Abstract
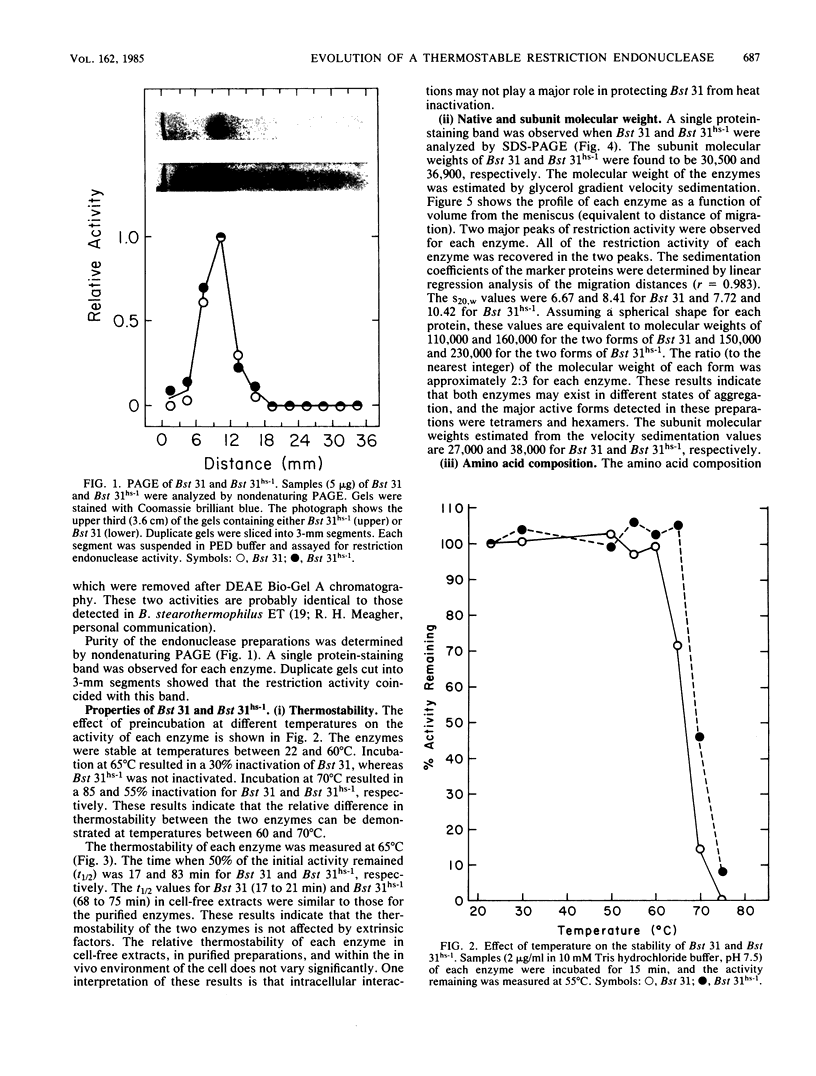
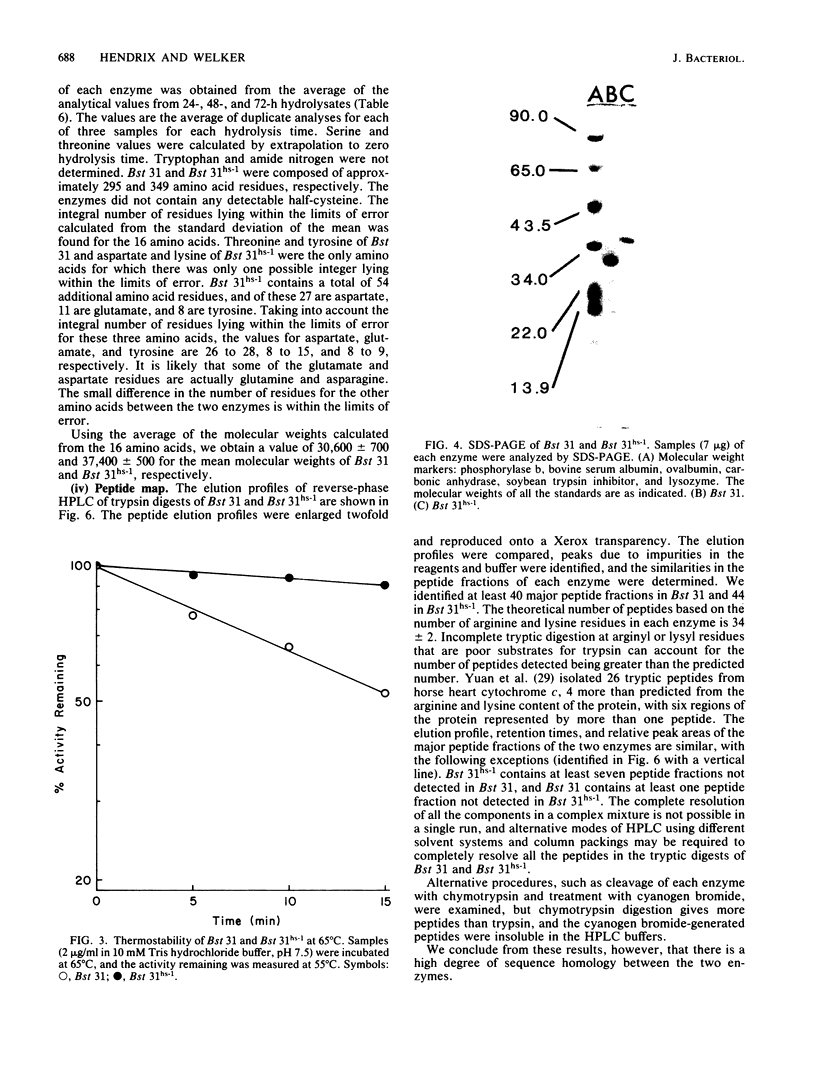
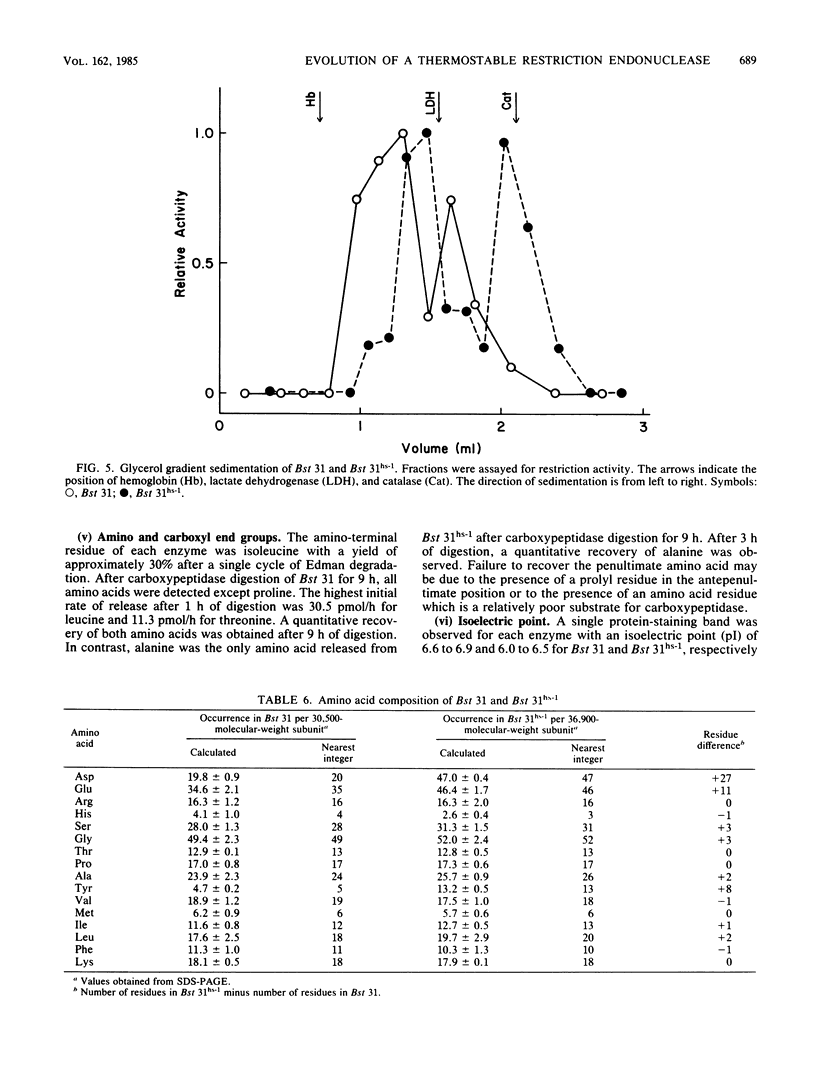
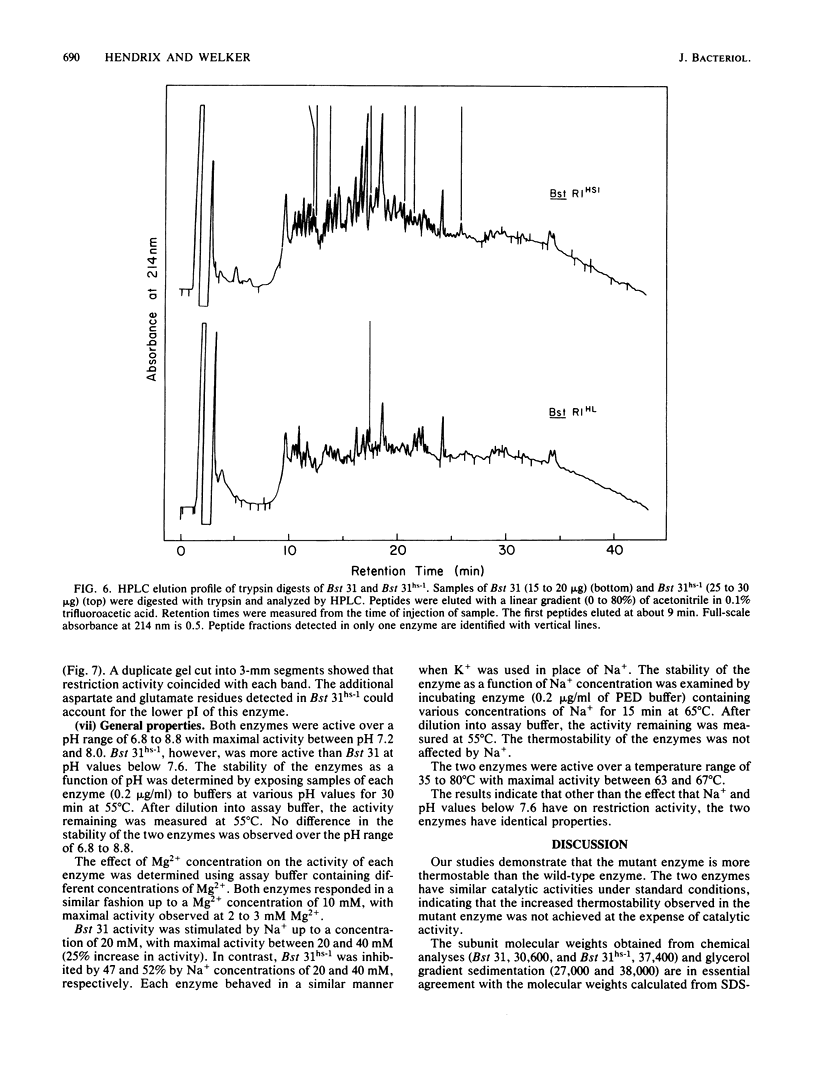
A procedure was developed for the selection of spontaneous mutants of Bacillus stearothermophilus NUB31 that are more efficient than the wild type in the restriction of phage at elevated temperatures. Inactivation studies revealed that two mutants contained a more thermostable restriction enzyme and one mutant contained three times more enzyme than the wild type. The restriction endonucleases from the wild type and one of the mutants were purified to apparent homogeneity. The mutant enzyme was more thermostable than the wild-type enzyme. The subunit molecular weight, amino acid composition, N-terminal and C-terminal amino acid residues, tryptic peptide map, and catalytic properties of the two enzymes were determined. The two enzymes have similar catalytic properties, but the molecular size of the mutant enzyme is approximately 6 to 7 kilodaltons larger than that of the wild-type enzyme. The mutant enzyme contains 54 additional amino acid residues, of which 26 to 28 are aspartate/asparagine, 8 to 15 are glutamate/glutamine, and 8 to 9 are tyrosine residues. The two enzymes contained similar amounts of the other amino acids, identical N-terminal residues, and different C-terminal residues. Tryptic peptide analyses revealed a high degree of homology between the two enzymes. The increased thermostability observed in the mutant enzyme appears to have been achieved by a mutation that resulted in the addition of amino acid residues to the wild-type enzyme. A number of mechanisms are discussed that could account for the observed difference between the mutant and wild-type enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amelunxen R. E., Murdock A. L. Mechanisms of thermophily. CRC Crit Rev Microbiol. 1978;6(4):343–393. doi: 10.3109/10408417809090626. [DOI] [PubMed] [Google Scholar]
- Argos P., Rossman M. G., Grau U. M., Zuber H., Frank G., Tratschin J. D. Thermal stability and protein structure. Biochemistry. 1979 Dec 11;18(25):5698–5703. doi: 10.1021/bi00592a028. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Welker N. E. Isolation and properties of a thermostable restriction endonuclease (ENDO R-Bst1503). J Bacteriol. 1977 Feb;129(2):1110–1120. doi: 10.1128/jb.129.2.1110-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell M. L., Schellman J. A. Stability of phage T4 lysozymes. I. Native properties and thermal stability of wild type and two mutant lysozymes. Biochim Biophys Acta. 1977 Oct 26;494(2):367–383. doi: 10.1016/0005-2795(77)90166-0. [DOI] [PubMed] [Google Scholar]
- Frank G., Haberstich H. U., Schaer H. P., Tratschin J. D., Zuber H. Thermophilic and mesophilic enzymes from B. caldotenax and B. stearothermophilus: properties, relationships and formation. Experientia Suppl. 1976;26:375–389. doi: 10.1007/978-3-0348-7675-9_30. [DOI] [PubMed] [Google Scholar]
- Gracy R. W. Two-dimensional thin-layer methods. Methods Enzymol. 1977;47:195–204. doi: 10.1016/0076-6879(77)47024-1. [DOI] [PubMed] [Google Scholar]
- Grütter M. G., Hawkes R. B., Matthews B. W. Molecular basis of thermostability in the lysozyme from bacteriophage T4. Nature. 1979 Feb 22;277(5698):667–669. doi: 10.1038/277667a0. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Langridge J. Genetic and enzymatic experiments relating to the tertiary structure of beta-galactosidase. J Bacteriol. 1968 Nov;96(5):1711–1717. doi: 10.1128/jb.96.5.1711-1717.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees N. D., Welker N. E. Restriction and modification of bacteriophage in Bacillus stearothermophilus. J Virol. 1973 Apr;11(4):606–609. doi: 10.1128/jvi.11.4.606-609.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy W. P., Welker N. E. Deoxyribonucleic acid modification methylase from Bacillus stearothermophilus. Biochemistry. 1981 Mar 3;20(5):1120–1127. doi: 10.1021/bi00508a012. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Pederson D. M., Goodman R. E. Isozymes of alpha-galactosidase from Bacillus stearothermophilus. Can J Microbiol. 1980 Aug;26(8):978–984. doi: 10.1139/m80-166. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1982 Mar 11;10(5):r117–r144. doi: 10.1093/nar/10.5.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. INDUCTION AND PROPERTIES OF A TEMPERATURE BACTERIOPHAGE FROM BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Jan;89:175–184. doi: 10.1128/jb.89.1.175-184.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Welply J. K., Fowler A. V., Zabin I. beta-Galactosidase alpha-complementation. Effect of single amino acid substitutions. J Biol Chem. 1981 Jul 10;256(13):6811–6816. [PubMed] [Google Scholar]
- Wisdom C., Welker N. E. Membranes of Bacillus stearothermophilus: factors affecting protoplast stability and thermostability of alkaline phosphatase and reduced nicotinamide adenine dinucleotide oxidase. J Bacteriol. 1973 Jun;114(3):1336–1345. doi: 10.1128/jb.114.3.1336-1345.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. Experimental evolution in bacteria. CRC Crit Rev Microbiol. 1978 Sep;6(1):33–51. doi: 10.3109/10408417809090619. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Horn V. Tryptophan synthetase chain positions affected by mutations near the ends of the genetic map of trpA of Escherichia coli. J Biol Chem. 1972 Jul 25;247(14):4494–4498. [PubMed] [Google Scholar]
- Yuan P. M., Pande H., Clark B. R., Shively J. E. Microsequence analysis of peptides and proteins. I. Preparation of samples by reverse-phase liquid chromatography. Anal Biochem. 1982 Mar 1;120(2):289–301. doi: 10.1016/0003-2697(82)90350-5. [DOI] [PubMed] [Google Scholar]
- Yutani K., Ogasahara K., Kimura A., Sugino Y. Effect of single amino acid substitutions at the same position on stability of a two-domain protein. J Mol Biol. 1982 Sep 15;160(2):387–390. doi: 10.1016/0022-2836(82)90184-x. [DOI] [PubMed] [Google Scholar]