Abstract
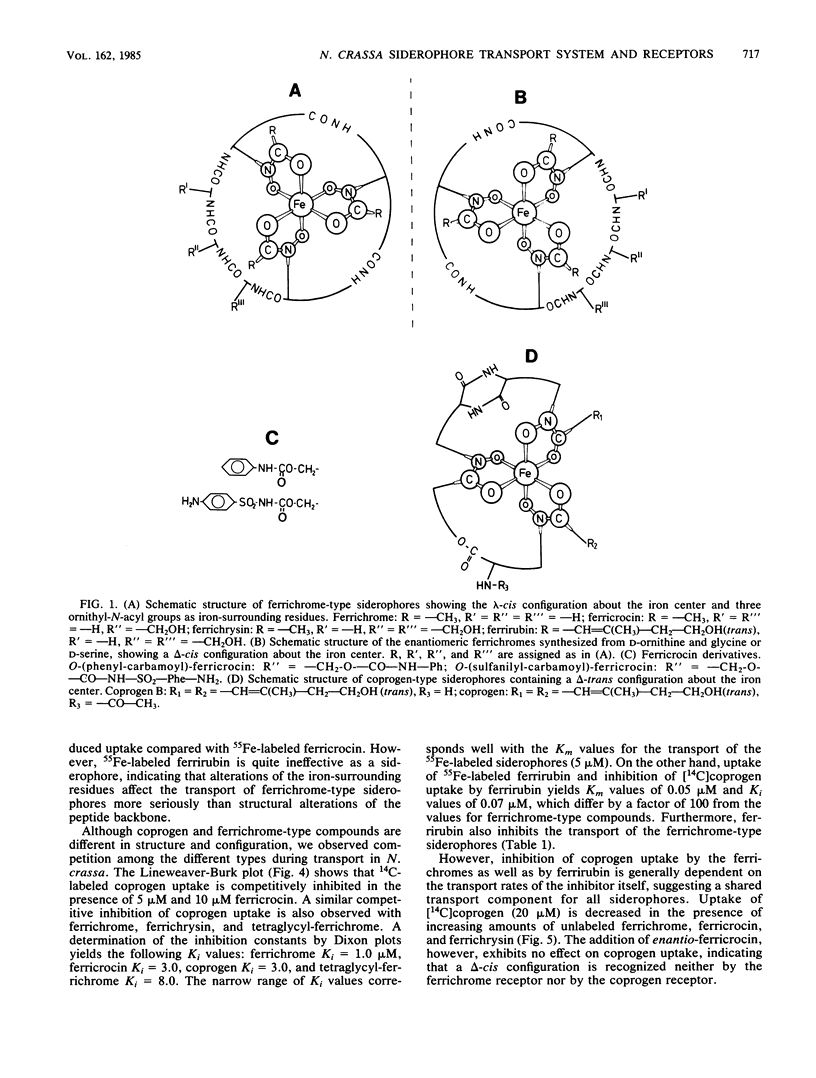
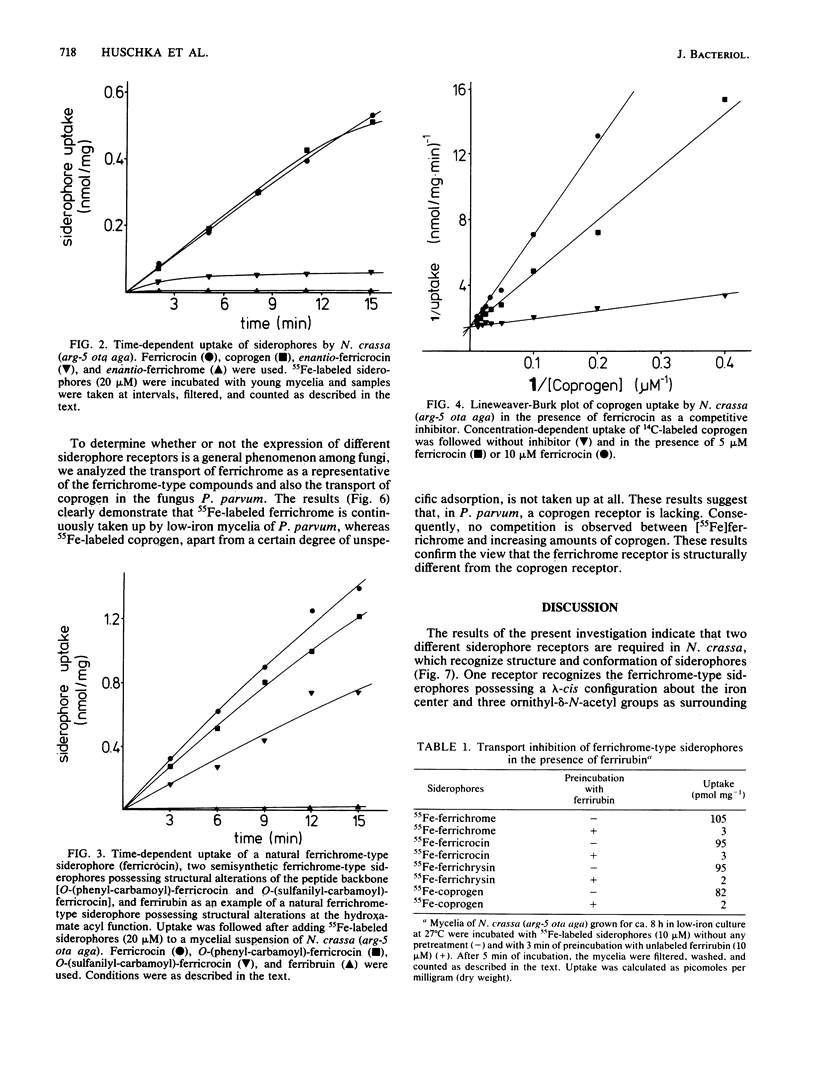
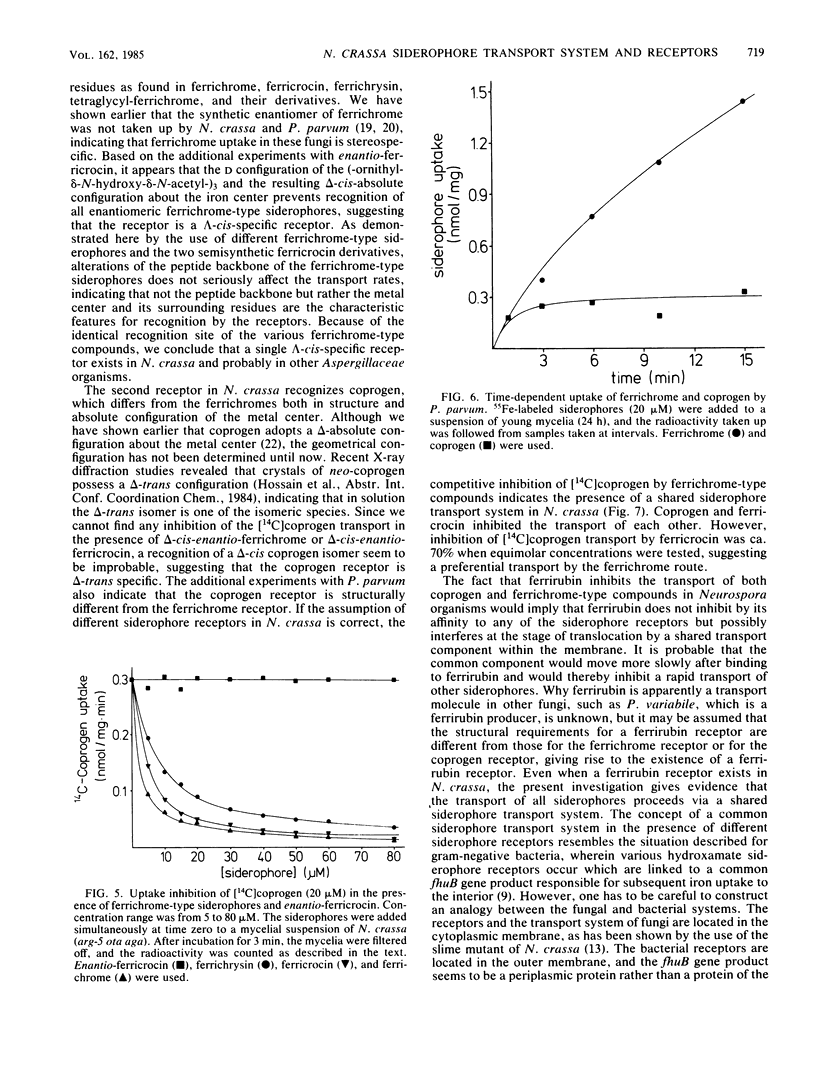
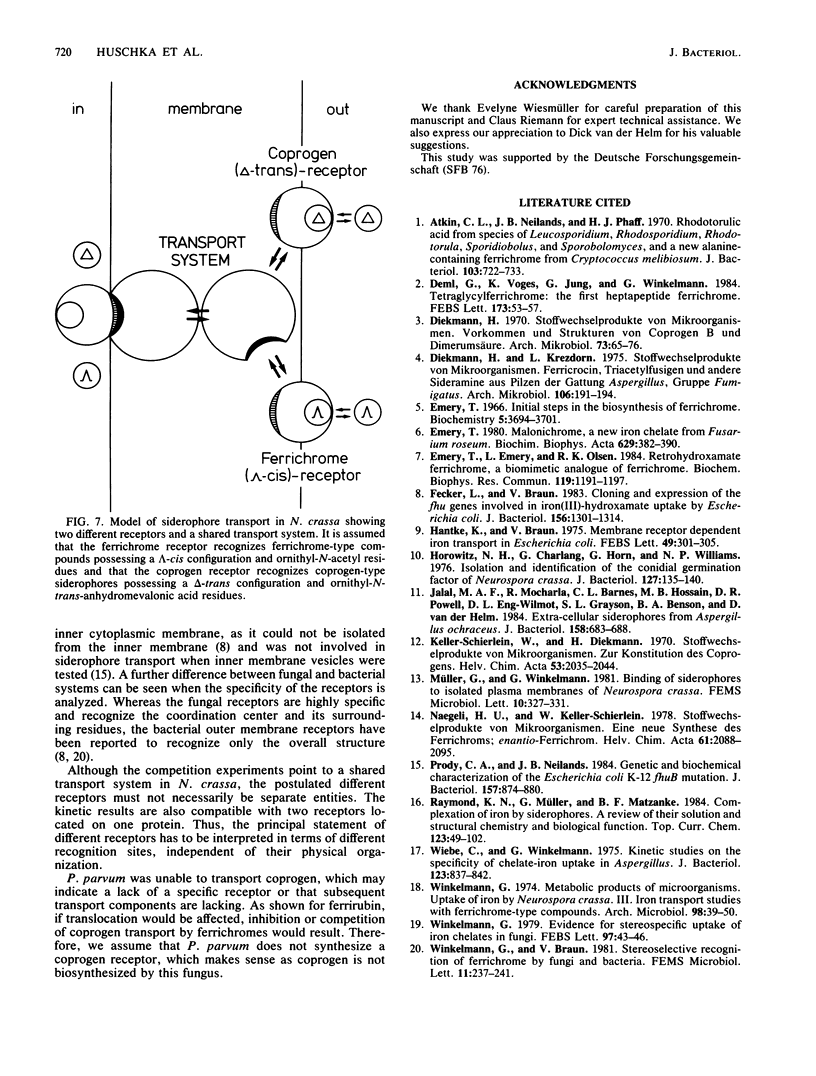
Uptake and competition experiments were performed with Neurospora crassa and Penicillium parvum by using 14C-labeled coprogen and 55Fe-labeled ferrichrome-type siderophores. Several siderophores of the ferrichrome family, such as ferrichrome, ferricrocin, ferrichrysin, and tetraglycyl-ferrichrome as well as the semisynthetic ferricrocin derivatives O-(phenyl-carbamoyl)-ferricrocin and O-(sulfanilyl-carbamoyl)-ferricrocin were taken up by N. crassa. The ferrichrome-type siderophores used vary in the structure of the peptide backbone but possess a common lambda-cis configuration about the iron center and three identical ornithyl-delta-N-acetyl groups as surrounding residues. This suggests that these ferrichrome-type siderophores are recognized by a common ferrichrome receptor. We also concluded that the ferrichrome receptor is lambda-cis specific from the inability to take up the synthetic enantiomers, enantio-ferrichrome and enantio-ferricrocin, possessing a delta-cis configuration about the iron center. On the other hand, we found that coprogen, possessing a delta-absolute configuration and two trans-anhydromevalonic acid residues around the metal center, was also taken up by N. crassa and was competitively inhibited by the ferrichrome-type siderophores. We therefore propose the existence of a common siderophore transport system but the presence of different siderophore receptors in N. crassa. In addition, ferrirubin, which is very slowly transported by N. crassa, inhibited both coprogen and ferrichrome-type siderophore transport. Contrary to the findings with N. crassa, transport experiments with P. parvum revealed the presence of a ferrichrome receptor but the absence of a coprogen receptor; coprogen was neither transported nor did it inhibit the ferrichrome transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Neilands J. B., Phaff H. J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970 Sep;103(3):722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann H., Krezdorn E. Stoffwechselprodukte von Mikroorganismen 150. Mitteilung. Ferricrocin, Triacetylfusigen und andere Sideramine aus Pilzen der Gattung Aspergillus, Gruppe Fumigatus. Arch Microbiol. 1975 Dec 31;106(3):191–194. doi: 10.1007/BF00446522. [DOI] [PubMed] [Google Scholar]
- Diekmann H. Stoffwechselprodukte von Mikroorganismen. 81. Vorkommen und Strukturen von Coprogen B und Dimerumsäure. Arch Mikrobiol. 1970;73(1):65–76. [PubMed] [Google Scholar]
- Emery T. F. Initial steps in the biosynthesis of ferrichrome. Incorporation of delta-N-hydroxyornithine and delta-N-acetyl-delta-N-hydroxyornithine. Biochemistry. 1966 Nov;5(11):3694–3701. doi: 10.1021/bi00875a045. [DOI] [PubMed] [Google Scholar]
- Emery T., Emery L., Olsen R. K. Retrohydroxamate ferrichrome, a biomimetic analogue of ferrichrome. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1191–1197. doi: 10.1016/0006-291x(84)90902-1. [DOI] [PubMed] [Google Scholar]
- Emery T. Malonichrome, a new iron chelate from Fusarium roseum. Biochim Biophys Acta. 1980 May 7;629(2):382–390. doi: 10.1016/0304-4165(80)90110-5. [DOI] [PubMed] [Google Scholar]
- Fecker L., Braun V. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J Bacteriol. 1983 Dec;156(3):1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H., Charlang G., Horn G., Williams N. P. Isolation and identification of the conidial germination factor of Neurospora crassa. J Bacteriol. 1976 Jul;127(1):135–140. doi: 10.1128/jb.127.1.135-140.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal M. A., Mocharla R., Barnes C. L., Hossain M. B., Powell D. R., Eng-Wilmot D. L., Grayson S. L., Benson B. A., van der Helm D. Extracellular siderophores from Aspergillus ochraceous. J Bacteriol. 1984 May;158(2):683–688. doi: 10.1128/jb.158.2.683-688.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody C. A., Neilands J. B. Genetic and biochemical characterization of the Escherichia coli K-12 fhuB mutation. J Bacteriol. 1984 Mar;157(3):874–880. doi: 10.1128/jb.157.3.874-880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe C., Winkelmann G. Kinetic studies on the specificity of chelate-iron uptake in Aspergillus. J Bacteriol. 1975 Sep;123(3):837–842. doi: 10.1128/jb.123.3.837-842.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann G. Metabolic products of microorganisms. 132. Uptake of iron by Neurospora crassa. 3. Iron transport studies with ferrichrome-type compounds. Arch Mikrobiol. 1974 Jun 7;98(1):39–50. [PubMed] [Google Scholar]



