Abstract
Although spermatogenesis is essential for reproduction, little is known about spermatogonial stem cells. These cells provide the basis for spermatogenesis throughout adult life by undergoing self-renewal and by providing progeny that differentiate into spermatozoa. A major impediment to our understanding of the biology of these stem cells is the inability to distinguish them from spermatogonia that are committed to differentiation. We made use of the known association of stem cells with basement membranes and our spermatogonial transplantation assay system to identify specific molecular markers on the stem cell surface. Selection of mouse testis cells with anti-β1- or anti-α6-integrin antibody, but not anti-c-kit antibody, produced cell populations with a significantly enhanced ability to colonize recipient testes and generate donor cell-derived spermatogenesis. We demonstrate spermatogonial stem cell-associated antigens by using an assay system based on biological function. Furthermore, the presence of surface integrins on spermatogonial stem cells suggests that these cells share elements of a common molecular machinery with stem cells in other tissues.
Spermatogenesis is a complex process that originates with and depends on spermatogonial stem cells. These primitive cells reside on the basement membrane of the seminiferous tubule and slowly proliferate to provide (a) additional stem cells by self-renewal, and (b) progeny cells that undergo significant amplification during the differentiation process to spermatozoa (1, 2). A single stem cell in the rat is capable of generating more than 4,000 spermatozoa, although many progeny cells are lost through programmed cell death during normal spermatogenesis (3–5). The differentiation process consists of three main phases: spermatogonial amplification, meiotic recombination, and spermatid differentiation. Although considerable information exists regarding differentiation stages of spermatogenesis, stem cell biology is poorly understood. The location of stem cells on the basement membrane and their general morphology are known; however, no specific biochemical or surface antigen markers have been identified (3–5). Consequently, detailed studies on the biology of this important cell have been severely handicapped.
The body also contains self-renewing stem cell systems in other tissues, including hematopoietic, intestinal epithelial, and epidermal (6). All are characterized by a stem-cell population and a differentiating cell population. The number of stem cells relative to differentiating cells is low in each system; in testes there are estimated to be about two stem cells per 104 total testis cells, making these rare cells difficult to isolate and study (7). Because stem cells of different tissues show certain similarities in biological behavior, we hypothesized that they might share similar molecular properties even though the cell types or number of lineages produced during differentiation of daughter cells might be quite variable (6, 8). For instance, stem cells divide slowly and are usually on the basement membrane situated in a protected region, often described as a niche, among supporting cells. After damage by irradiation or chemicals, the stem cell is the last cell type destroyed and can regenerate the entire system in a relatively short time and in a regulated manner. Also, the stem cell often has the capacity to migrate during development, after transplantation, or when part of the population is destroyed (5, 6, 8). Thus, although the molecular mechanisms that control these events are not well understood for any of the self-renewing systems, it seems reasonable to assume that similar characteristics of stem cell activity may reflect shared molecular properties. For example, both steel factor and its surface tyrosine kinase receptor, c-kit, are critical for development and maintenance of both hematopoiesis and spermatogenesis (9), and c-kit appears on early stages of spermatogonia (10). Moreover, mice deficient in telomerase have defects in several systems that depend on stem cell self-renewal (11). These facts strongly argue for the existence of other shared molecules.
Among self-renewing systems, hematopoiesis has been the best characterized. Identification of several stem cell markers now has facilitated purification of stem cells in a definitive manner (12) and led to isolation of single hematopoietic stem cells (HSC) (13). Success in this endeavor was possible only because a functional assay system was developed. Initially, donor cells were injected into irradiated animals, and spleen colony formation was assessed to indicate putative stem cells (14). Although considerable information evolved from this assay system, these cells proved to be progenitor cells rather than true stem cells. Definitive identification of the HSC was achieved by using the more laborious competitive long-term reconstitution analysis, in which transplanted cells repopulate the hematopoietic system (15). Thus, only a functional assay system capable of determining both long-term regenerative ability and adequacy of differentiated cell types is able to definitively identify the presence of a stem cell population.
Recently, a system to transplant mouse testis cells from a fertile donor male to the seminiferous tubules of an infertile recipient male has been developed (16, 17). Spermatogenesis is generated from transplanted cells, and recipients are capable of transmitting the donor haplotype to progeny (17). After transplantation, primitive donor spermatogonia migrate to the basement membrane of recipient seminiferous tubules and begin proliferating (18, 19). Eventually, these cells establish stable colonies with a characteristic appearance, which expands and produces differentiating germ cells, including mature spermatozoa (18, 19). Thus, the transplanted cells self-renew and produce progeny that differentiate into fully functional spermatozoa. By definition, only stem cells could produce this result. Therefore, the spermatogonial transplantation system provides a method to determine the spermatogonial stem cell potential of any donor cell population. In particular, the spermatogonial transplantation technique allows identification of individual colonization events, a strength not available in bone marrow stem cell transplantation assays (19). In this report, we describe the use of spermatogonial transplantation to test the hypothesis that spermatogonial stem cells, like those of other self-renewing systems, display certain biochemical or antigenic markers, including cell surface molecules associated with attachment to basement membranes.
MATERIALS AND METHODS
Donor Mice and Cell Collection.
Donor cells were isolated from the ROSA26 transgenic mouse line, designated B6,129-TgR(ROSA26)26Sor, purchased from The Jackson Laboratory. These mice express the Escherichia coli LacZ gene in many cell types, including all stages of spermatogenesis (20). Expressing cells can be stained blue after incubation with the substrate 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal). Mice were maintained on a C57BL/6 × 129/Sv genetic background. Cells for transplantation were obtained from the testes of 4- to 6-week-old adult mice by using an enzymatic digestion procedure to produce a suspension of cells as described (20, 21).
Selection of Testis Cell Subpopulations.
Several techniques were used to isolate or enrich for cells with specific characteristics. For selection by extracellular matrix (ECM) molecules, 60-mm Falcon Petri dishes were incubated overnight at 37°C with laminin, fibronectin, or collagen type IV (all from Sigma) at a concentration of 20 μg/ml of PBS. The solution was removed, and the dishes were washed three times with 2 ml of PBS. Nonspecific binding was prevented by preincubating the dishes with 0.5 mg/ml of BSA for 1 hr at 37°C. The dishes then were washed three times with PBS before adding cells. In each experiment, 4 × 107 cells were suspended in 8 ml of DMEM plus 10% FBS, which was divided among four coated dishes. The cells were incubated for 1 hr at 32°C, and the plates washed five times with PBS to remove unbound cells. The attached cells then were removed by trypsin (0.25%)-EDTA (1 mM) digestion for 5 min followed by strong pipetting.
In some experiments, magnetic beads (Dynabeads, Dynal) were used to isolate subpopulations of cells enriched for β1-integrin, c-kit receptor, or α6-integrin. For positive selection, 4 × 107 testis cells were suspended in 1 ml of DMEM containing 1% FBS (DMEM/FBS) with 15 μg/ml of primary antibody. Biotinylated hamster anti-β1-integrin (Ha2/5) antibody (PharMingen) was used with Dynabeads M-280 streptavidin (32 μl) to select cells expressing β1-integrin molecules. A rat anti-c-kit (ACK4) antibody (a gift from S.-I. Nishikawa, Kyoto University, Kyoto, Japan) was used with Dynabeads M-450 sheep anti-rat IgG (84 μl) to select cells expressing c-kit receptor. A rat anti-α6-integrin (GoH3) antibody (PharMingen) was used with Dynabeads M-450 sheep anti-rat IgG (84 μl) to select cells expressing α6-integrin molecules. The cells plus antibody were incubated in a 16-ml plastic tube for 30 min at 4°C with agitation (20 rpm). After washing three times with 5 ml of PBS containing 1% FBS (PBS/FBS), the cells were suspended in 1 ml of DMEM/FBS, and the magnetic beads were added. The cells were incubated for 1 hr at 4°C, then 4 ml of PBS/FBS was added, and the cells were isolated according to the manufacturer’s recommendation. To remove as many cells as possible that express β1-integrin before injecting the remaining cell population, negative selection was performed as described above, except that a larger quantity of magnetic beads (1 ml) was used in 5 ml of medium containing 4 × 107 cells.
Control cells for each experiment were taken from the testis cell population before selection. Cells for injection into testes were suspended in 100 μl of DMEM containing 10% FBS; viability (trypan blue exclusion) was greater than 90%. Because the number of cells selected by ECM or beads was small, 4–5 × 106 STO (SIM mouse fibroblasts) cells were added as carriers. Experimental and control donor cells were maintained on ice, and then microinjected at equal concentrations into the efferent ducts of recipient testes (21). Approximately 10 μl could be introduced into the seminiferous tubules of a busulfan-treated mouse, and 75–85% of the tubules in each testis was filled with the cell suspension (21).
Flow Cytometry.
Flow cytometric analyses were performed on enriched populations of testis cells by using standard procedures (22). Briefly, 106 cells were suspended in 0.1 ml of PBS/FBS. To identify β1-integrin-positive cells from the population of ECM-adherent cells, 1 μg hamster anti-β1-integrin antibody in 20 μl of PBS/FBS was added to the cells collected by trypsin digestion for 20 min at 4°C. The cells were then washed twice in 1 ml of PBS/FBS and 1 μg FITC-conjugated goat anti-hamster IgG in 20 μl of PBS/FBS was added for 20 min at 4°C. To identify c-kit-positive cells from the population of ECM-adherent cells, 1 μg R-phycoerythrin (PE)-conjugated rat anti-c-kit antibody (2B8, PharMingen) in 5 μl of PBS/FBS was added to the cells collected by trypsin digestion for 20 min at 4°C. To remove excess FITC- or PE-conjugated antibody, the cells were washed twice with 1 ml of PBS/FBS. Control cells were not treated with anti-β1-integrin antibody or PE anti-c-kit antibody. Unselected cells used as reference standards were treated the same as selected cells. Similar procedures were used to examine cell populations isolated via magnetic bead enrichment. For β1-integrin, cells were treated with 1 μg of FITC-conjugated goat anti-hamster IgG; for c-kit, cells were treated with 1 μg of PE-conjugated rat anti-c-kit antibody; and for α6-integrin, cells were treated with 1 μg of FITC-conjugated donkey anti-rat IgG. Control cells were not treated with FITC anti-hamster IgG, PE anti-c-kit antibody, or FITC anti-rat IgG. Unselected cells for reference were treated the same as in ECM flow cytometry. Cells were kept in the dark on ice until analysis on a Becton-Dickinson FACSCalibur. At least 10,000 events were acquired for each sample.
Recipient Mice and Analysis of Testes.
LacZ-marked donor cells were transplanted into testes of immunologically compatible C57BL/6 × 129/Sv F1 hybrid mice that had been treated with busulfan (50 mg/kg body weight) at 4–6 weeks of age (19). Experimental and control recipient mice received a single injection of a gonadotrophin-releasing hormone agonist (leuprolide acetate, TAP Pharmaceuticals, Deerfield, IL, 7.6 mg/kg, s.c.) at −44 to +1 day relative to cell transplantation to enhance colonization (23). Recipient testes were collected 2 months after donor cell transplantation and analyzed by X-gal staining as described (19, 21). Occasionally, injected recipient testes showed fibrosis or were firm at the time of recovery, most likely associated with transplantation-related inflammation; these testes generally are not colonized by donor cells, thus were excluded from analysis. Seminiferous tubules were spread on a microscope slide, and images were acquired via a computer imaging system (24). Appearance of individual blue-stained stretches of seminiferous tubules in recipient testes reflects colonization and generation of donor stem cell-derived spermatogenesis (19, 21, 24). Other types of testis cells cannot regenerate spermatogenesis, and any remaining endogenous recipient spermatogenesis will not produce blue-stained cells. A typical result is shown in Fig. 1. The most useful parameters to measure were the number of individual colonies and the total blue area (mm2) colonized by donor cells, which are measures of stem cell potential of the donor cell population (24). However, when a large number of colonies is present, the ends of some colonies are likely to fuse; in addition, artificial breakage of long colonies during seminiferous tubule spreading may bias the number of separate colonized segments of tubule. These artifacts may influence colony number when large numbers or long stretches of colonies are present. Therefore, both colony number and blue area were determined and used in analysis of results. Because the number of cells that could be recovered and injected on any day varied, results were normalized to number of colonies or colonized area for donor cells injected at a concentration of 107 cell/ml. Statistical analysis was performed by Student’s t test using the systat program (SPSS, Chicago).
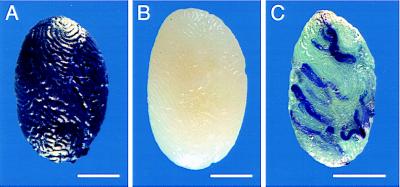
Figure 1.
Transplantation of ROSA26 testis cells. (A) Testis from ROSA26 transgenic mouse incubated in X-gal showing blue staining of donor cells. (B) Testis from a C57BL/6 × 129/SvF1 recipient mouse demonstrating that cells do not stain. (C) Recipient testis 2 months after transplantation of ROSA26 donor cells. Blue stretches of seminiferous tubules represent colonies formed by donor spermatogonial stem cells that have expanded to produce areas of spermatogenesis. (Bar = 2 mm.)
RESULTS
Analysis of Testis Cells Based on Surface Antigens with Affinity for ECM Molecules.
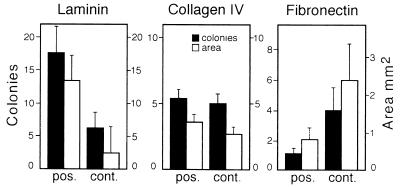
Because many types of stem cells adhere to basement membranes, testis cells initially were plated on Petri dishes coated with ECM molecules. On the basis of studies with other cell types, laminin, collagen type IV, and fibronectin were selected as the most likely surfaces to which spermatogonial stem cells might bind. Two experiments were performed with each ECM molecule, and the average recovery of adherent cells was approximately 7%, 10%, and 6% for laminin, collagen, and fibronectin, respectively, and total recovered cells ranged from 1 to 5 × 106. Adherent and control cells were transplanted, and the recipient testes were analyzed 2 months later by X-gal staining. The number of blue stretches of tubule (colonies) and the total blue area (mm2) were determined for each experimental and control testis (Fig. 2). Laminin-selected cells colonized recipient testes 3- to 4-fold better than control cells. The effect was significant for both colony number and total blue area (P < 0.05). Collagen type IV-selected testis cells were not significantly different from control cells. Fibronectin-selected testis cells colonized recipient testes only about one-third as well as control cells, but the effect was not significant for either colony number or blue area.
Figure 2.
Colonization of recipient testes by transplanted ROSA26 donor cells that adhered to laminin, collagen type IV, or fibronectin-coated plates. The degree of colonization in two experiments is represented by number of individual blue colonies (solid bars) and by total blue area (open bars). The values are mean ± SEM (n = 8 testes, except fibronectin control n = 7). The difference between adherent and control values is significant (P < 0.05) only for laminin.
Because β1-integrin often is involved in attachment of cells to ECM molecules, flow cytometry was used to determine whether testis cells selected on coated Petri dishes were enriched for β1-integrin. Cells that adhered to laminin, collagen type IV, and fibronectin all were enriched for β1-integrin-positive cells, but enrichment was best with laminin (Table 1). When testis cells selected by adhesion to laminin were treated with FITC-conjugated antibody to identify β1-integrin, 23% demonstrated higher fluorescence than similarly selected cells not treated with specific antibody (controls). Because many studies have indicated that the c-kit receptor is present on spermatogonia, similar flow cytometric analysis was performed for this antigen. Both fibronectin and collagen type IV were superior to laminin for selection of cells with c-kit on their surface (Table 1).
Table 1.
Presence of β1-integrin and c-kit surface antigens on testis cells selected with ECM molecules
| Type of selection | Antigen
|
|
|---|---|---|
| β1-integrin | c-kit | |
| Laminin | 23.0 ± 3.8 | 25.5 ± 2.9 |
| Collagen IV | 15.6 ± 0.8 | 34.0 ± 3.2 |
| Fibronectin | 16.8 ± 2.0 | 43.2 ± 3.4 |
| None | 2.5 ± 0.4 | 7.0 ± 1.1 |
Values are percent (mean ± SEM, n = 3) of mouse testis cells selected by the indicated ECM molecule that demonstrated high fluorescence (above control cell fluorescence) when treated with FITC- or PE-conjugated antibody to the designated antigen, and then analyzed by flow cytometry.
Transplantation of Cells Selected for β1-Integrin Surface Antigen.
To examine the possibility that testis cells with β1-integrin on their surface might be more effective in colonizing recipient testes, a magnetic bead procedure was used to select testis cells positive for this surface molecule. In these experiments, 1.1–1.4 × 106 β1-integrin-positive cells, about 3% of total cells, could be selected. After separate transplantation of equal numbers of selected versus control cells, recipient testes were analyzed for donor cell-derived colonies of spermatogenesis (Fig. 3A). The number of blue colonies and total blue area were increased by a factor of 3.8 and 5.0, respectively, (P < 0.005) for anti-β-integrin antibody-selected cells compared with controls.
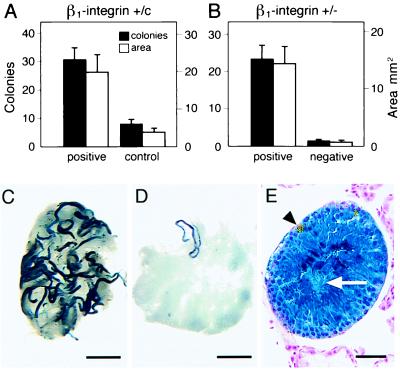
Figure 3.
Expression of β1-integrin on spermatogonial stem cells. (A) Enhanced colonization of recipient testes by transplanted ROSA26 donor cells selected with anti-β1-integrin antibody. Approximately 10 μl of cell suspension (107 cells per ml) was injected into each testis (see Materials and Methods). Degree of colonization in three experiments is represented by number of individual blue colonies (solid bars) and by total blue area (open bars). The values are mean ± SEM (n = 12 experimental and 14 control testes). Difference between selected (positive) and unselected cells (control) is significant (P < 0.005). (B) Colonization by cells selected with anti-β1-integrin antibody (positive cells) and testis cells from which β1-integrin positive cells were removed with anti-β1-integrin antibody (negative cells). Degree of colonization in three experiments is represented by number of blue colonies and total blue area (mean ± SEM; n = 12 and 14 testes for positive and negative selection, respectively). Difference between positive and negative selected cells is significant (P < 0.001). (C) Appearance of recipient testis 2 months after β1-integrin-positive donor cell transplantation. (D) Appearance of recipient testis 2 months after β1-integrin-negative cell transplantation. Stain C and D: X-gal. (Bar = 2 mm.) (E) Donor cell-derived spermatogenesis from β1-integrin-positive cells. Note normal appearance and organization of germ cells and presence of spermatozoa tails in center of seminiferous tubules (arrow). Small gold objects are magnetic beads in Sertoli cells (arrowhead). Stain: X-gal and nuclear fast red. (Bar = 50 μm.)
To further examine the association between β1-integrin and spermatogonial stem cells, testis cells were either positively or negatively selected for β1-integrin. In three experiments, 0.74–1.1 × 106 β1-integrin-positive cells were selected, and an equal number of β1-integrin negatively selected cells, from the same three testis cell preparations, was used for injection. After transplantation to recipient testes, the number of blue colonies and blue area was increased by a factor of 14.6 and 19.3, respectively (P < 0.001) for β1-integrin-positive compared with -negative cells (Fig. 3B). These ratios were greater for both the number of colonies and blue area than were found for β1-integrin-positive compared with control cells (Fig. 3A).
The difference in both number of colonies and blue area generated by anti-β1-integrin antibody-selected cells compared with nonselected or negatively selected cells was significant, and the difference in appearance of the recipient testes was dramatic (Fig. 3 C and D). However, individual colonies generated by experimental and control cells appeared similar and suggested that the colonization process and subsequent spermatogenesis were unaffected by selection. In addition, histological examination of blue areas resulting from anti-β1-integrin antibody selected cells confirmed that normal spermatogenesis was present (Fig. 3E). Interestingly, magnetic beads, which were injected with donor cells, were still present in Sertoli cells of recipient testes 2 months after transplantation (Fig. 3E).
Transplantation of Testis Cells Selected for c-kit Surface Antigen.
The importance of c-kit in early spermatogenesis led us to examine the ability of cells with this surface antigen to colonize testes. In three separate experiments, 0.71–1.27 × 106 c-kit-positive cells were recovered and injected into recipient testes. The number of colonies generated per testis was 5.8 ± 1.7 (n = 13) and 3.4 ± 0.7 (n = 15) from c-kit-positive and control cells, respectively, and the total blue area (mm2) was 2.9 ± 0.9 (n = 13) and 1.6 ± 0.3 (n = 15) from positive and control cells, respectively (means ± SEM). Thus, cells positive for c-kit were not significantly better than controls in their ability to colonize testes. As an additional measure of the presence of c-kit on spermatogonial stem cells, β1-integrin selected cells were analyzed by flow cytometry to determine whether they also contained c-kit-positive cells (Fig. 4). Flow cytometric analysis with anti-β1-integrin antibody demonstrated a 13.9-fold enrichment for these cells. However, the levels of c-kit expression in β1-integrin selected and nonselected cells were the same. Thus, the population of testis cells with β1-integrin surface antigen are not enriched for c-kit-positive cells.
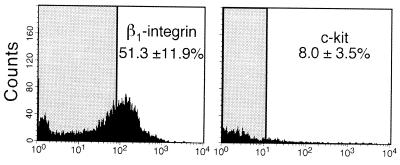
Figure 4.
Flow cytometric analysis of anti-β1-integrin antibody selected cells. (A) Fluorescence of β1-integrin selected cells treated with FITC-conjugated antibody was compared with fluorescence of similar cells (controls) not treated with FITC-conjugated antibody. The shaded area shows the range of fluorescence of controls. Fluorescence was measured in arbitrary units on a log scale. The average percent of high fluorescing β1-integrin cells above the control range (shaded area) in three experiments was 51.3 ± 11.9 (mean ± SEM). The value for unselected cells was 3.7 ± 1.2 (not shown). (B) Cells selected as in A were treated with anti-c-kit antibody conjugated to PE and analyzed as above. In three experiments, the average percent of high fluorescing c-kit-expressing cells above controls in the anti-β1-integrin antibody selected cell population was 8.0 ± 3.5 (mean ± SEM). The value for unselected cells was 7.8 ± 2.1 (not shown).
Transplantation of Testis Cells Selected for α6-Integrin Surface Antigen.
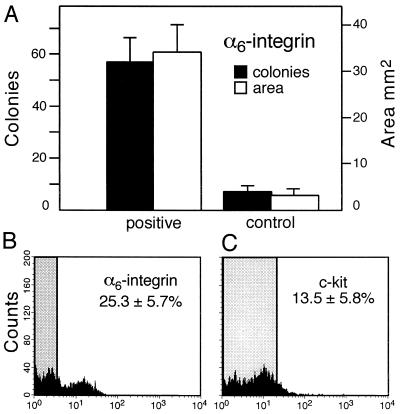
Because it is well established that β-integrins form heterodimeric complexes with α-chains (25), we used magnetic beads to screen several anti-α-integrin antibodies (α1,2,4,5,6) for their ability to select subpopulations of testis cells with an increased potential to colonize recipient testes. Of these, only anti-α6 antibody showed an effect (data not shown). Therefore, more extensive studies were undertaken with anti-α6-integrin antibody by using magnetic beads to enrich for this cell population. In three separate experiments, 0.73–0.85 × 106 cells were selected and transplanted to recipient testes (Fig. 5A). The number of blue colonies was increased by a factor of 8.4 and blue area by a factor of 10.2 (P < 0.001). Testis cells selected by anti-α6-integrin antibody were subjected to flow cytometric analysis to determine the degree of enrichment. In cells selected with anti-α6-integrin antibody, the ratio of α6-integrin cells with high fluorescence compared with unselected cells with high fluorescence was 13.2, whereas the ratio of c-kit cells with high fluorescence to unselected cells was 2.2 (Fig. 5 B and C). Thus, as found for β1-integrin, the α6-integrin-enriched cell population was enriched little, if at all, for c-kit-positive cells.
Figure 5.
Expression of α6-integrin on spermatogonial stem cells. (A) Enhanced colonization of recipient testes by transplanted ROSA26 donor cells selected with anti-α6-integrin antibody. Approximately 10 μl of cell suspension (107 cells per ml) was injected into each testis (see Materials and Methods). Degree of colonization in three experiments is represented by number of individual blue colonies and by total blue area (mean ± SEM; n = 15 experimental and control testes). Difference between selected and unselected cells is significant (P < 0.001). (B) For flow cytometric analysis, cells selected with anti-α6-integrin antibody were treated with FITC-conjugated antibody. The fluorescence of α6-integrin selected cells was compared with similar cells not treated with FITC-conjugated antibody. The shaded area shows the range of fluorescence of controls. The average percent of high fluorescing α6-integrin cells above the control range in three experiments was 25.3 ± 5.7 (mean ± SEM). The value for unselected cells was 1.9 ± 0.4 (not shown). (C) Cells selected as in B were treated with anti-c-kit antibody conjugated to PE and analyzed as above. The average percent of high fluorescing c-kit-expressing cells above the control range (shaded area) in the α6-integrin selected cell population in three experiments was 13.5 ± 5.8 (mean ± SEM). The value for unselected cells was 6.1 ± 1.3 (not shown).
DISCUSSION
Because stem cells are thought to be associated with basement membranes, we investigated whether there was a correlation between the adhesiveness of testis cells to ECM molecules and their ability to form spermatogenic colonies. Bone marrow stem cells have an affinity for fibronectin, keratinocytes for type IV collagen, and postmigratory primordial germ cells (PGC) for laminin (26–28). Thus, these were logical candidate molecules to examine. The results with testis cells demonstrated that spermatogonial stem cells adhere preferentially to laminin, but not to type IV collagen or fibronectin. The primordial germ cell migrates from the base of the allantois to the genital ridge during early fetal development, and this process seems to depend on interstitial laminin and fibronectin on the route to the genital ridge (28). It has been demonstrated that PGC adhesiveness changes during migration to the genital ridge and that germ cells adhere very strongly to laminin at the end of the migratory journey (28).
The adhesiveness of spermatogonial stem cells to laminin suggested the involvement of integrin for several reasons. First, it has been established that the α6- and β1-integrin dimer acts as a laminin receptor (25). Second, flow cytometric analysis of testis cells that adhered to laminin indicated that this population of cells was enriched for cell surface β1-integrin. Interestingly, the α6- and β1-integrin dimer is expressed on the early female primordial germ cells, continues to appear on the surface of the oocyte, and mediates egg-sperm fusion at fertilization (29, 30). We reasoned that α6- and β1-integrin also may continue to be expressed in postnatal stages of male germ-line stem cells. Therefore, specific antibody for β1-integrin was used to select spermatogonial stem cells, which proved to be very effective at enriching for these cells in our functional assay. Furthermore, exposing testis cells to anti-β1-integrin antibody and then removing positive cells with magnetic beads (negative selection) reduced the ability of the remaining cells to colonize recipient testes. These findings are in agreement with several reports that the stem cell or progenitor cell populations of other self-renewing tissues express integrin molecules. For example, β1-integrin is required for hematopoietic precursor cell migration into the fetal liver during development (31), colonization of hematopoietic organs after transplantation (26), and mobilization of these cells from bone marrow (32). In epithelium, keratinocyte stem cells can be isolated based on high expression of the integrin molecule (27). In addition, β1-integrins are expressed on cultured brain stem cells and regulate chain migration and division in these cells (33). The spermatogonial stem cell now can be added to the list of stem cells that express β1-integrin molecules on their surface.
Because the c-kit receptor is critical for spermatogonial division and early stages of male germ cell differentiation (10), we examined the colonizing ability of testis cells selected by anti-c-kit antibody, but failed to demonstrate significant stem cell enrichment by this approach. In addition, flow cytometric analysis of the β1-integrin-enriched and α6-integrin-enriched cell populations did not indicate a high number of c-kit-positive cells in these integrin-selected cells. It is possible that a small number of c-kit receptor molecules might not have been detected in our selection procedures, but other studies have questioned whether c-kit is expressed by or is important to the spermatogonial stem cell. For example, primordial germ cells are present in dominant-white-spotting mutants that lack the c-kit receptor, and spermatogonia are found on the basement membrane of the seminiferous tubules in these animals for a period of time after birth (9, 34). Furthermore, in adult wild-type mice, undifferentiated spermatogonia proliferate normally even when the receptor is blocked with antibody, although more mature spermatogonia and other germ cells disappear (10). On the other hand, there is evidence that c-kit is expressed and important during fetal stem cell development, because c-kit ligand is necessary for embryonic germ cell formation (35), and the receptor can be identified on migrating primordial germ cells (36). However, in vitro culture studies indicate that proliferation of primitive type A spermatogonia 2 days after birth is independent of c-kit ligand (37). Taken together, these results suggest that the molecule is present in the fetal stage but disappears from the stem cell by 2 days after birth. Our studies add additional evidence that, in the adult, c-kit may be absent from or present in only low levels on the spermatogonial stem cell.
The enrichment of stem cells in laminin-selected testis cell populations suggested that an α-β-integrin molecule could be involved in adhesion, because these molecules have been demonstrated to be important for cell binding to laminin (25). Therefore, after identifying β1-integrin as a surface molecule on spermatogonial stem cells, we screened for the presence of several α-integrins, including α1, α2, and α6, which are known to be important in binding other cell types to laminin. Only α6-integrin appeared promising as a surface marker, and further selection studies with anti-α6-integrin antibody demonstrated a definite enrichment of stem cell potential in this subpopulation of cells. In fact, cells selected by anti-α6-integrin antibody appeared to be twice as effective in colonizing testes as those selected by anti-β1-integrin antibody (Figs. 3A and 5A). Perhaps β1-integrin combines with α-integrin molecules other than α6 on different cell types that do not have stem cell potential. Immunohistochemical studies on rat testes demonstrate that Sertoli cells and several stages of germ cell differentiation express β1-integrin (38). Alternatively, anti-α6-integrin antibody may be more effective than anti-β1-integrin antibody in selection of cells with the target antigen. Nonetheless, the results of our studies indicate that β1- and α6-integrins are markers to identify spermatogonial stem cells and suggest they form a dimer on the surface of the spermatogonial stem cell.
The identification of β1- and α6-integrins as marker molecules on spermatogonial stem cells provides evidence to support our hypothesis that stem cells of many self-renewing systems share molecular properties. We used this putative similarity among stem cells of different tissues and a functional assay to identify surface markers of spermatogonial stem cells. The degree of enrichment of stem cells attainable by this method will allow further fractionation and analysis of the enriched cell population to identify a set of additional antigens characteristic of and unique for spermatogonial stem cells. A systematic evaluation of surface molecules on the stem cell will facilitate identification and purification of these cells and greatly contribute to our understanding of their biology and survival requirements. This approach mirrors developments made with hematopoietic stem cells during the last decade. As stem cell markers are identified, enrichment for stem cell populations can be increased, facilitating their eventual purification. A primary goal will be to culture and genetically modify purified stem cells from many species.
Acknowledgments
We thank Drs. I. Dobrinski, M. Kotlikoff, M. Nagano, K. Orwig, and E. Sandgren for critical evaluation of the manuscript, C. Freeman and R. Naroznowski for assistance with animal maintenance, and J. Hayden for help with photography. T.S. was supported by the Japan Society for the Promotion of Science. Financial support was from the National Institutes of Health (National Institute of Child Health and Human Development Grant 36504), U.S. Department of Agriculture, Commonwealth and General Assembly of Pennsylvania, and Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation.
ABBREVIATIONS
- ECM
extracellular matrix
- PE
R-phycoerythrin
- X-gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Huckins C. Anat Rec. 1971;169:533–558. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 2.Clermont Y. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 3.Russell L D, Ettlin R A, Sinha Hikim A P, Clegg E D. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. pp. 1–40. [Google Scholar]
- 4.de Kretser D M, Kerr J B. In: The Physiology of Reproduction. 2nd Ed. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 1177–1290. [Google Scholar]
- 5.de Rooij D G, Grootegoed J A. Curr Opin Cell Biol. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- 6.Potten C S. In: Oxford Textbook of Pathology. McGee J O, Isaacson P G, Wright N A, editors. Oxford: Oxford Univ. Press; 1992. pp. 43–52. [Google Scholar]
- 7.Meistrich M L, van Beek M E A B. In: Cell and Molecular Biology of the Testis. Desjardins C, Ewing L L, editors. New York: Oxford Univ. Press; 1993. pp. 266–295. [Google Scholar]
- 8.Morrison S J, Shah N M, Anderson D J. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 9.Huang E, Nocka K, Beier D R, Chu T-Y, Buck J, Lahm H-W, Wellner D, Leder P, Besmer P. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 10.Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S-I, Kunisada T, Fujimoto T, Nishikawa S-I. Development (Cambridge, UK) 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 11.Lee H-W, Blasco M A, Gottlieb G J, Horner II J W, Greider C W, DePinho R A. Nature (London) 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 12.Morrison S J, Weissman I L. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 13.Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 14.Till J E, McCulloch E A. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 15.Harrison D E. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 16.Brinster R L, Zimmermann J W. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinster R L, Avarbock M R. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parreira G G, Ogawa T, Avarbock M R, França L R, Brinster R L, Russell L D. Biol Reprod. 1998;59:1360–1370. doi: 10.1095/biolreprod59.6.1360. [DOI] [PubMed] [Google Scholar]
- 19.Nagano M, Avarbock M R, Brinster R L. Biol Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagano M, Brinster R L. Acta Pathol Microsc Immunol Scand. 1998;106:47–57. doi: 10.1111/j.1699-0463.1998.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa T, Aréchaga J M, Avarbock M R, Brinster R L. Intl J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 22.Kanatsu M, Nishikawa S-I. Development (Cambridge, UK) 1996;122:823–830. doi: 10.1242/dev.122.3.823. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa T, Dobrinski I, Avarbock M R, Brinster R L. Tissue Cell. 1998;30:583–588. doi: 10.1016/s0040-8166(98)80039-6. [DOI] [PubMed] [Google Scholar]
- 24.Dobrinski I, Ogawa T, Avarbock M R, Brinster R L. Mol Reprod Dev. 1999;53:142–148. doi: 10.1002/(SICI)1098-2795(199906)53:2<142::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 26.Williams D A, Rios M, Stephens C, Patel V P. Nature (London) 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 27.Jones P H, Watt F M. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 28.García-Castro M I, Anderson R, Heasman J, Wylie C C. J Cell Biol. 1997;138:471–480. doi: 10.1083/jcb.138.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida E A C, Huovila A-P J, Sutherland A E, Stephens L E, Calarco P G, Shaw L M, Mercurio A M, Sonnenberg A, Primakoff P, Myles D G, White J M. Cell. 1995;81:1095–1104. doi: 10.1016/s0092-8674(05)80014-5. [DOI] [PubMed] [Google Scholar]
- 30.Zuccotti M, Rossi P G, Fiorillo E, Garagna S, Forabosco A, Redi C A. Dev Biol. 1998;200:27–34. doi: 10.1006/dbio.1998.8923. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch E, Iglesias A, Potocnik A J, Hartmann U, Fässler R. Nature (London) 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 32.Craddock C F, Nakamoto B, Andrews R G, Priestley G V, Papayannoupoulou T. Blood. 1997;12:4779–4788. [PubMed] [Google Scholar]
- 33.Jacques T S, Relvas J B, Nishimura S, Pytela R, Edwards G M, Streuli C H, ffrench-Constant C. Development (Cambridge, UK) 1998;125:3167–3177. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- 34.Silvers W K. The Coat Colors of Mice: A Model for Mammalian Gene Action and Interaction. New York: Springer; 1979. pp. 206–267. [Google Scholar]
- 35.Matsui Y, Zsebo K, Hogan B L M. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 36.Orr-Urtreger A, Avivi A, Zimmer Y, Givol D, Yarden Y, Lonai P. Development (Cambridge, UK) 1990;109:911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- 37.Tajima Y, Sawada K, Morimoto T, Nishimune Y. J Reprod Fertil. 1994;102:117–122. doi: 10.1530/jrf.0.1020117. [DOI] [PubMed] [Google Scholar]
- 38.Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Biol Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]