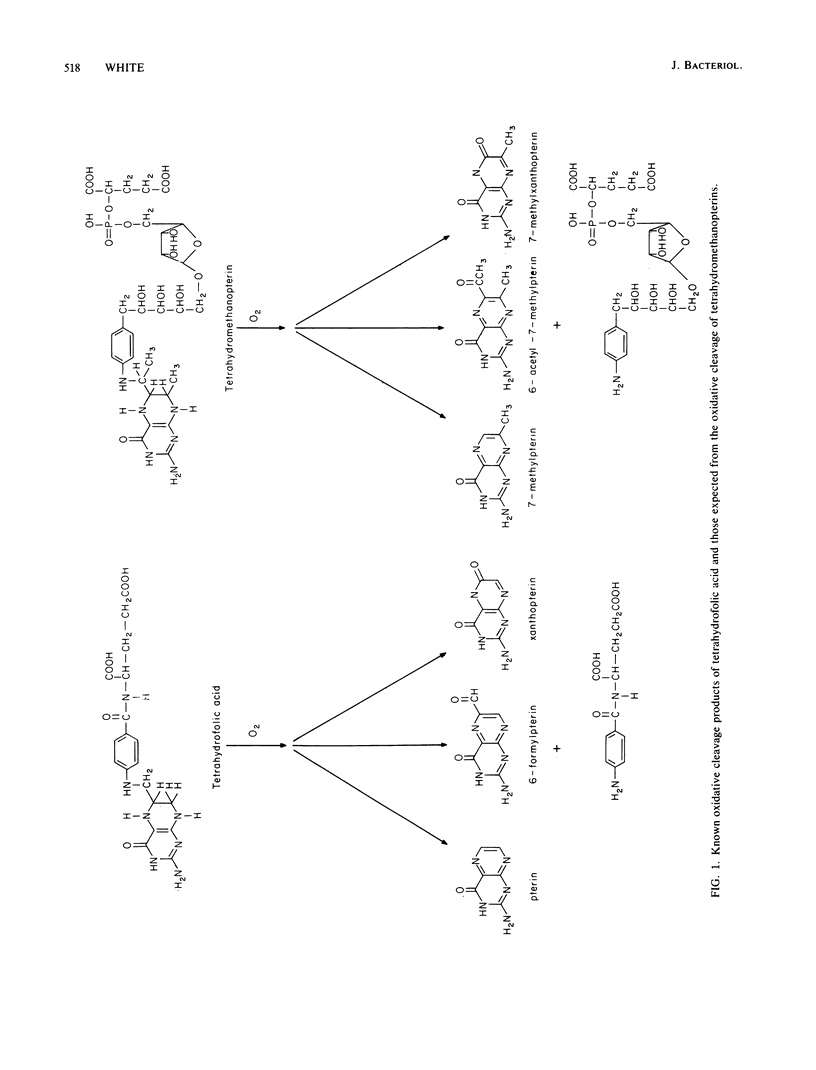
Abstract
7-Methylpterin and 7-methyllumizine were isolated and identified in extracts of methanogenic bacteria which had been extracted in air with ethanol-water. Ethanol-water preparations of cells extracted under nitrogen or hydrogen were devoid of these compounds. Extracts of cells obtained in the presence of air also had an increased amount of a complex arylamine which, on acid hydrolysis, gave 1 mol each of phosphate, 5-(p-aminophenyl)-1,2,3,4-tetrahydroxypentane, and alpha-hydroxyglutaric acid. Gas chromatography-mass spectrometry was used to identify the 5-(p-aminophenyl)-1,2,3,4-tetrahydroxypentane as its tetratrimethylsilyl derivative and the alpha-hydroxyglutaric acid as the n-butyl ester derivative of its gamma-lactone. When exposed to air, extracts of cells prepared in the absence of air produced 6-acetyl-7-methylpterin and 7-methylxanthopterin in addition to 7-methylpterin and 7-methyllumizine. It is concluded that these compounds are derived from the oxidative cleavage of the tetrahydromethanopterin, which is present in these bacteria, by a series of reactions analogous to those known to occur in the oxidative cleavage of tetrahydrofolic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAKLEY R. L. The interconversion of serine and glycine; preparation and properties of catalytic derivatives of pteroylglutamic acid. Biochem J. 1957 Feb;65(2):331–342. doi: 10.1042/bj0650331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Leigh J. A., Rinehart K. L., Wolfe R. S. Formaldehyde activation factor, tetrahydromethanopterin, a coenzyme of methanogenesis. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1976–1980. doi: 10.1073/pnas.81.7.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto I., Krumdieck C. L. Determination of three different pools of reduced one-carbon-substituted folates. 1. A study of the fundamental chemical reactions. Anal Biochem. 1980 Nov 15;109(1):167–184. doi: 10.1016/0003-2697(80)90026-3. [DOI] [PubMed] [Google Scholar]
- GOTO M., FORREST H. S. Identification of a new phosphorylated pteridine from E. coli. Biochem Biophys Res Commun. 1961 Nov 20;6:180–183. doi: 10.1016/0006-291x(61)90125-5. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V. Characterization of the molybdenum cofactor of sulfite oxidase, xanthine, oxidase, and nitrate reductase. Identification of a pteridine as a structural component. J Biol Chem. 1980 Mar 10;255(5):1783–1786. [PubMed] [Google Scholar]
- Keltjens J. T., Huberts M. J., Laarhoven W. H., Vogels G. D. Structural elements of methanopterin, a novel pterin present in Methanobacterium thermoautotrophicum. Eur J Biochem. 1983 Feb 15;130(3):537–544. doi: 10.1111/j.1432-1033.1983.tb07183.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Forrest H. S. Identification of pteridines produced by three species of photosynthetic bacteria. Biochim Biophys Acta. 1967 Aug 29;141(3):642–644. doi: 10.1016/0304-4165(67)90195-x. [DOI] [PubMed] [Google Scholar]
- LEVENBERG B., HAYAISHI O. A bacterial pterin deaminase. J Biol Chem. 1959 Apr;234(4):955–961. [PubMed] [Google Scholar]
- Lancaster J. R., Jr Membrane-bound flavin adenine dinucleotide in Methanobacterium Bryantii. Biochem Biophys Res Commun. 1981 May 15;100(1):240–246. doi: 10.1016/s0006-291x(81)80088-5. [DOI] [PubMed] [Google Scholar]
- Lloyd T., Mori T., Kaufman S. 6-Methyltetrahydropterin. Isolation and identification as the highly active hydroxylase cofactor from tetrahydrofolate. Biochemistry. 1971 Jun 8;10(12):2330–2336. doi: 10.1021/bi00788a024. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Greening R. C., Ferry J. G. Rapidly growing rumen methanogenic organism that synthesizes coenzyme M and has a high affinity for formate. Appl Environ Microbiol. 1984 Jul;48(1):81–87. doi: 10.1128/aem.48.1.81-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., White R. H., Ferry J. G. Identification of methyl coenzyme M as an intermediate in methanogenesis from acetate in Methanosarcina spp. J Bacteriol. 1984 Nov;160(2):521–525. doi: 10.1128/jb.160.2.521-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar R. C., Shaw K. M., Sprott G. D. Isolation and characterization of a FAD-dependent NADH diaphorase from Methanospirillum hungatei strain GP1. Can J Biochem. 1981 Feb;59(2):83–91. doi: 10.1139/o81-013. [DOI] [PubMed] [Google Scholar]
- Ortiz P. J., Hotchkiss R. D. The enzymatic synthesis of dihydrofolate and dihydropteroate in cell-free preparations from wild-type and sulfonamide-resistant pneumococcus. Biochemistry. 1966 Jan;5(1):67–74. doi: 10.1021/bi00865a010. [DOI] [PubMed] [Google Scholar]
- SHIOTA T., DISRAELY M. N., MCCANN M. P. THE ENZYMATIC SYNTHESIS OF FOLATE-LIKE COMPOUNDS FROM HYDROXYMETHYLDIHYDROPTERIDINE PYROPHOSPHATE. J Biol Chem. 1964 Jul;239:2259–2266. [PubMed] [Google Scholar]
- SMYTH R. B., MCKEOWN G. G. THE ANALYSIS OF ARYLAMINES AND PHENOLS IN OXIDATION-TYPE HAIR DYES BY PAPER CHROMATOGRAPHY. J Chromatogr. 1964 Dec;16:454–459. doi: 10.1016/s0021-9673(01)82515-2. [DOI] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. FAD requirement for the reduction of coenzyme F420 by formate dehydrogenase from Methanobacterium formicicum. J Bacteriol. 1983 Aug;155(2):467–472. doi: 10.1128/jb.155.2.467-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Properties of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1982 Apr;150(1):1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushibara T., Forrest H. S., Hoare D. S., Patel R. N. Pteridines produced by Methylococcus capsulatus. Isolation and identification of a neopterin 2':3'-phosphate. Biochem J. 1971 Nov;125(1):141–146. doi: 10.1042/bj1250141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushibara T., Forrest H. S. Identification of 2-amino-4-hydroxy-6-methylpteridine as a naturally-occurring compound in two methane-oxidizing bacteria. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1189–1193. doi: 10.1016/0006-291x(70)90921-6. [DOI] [PubMed] [Google Scholar]
- Van Beelen P., Geerts W. J., Pol A., Vogels G. D. Quantification of coenzymes and related compounds from methanogenic bacteria by high-performance liquid chromatography. Anal Biochem. 1983 Jun;131(2):285–290. doi: 10.1016/0003-2697(83)90171-9. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski S. F. Evidence for the chemical interaction between 2-mercaptoethanol and tetrahydrofolate. J Biol Chem. 1966 Jun 25;241(12):2957–2961. [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beelen P., Stassen A. P., Bosch J. W., Vogels G. D., Guijt W., Haasnoot C. A. Elucidation of the structure of methanopterin, a coenzyme from Methanobacterium thermoautotrophicum, using two-dimensional nuclear-magnetic-resonance techniques. Eur J Biochem. 1984 Feb 1;138(3):563–571. doi: 10.1111/j.1432-1033.1984.tb07951.x. [DOI] [PubMed] [Google Scholar]



