Abstract
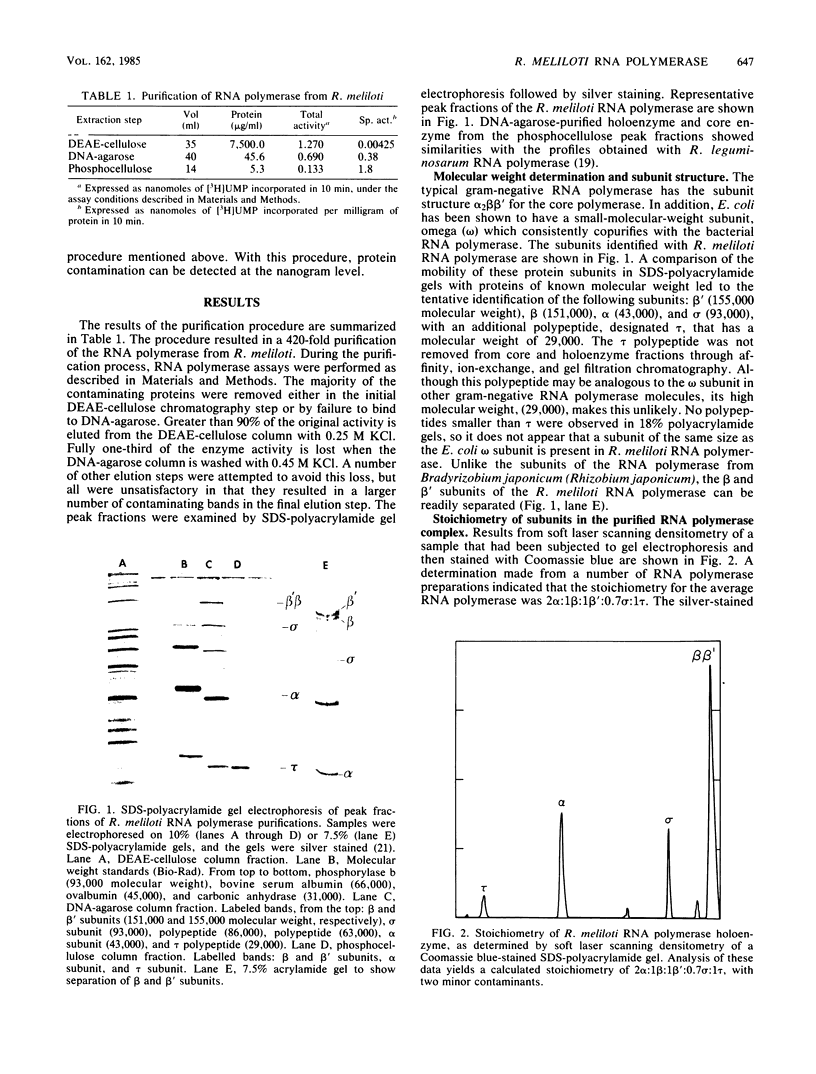
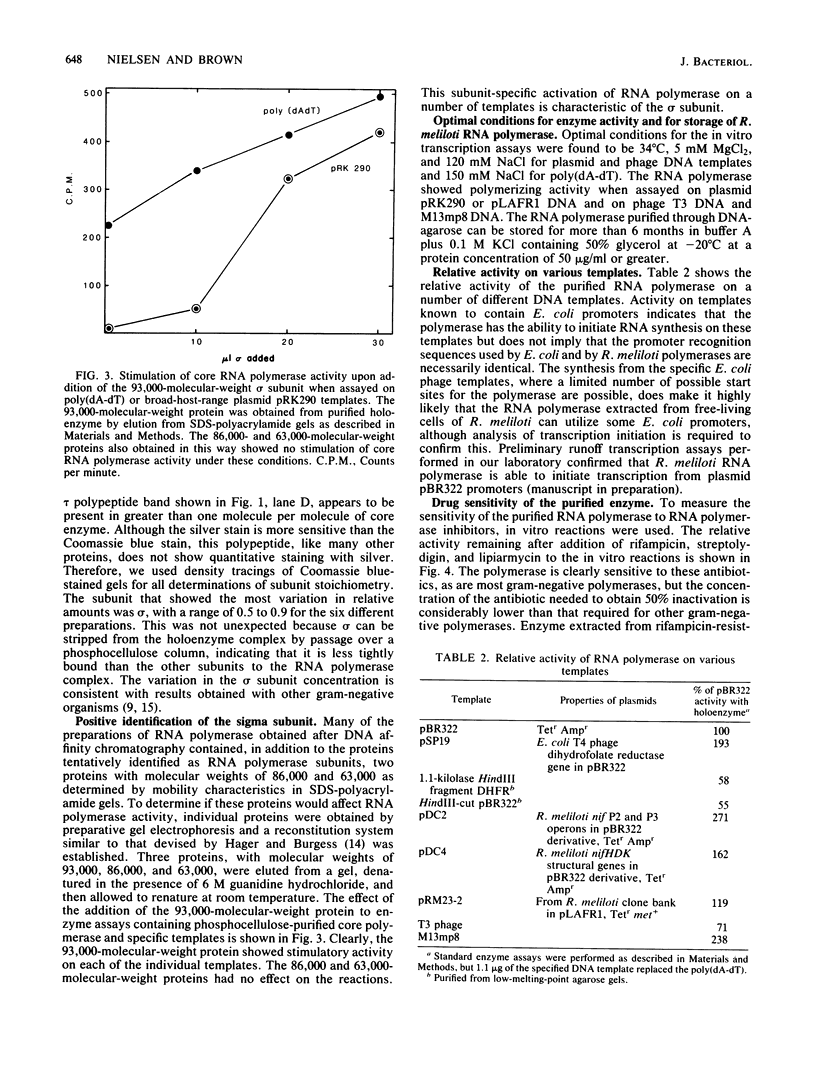
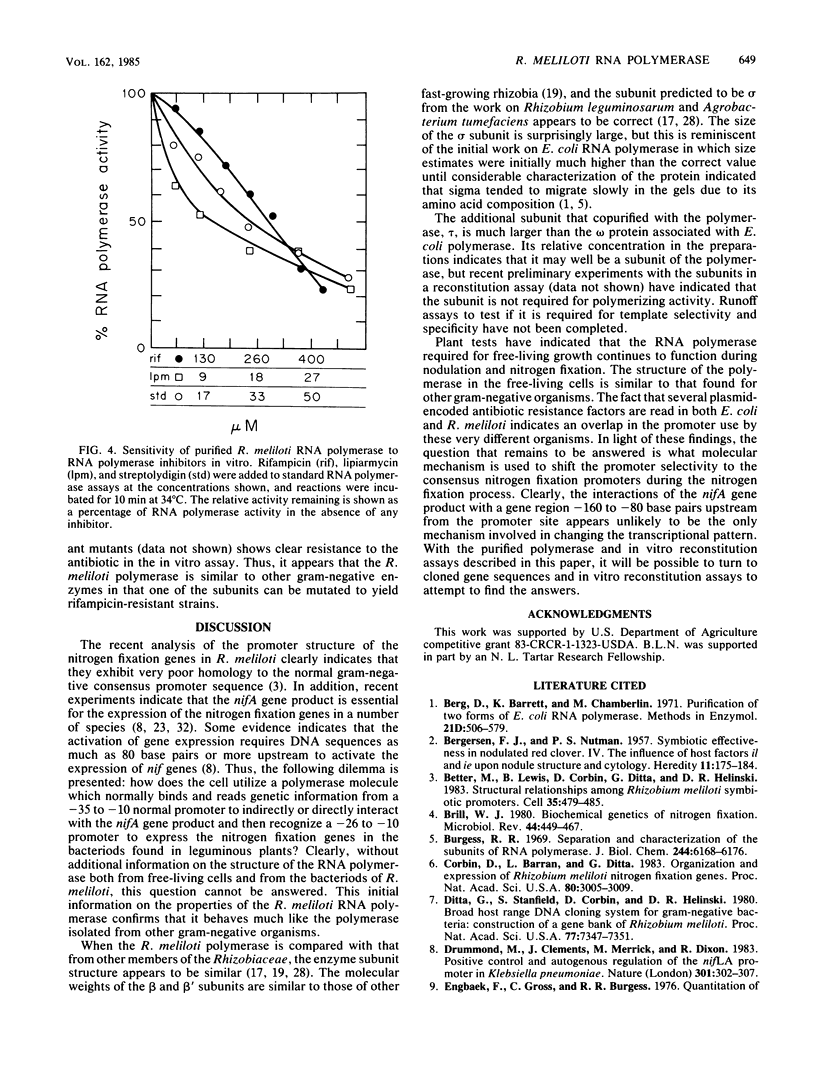
The RNA polymerase of the symbiotic, nitrogen-fixing bacterium Rhizobium meliloti was purified, and its subunit composition was determined. The cells were disrupted in the presence of protease inhibitors, and holoenzyme fractions were purified by fractionation by using DEAE-cellulose and DNA-agarose chromatography. The core polymerase was purified by additional chromatography on phosphocellulose. The subunit structure is beta prime (155,000 molecular weight), beta (151,000), alpha (43,000), and sigma (93,000), with an additional polypeptide of 29,000 molecular weight, which we have designated tau, found associated with both core and holoenzyme fractions. The measured stoichiometry of the holoenzyme complex was found to be 2 alpha:1 beta':1 beta:0.7 sigma:1 tau. The 93,000 molecular-weight protein subunit was identified as the sigma subunit based upon stimulation of specific transcription in assays with reconstituted polymerase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Brill W. J. Biochemical genetics of nitrogen fixation. Microbiol Rev. 1980 Sep;44(3):449–467. doi: 10.1128/mr.44.3.449-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- Corbin D., Barran L., Ditta G. Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci U S A. 1983 May;80(10):3005–3009. doi: 10.1073/pnas.80.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M., Clements J., Merrick M., Dixon R. Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):302–307. doi: 10.1038/301302a0. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M., Hennecke H. Rhizobium japonicum nitrogenase Fe protein gene (nifH). J Bacteriol. 1984 Jun;158(3):1005–1011. doi: 10.1128/jb.158.3.1005-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C., Engbaek F., Flammang T., Burgess R. Rapid micromethod for the purification of Escherichia coli ribonucleic acid polymerase and the preparation of bacterial extracts active in ribonucleic acid synthesis. J Bacteriol. 1976 Oct;128(1):382–389. doi: 10.1128/jb.128.1.382-389.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Kaluza K., Fuhrmann M., Hahn M., Regensburger B., Hennecke H. In Rhizobium japonicum the nitrogenase genes nifH and nifDK are separated. J Bacteriol. 1983 Aug;155(2):915–918. doi: 10.1128/jb.155.2.915-918.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf U. C. The nucleoside triphosphate-ribonucleic acid nucleotidyltransferase (EC 2.7.7.6) of Agrobacterium tumefaciens (Smith and Townsend) Conn. Purification and properties of the enzyme from the tumorigenic strain B6806. Biochem J. 1974 Dec;143(3):511–520. doi: 10.1042/bj1430511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B. L., Brown L. R. The basis for colored silver-protein complex formation in stained polyacrylamide gels. Anal Biochem. 1984 Sep;141(2):311–315. doi: 10.1016/0003-2697(84)90047-2. [DOI] [PubMed] [Google Scholar]
- Ow D. W., Ausubel F. M. Regulation of nitrogen metabolism genes by nifA gene product in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):307–313. doi: 10.1038/301307a0. [DOI] [PubMed] [Google Scholar]
- Pankhurst C. E. Symbiotic effectiveness of antibiotic-resistant mutants of fast- and slow-growing strains of Rhizobium nodulating Lotus species. Can J Microbiol. 1977 Aug;23(8):1026–1033. doi: 10.1139/m77-152. [DOI] [PubMed] [Google Scholar]
- Purohit S., Mathews C. K. Nucleotide sequence reveals overlap between T4 phage genes encoding dihydrofolate reductase and thymidylate synthase. J Biol Chem. 1984 May 25;259(10):6261–6266. [PubMed] [Google Scholar]
- Regensburger B., Hennecke H. RNA polymerase from Rhizobium japonicum. Arch Microbiol. 1983 Aug;135(2):103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Nüsslein C., Bonhoeffer F. J., Kurz C., Nietzschmann I. Affinity chromatography of DNA-binding enzymes on single-stranded DNA-agarose columns. Eur J Biochem. 1972 Apr 24;26(4):474–481. doi: 10.1111/j.1432-1033.1972.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Jones J. D., Ow D. W., Ausubel F. M. Klebsiella pneumoniae nifA product activates the Rhizobium meliloti nitrogenase promoter. Nature. 1983 Feb 24;301(5902):728–732. doi: 10.1038/301728a0. [DOI] [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Vance C. P. Rhizobium infection and nodulation: a beneficial plant disease? Annu Rev Microbiol. 1983;37:399–424. doi: 10.1146/annurev.mi.37.100183.002151. [DOI] [PubMed] [Google Scholar]
- Werner D. Differentiation of Rhizobium japonicum, III. Inhibition of nitrogenase derepression by chloramphenicol and rifampicin concentrations, not inhibiting growth. Z Naturforsch C. 1978 Nov-Dec;33(11-12):859–862. doi: 10.1515/znc-1978-11-1210. [DOI] [PubMed] [Google Scholar]