Abstract
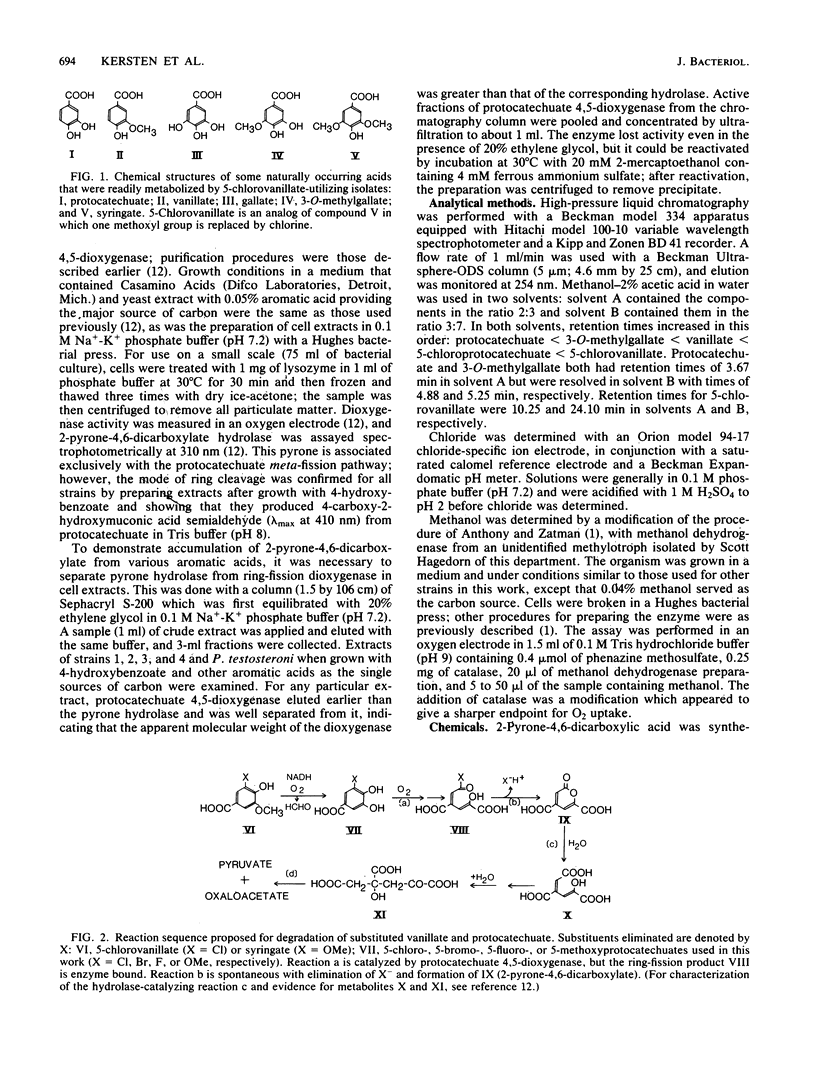
Four strains of gram-negative bacteria capable of growing at the expense of 5-chlorovanillate were isolated from soil, and the metabolism of one strain was studied in particular detail. In the presence of alpha, alpha'-bipyridyl, a suspension of 5-chlorovanillate-grown cells accumulated 5-chloroprotocatechuate from 5-chlorovanillate; in the absence of inhibitor these compounds, and various other 5-substituted protocatechuates and vanillates, were oxidized to completion. Cell suspensions of this strain grown on 5-chlorovanillate or vanillate released chloride quantitatively from 5-chlorovanillate and released methanol from syringate. Extracts of cells grown with 4-hydroxybenzoate, vanillate, or syringate possessed high levels of both protocatechuate 4,5-dioxygenase and 2-pyrone-4,6-dicarboxylate hydrolase; extracts from acetate-grown cells did not. Protocatechuate 4,5-dioxygenase, purified from strains that could grow with 5-chlorovanillate, oxidized 5-halogeno-protocatechuates and 3-O-methylgallate with the formation of 2-pyrone-4,6-dicarboxylate. A crude extract converted 5-chloroprotocatechuate into pyruvate plus oxaloacetate. On the basis of these observations, a meta-fission reaction sequence is proposed for the bacterial degradation of vanillate and protocatechuate substituted at C-5 of the benzene ring with halogen or methoxyl.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The alcohol dehydrogenase of Pseudomonas sp. M27. Biochem J. 1965 Sep;96(3):808–812. doi: 10.1042/bj0960808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels I., Knackmuss H. J., Reineke W. Suicide Inactivation of Catechol 2,3-Dioxygenase from Pseudomonas putida mt-2 by 3-Halocatechols. Appl Environ Microbiol. 1984 Mar;47(3):500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt F. H., Erdin N., Staudinger H., Ullrich V. Interactions of substrates with a purified 4-methoxybenzoate monooxygenase system (O-demethylating) from Pseudomonas putida. Eur J Biochem. 1973 May;35(1):126–134. doi: 10.1111/j.1432-1033.1973.tb02818.x. [DOI] [PubMed] [Google Scholar]
- Crawford R. L., McCoy E., Harkin J. M., Kirk T. K., Obst J. R. Degradation of methoxylated benzoic acids by a Nocardia from a lignin-rich environment: significance to lignin degradation and effect of chloro substituents. Appl Microbiol. 1973 Aug;26(2):176–184. doi: 10.1128/am.26.2.176-184.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M. I., Dagley S. Production of methanol from aromatic acids by Pseudomonas putida. J Bacteriol. 1980 Jun;142(3):916–924. doi: 10.1128/jb.142.3.916-924.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Fernley H. N., Davies J. I. Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem J. 1971 May;122(4):543–551. doi: 10.1042/bj1220543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow M., Alderson G. The actinomycete-genus Rhodococcus: a home for the "rhodochrous" complex. J Gen Microbiol. 1977 May;100(1):99–122. doi: 10.1099/00221287-100-1-99. [DOI] [PubMed] [Google Scholar]
- Husain M., Entsch B., Ballou D. P., Massey V., Chapman P. J. Fluoride elimination from substrates in hydroxylation reactions catalyzed by p-hydroxybenzoate hydroxylase. J Biol Chem. 1980 May 10;255(9):4189–4197. [PubMed] [Google Scholar]
- Kersten P. J., Dagley S., Whittaker J. W., Arciero D. M., Lipscomb J. D. 2-pyrone-4,6-dicarboxylic acid, a catabolite of gallic acids in Pseudomonas species. J Bacteriol. 1982 Dec;152(3):1154–1162. doi: 10.1128/jb.152.3.1154-1162.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W. Stoicheiometry of O-demethylase activity in Pseudomonas aeruginosa. FEBS Lett. 1970 May 25;8(2):101–104. doi: 10.1016/0014-5793(70)80235-6. [DOI] [PubMed] [Google Scholar]
- Suflita J. M., Horowitz A., Shelton D. R., Tiedje J. M. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science. 1982 Dec 10;218(4577):1115–1117. doi: 10.1126/science.218.4577.1115. [DOI] [PubMed] [Google Scholar]



