Abstract
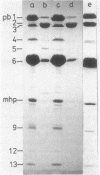
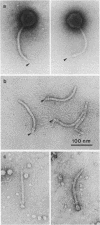
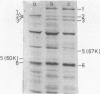
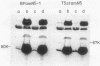
Treatment of purified tails of bacteriophage T5 with 0.05% sodium dodecyl sulfate specifically removed pb2, a protein of 108,000 molecular weight (108K), from the tail. Although these tails were devoid of the single straight tail fiber, they still inhibited adsorption of T5 to Escherichia coli cells. Reconstitution of these tails with pb2 increased the efficiency of inhibition of T5 adsorption. Treatment of tails with 0.1% sodium dodecyl sulfate removed, in addition to pb2, a protein of 67K from phage T5 and one of 60K from phage BF23. These tails failed to inhibit phage adsorption, and no reconstitution was achieved. Reconstitution of T5 tails with pb2 from BF23, and of BF23 tails with pb2 from T5, did not alter the host receptor specificity of the tails. Binding of untreated T5 tails to small FhuA receptor particles revealed that binding occurred with the conical part of the tail and that pb2 was most likely released from the tail upon binding. From these results and from recent observations with T5-BF23 hybrid phages (K.J. Heller, Virology 139:11-21, 1984), we conclude that the receptor-binding proteins of T5 and BF23 are the 67K and 60K proteins, respectively, and that they are not located at the tip of the tail but rather at or near the site where the straight tail fiber is attached to the conical part of the tail.
Full text
PDF

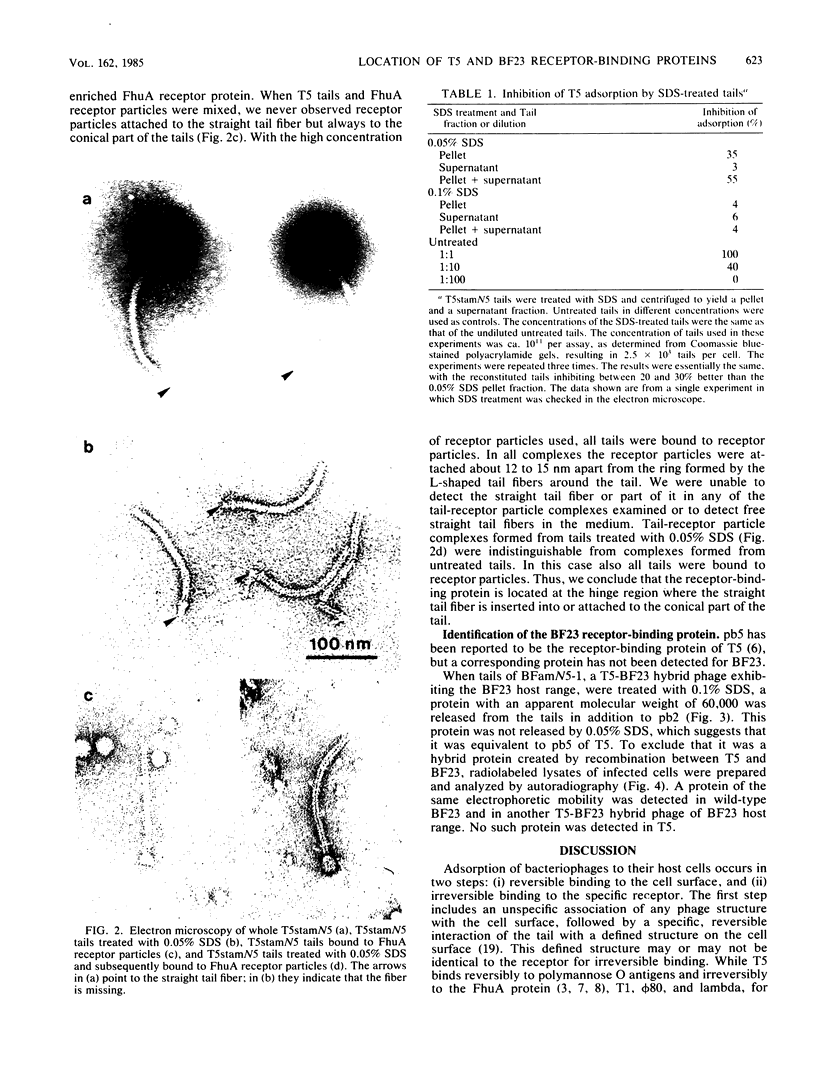
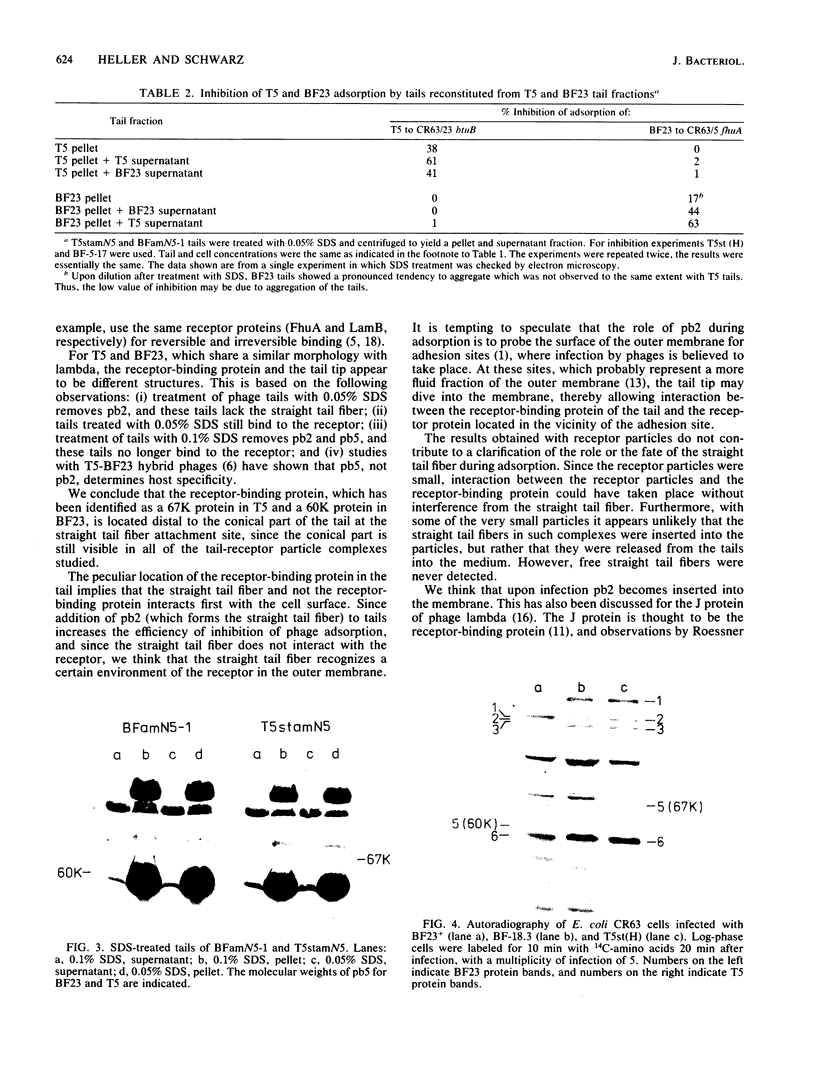

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. J., Bryniok D. O antigen-dependent mutant of bacteriophage T5. J Virol. 1984 Jan;49(1):20–25. doi: 10.1128/jvi.49.1.20-25.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. J. Identification of the phage gene for host receptor specificity by analyzing hybrid phages of T5 and BF23. Virology. 1984 Nov;139(1):11–21. doi: 10.1016/0042-6822(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Heller K., Braun V. Accelerated adsorption of bacteriophage T5 to Escherichia coli F, resulting from reversible tail fiber-lipopolysaccharide binding. J Bacteriol. 1979 Jul;139(1):32–38. doi: 10.1128/jb.139.1.32-38.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Braun V. Polymannose O-antigens of Escherichia coli, the binding sites for the reversible adsorption of bacteriophage T5+ via the L-shaped tail fibers. J Virol. 1982 Jan;41(1):222–227. doi: 10.1128/jvi.41.1.222-227.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson H. E., McCorquodale D. J. Genetic and physiological studies of bacteriophage t5 I. An expanded genetic map of t5. J Virol. 1971 May;7(5):612–618. doi: 10.1128/jvi.7.5.612-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- Roessner C. A., Ihler G. M. Proteinase sensitivity of bacteriophage lambda tail proteins gpJ and pH in complexes with the lambda receptor. J Bacteriol. 1984 Jan;157(1):165–170. doi: 10.1128/jb.157.1.165-170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner C. A., Struck D. K., Ihler G. M. Morphology of complexes formed between bacteriophage lambda and structures containing the lambda receptor. J Bacteriol. 1983 Mar;153(3):1528–1534. doi: 10.1128/jb.153.3.1528-1534.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigo K. Isolation of high-density mutants and identification of nonessential structural proteins in bacteriophage T5; dispensability of L-shaped tail fibers and a secondary major head protein. Virology. 1978 Apr;85(2):422–433. doi: 10.1016/0042-6822(78)90449-x. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Reversible interaction between coliphage lambda and its receptor protein. J Mol Biol. 1975 Nov 25;99(1):185–201. doi: 10.1016/s0022-2836(75)80167-7. [DOI] [PubMed] [Google Scholar]
- Schwartz M. The adsorption of coliphage lambda to its host: effect of variations in the surface density of receptor and in phage-receptor affinity. J Mol Biol. 1976 May 25;103(3):521–536. doi: 10.1016/0022-2836(76)90215-1. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Structural proteins of bacteriophage T5. Virology. 1973 Feb;51(2):443–453. doi: 10.1016/0042-6822(73)90443-1. [DOI] [PubMed] [Google Scholar]