Abstract
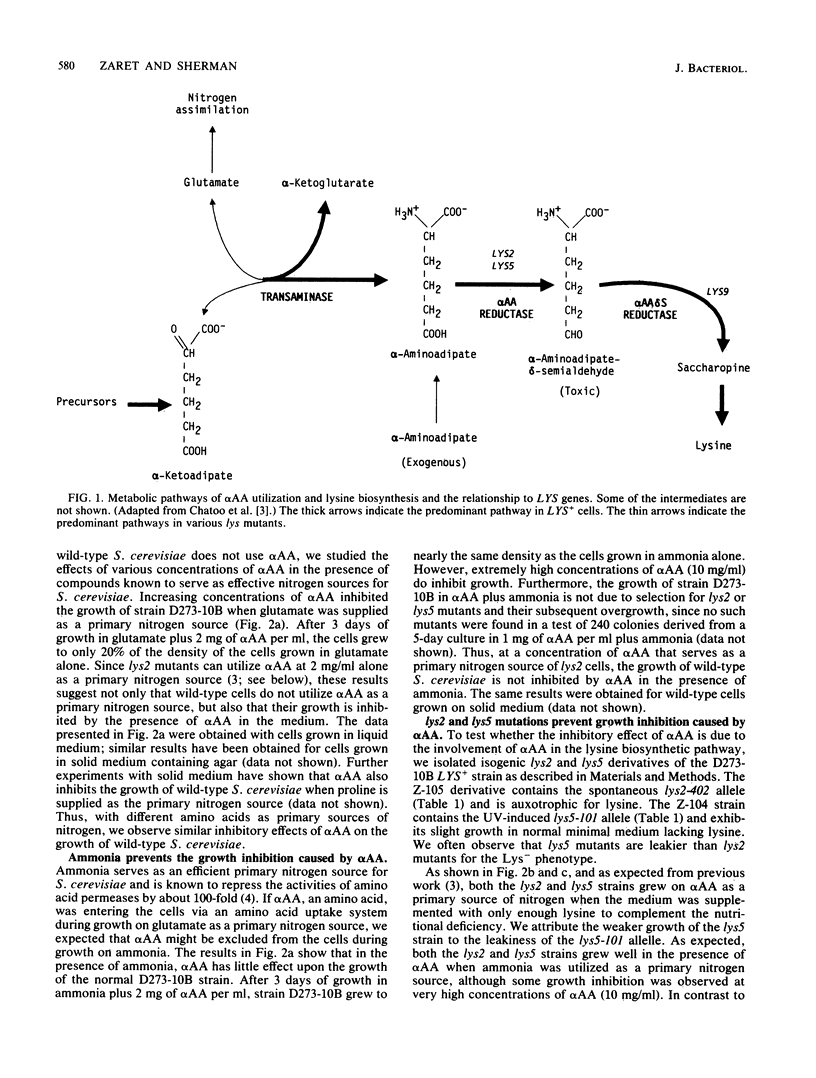
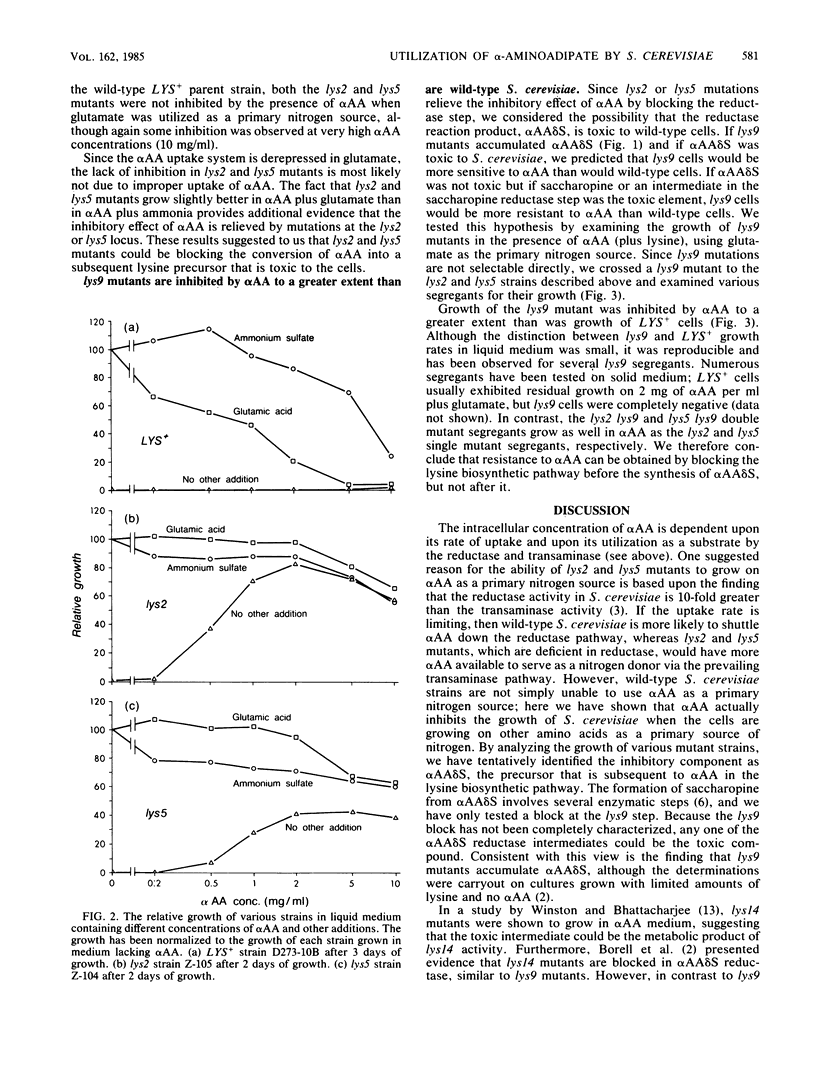
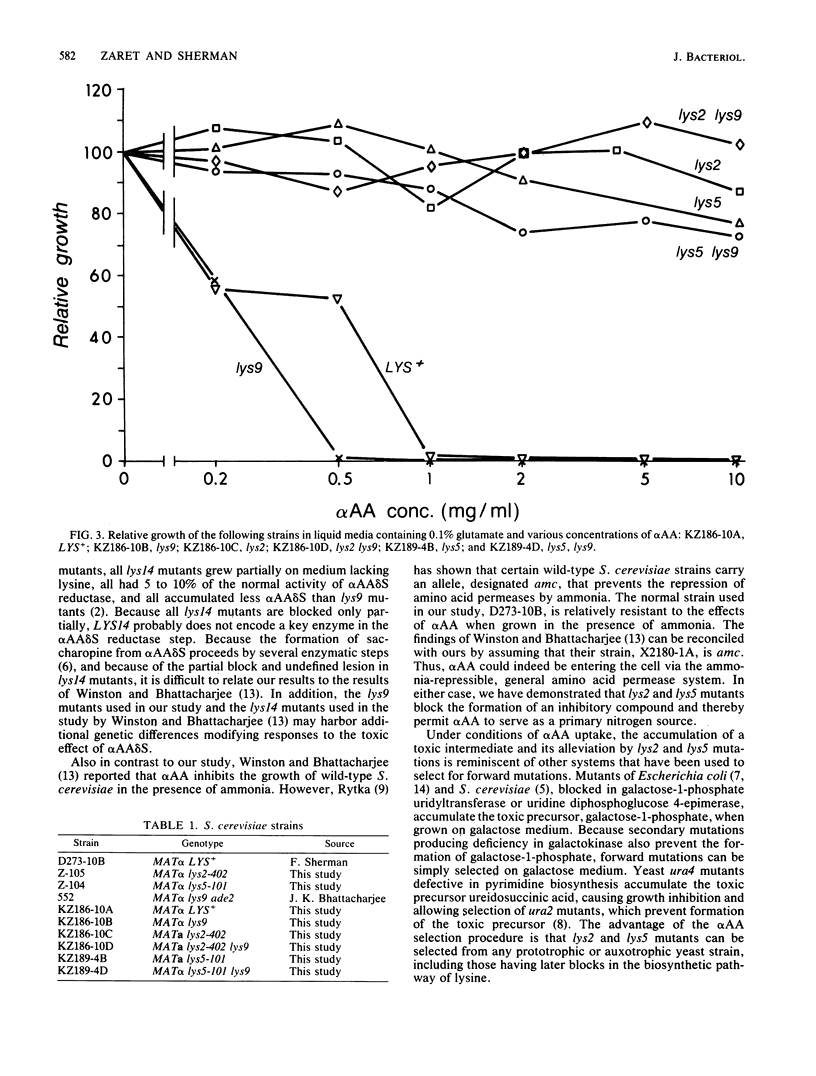
In contrast to wild-type strains of the yeast Saccharomyces cerevisiae, lys2 and lys5 mutants are able to utilize alpha-aminoadipate as a primary source of nitrogen. Chattoo et al. (B. B. Chattoo, F. Sherman, D. A. Azubalis, T. A. Fjellstedt, D. Mehnert, and M. Ogur, Genetics 93:51-65, 1979) relied on this difference in the effective utilization of alpha-aminoadipate to develop a procedure for directly selecting lys2 and lys5 mutants. In this study we used a range of mutant strains and various media to determine why normal strains are unable to utilize alpha-aminoadipate as a nitrogen source. Our results demonstrate that the anabolism of high levels of alpha-aminoadipate through the biosynthetic pathway of lysine results in the accumulation of a toxic intermediate and, furthermore, that lys2 and lys5 mutants contain blocks leading to the formation of this intermediate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee J. K., Sinha A. K. Relationship among the genes, enzymes, and intermediates of the biosynthetic pathway of lysine in Saccharomyces. Mol Gen Genet. 1972;115(1):26–30. doi: 10.1007/BF00272214. [DOI] [PubMed] [Google Scholar]
- Borell C. W., Urrestarazu L. A., Bhattacharjee J. K. Two unlinked lysine genes (LYS9 and LYS14) are required for the synthesis of saccharopine reductase in Saccharomyces cerevisiae. J Bacteriol. 1984 Jul;159(1):429–432. doi: 10.1128/jb.159.1.429-432.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoo B. B., Sherman F., Azubalis D. A., Fjellstedt T. A., Mehnert D., Ogur M. Selection of lys2 Mutants of the Yeast SACCHAROMYCES CEREVISIAE by the Utilization of alpha-AMINOADIPATE. Genetics. 1979 Sep;93(1):51–65. doi: 10.1093/genetics/93.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Magasanik B. Ammonia regulation of amino acid permeases in Saccharomyces cerevisiae. Mol Cell Biol. 1983 Apr;3(4):672–683. doi: 10.1128/mcb.3.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS H. C., HAWTHORNE D. C. ENZYMATIC EXPRESSION AND GENETIC LINKAGE OF GENES CONTROLLING GALACTOSE UTILIZATION IN SACCHAROMYCES. Genetics. 1964 May;49:837–844. doi: 10.1093/genetics/49.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES E. E., BROQUIST H. P. SACCHAROPINE, AN INTERMEDIATE OF THE AMINOADIPIC ACID PATHWAY OF LYSINE BIOSYNTHESIS. II. STUDIES IN SACCHAROMYCES CEREVISEAE. J Biol Chem. 1965 Jun;240:2531–2536. [PubMed] [Google Scholar]
- KURAHASHI K., WAHBA A. J. Interference with growth of certain Escherichia coli mutants by galactose. Biochim Biophys Acta. 1958 Nov;30(2):298–302. doi: 10.1016/0006-3002(58)90054-4. [DOI] [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytka J. Positive selection of general amino acid permease mutants in Saccharomyces cerevisiae. J Bacteriol. 1975 Feb;121(2):562–570. doi: 10.1128/jb.121.2.562-570.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A. K., Bhattacharjee J. K. Control of a lysine-biosynthetic step by two unlinked genes of Saccharomyces. Biochem Biophys Res Commun. 1970;39(6):1205–1210. doi: 10.1016/0006-291x(70)90689-3. [DOI] [PubMed] [Google Scholar]
- Winston M. K., Bhattacharjee J. K. Growth inhibition by alpha-aminoadipate and reversal of the effect by specific amino acid supplements in Saccharomyces cerevisiae. J Bacteriol. 1982 Nov;152(2):874–879. doi: 10.1128/jb.152.2.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]


