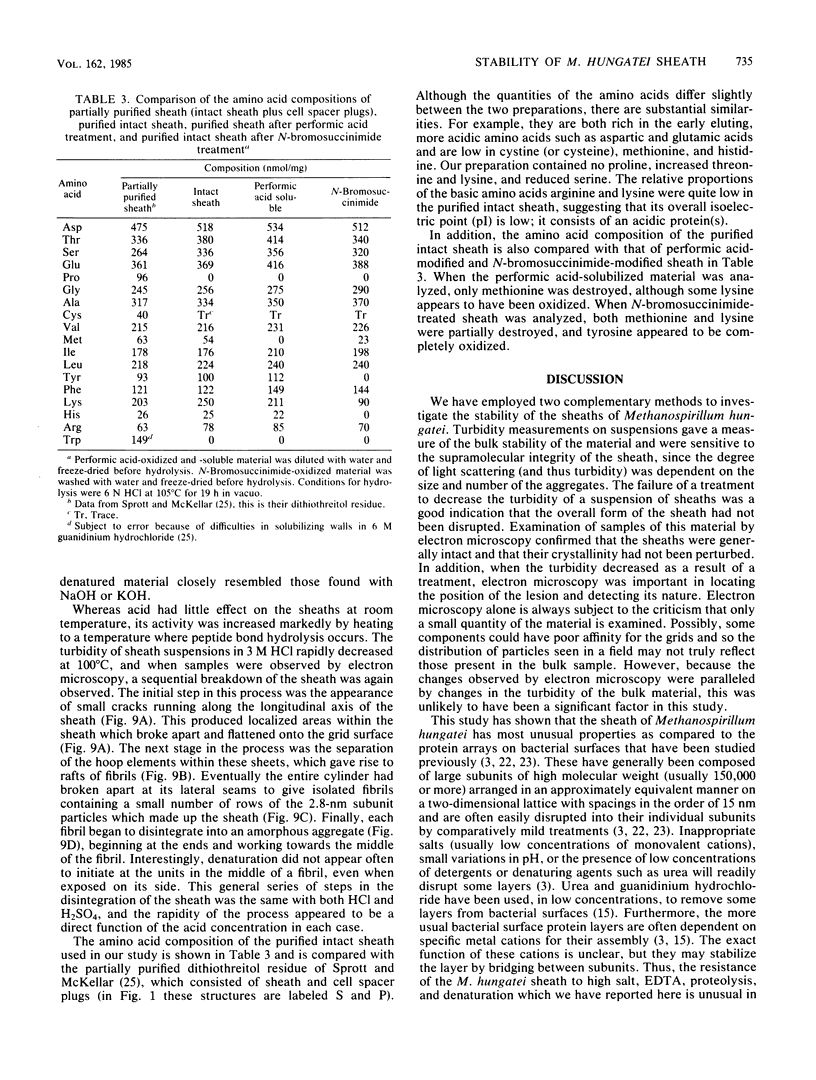
Abstract
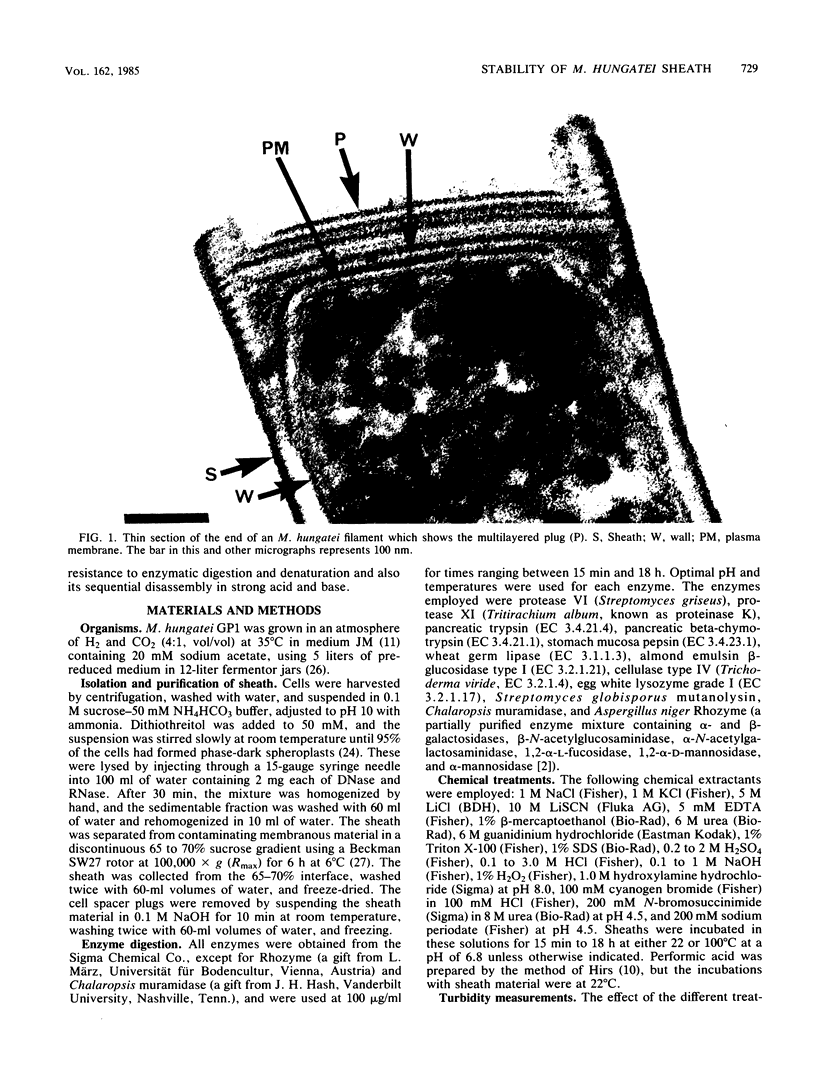
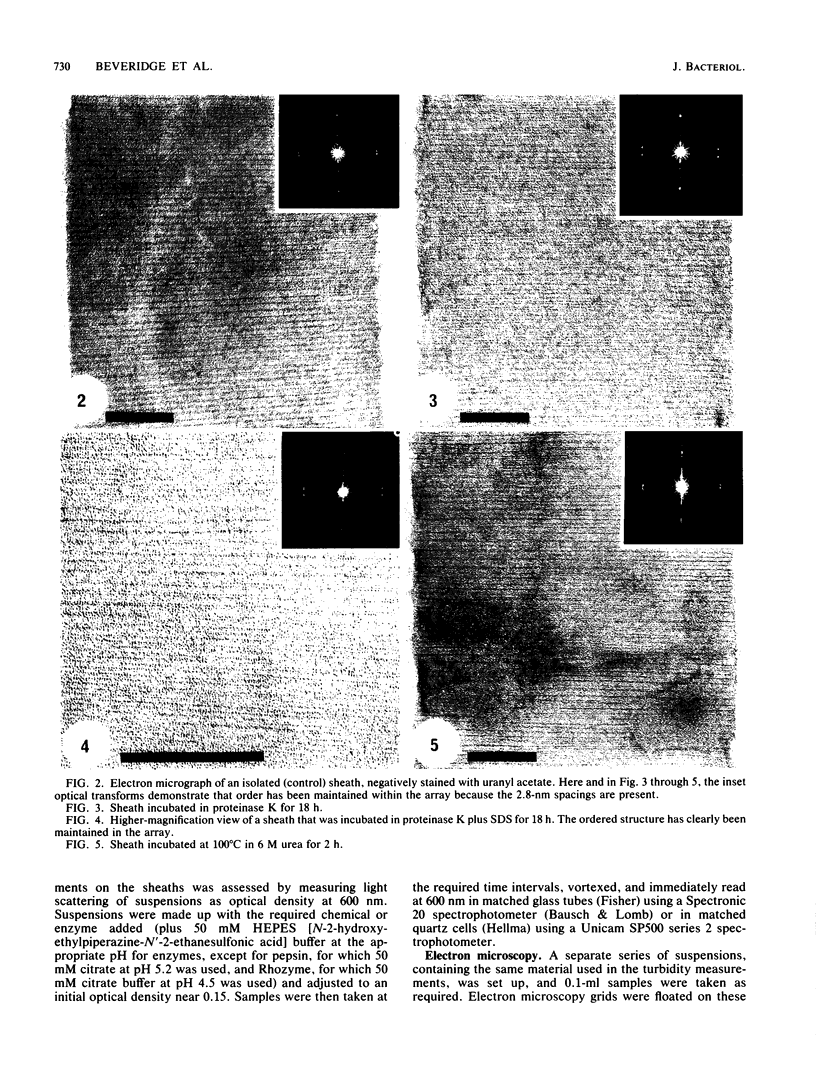
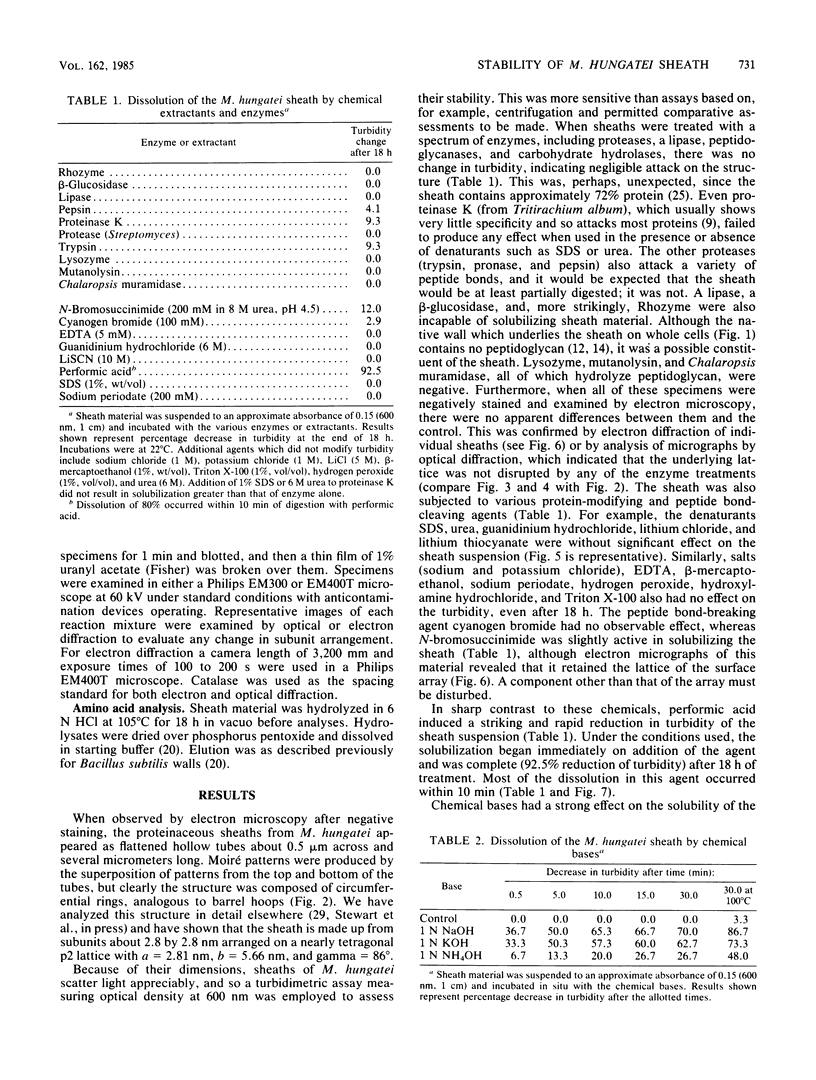
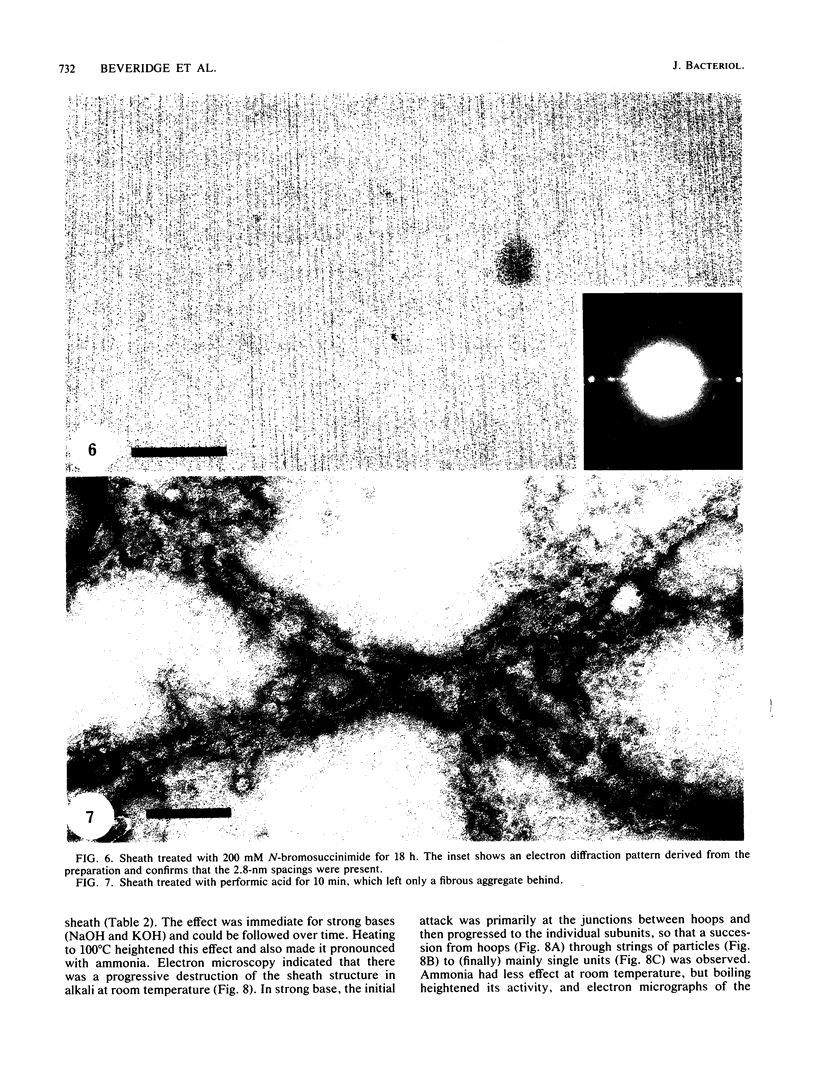
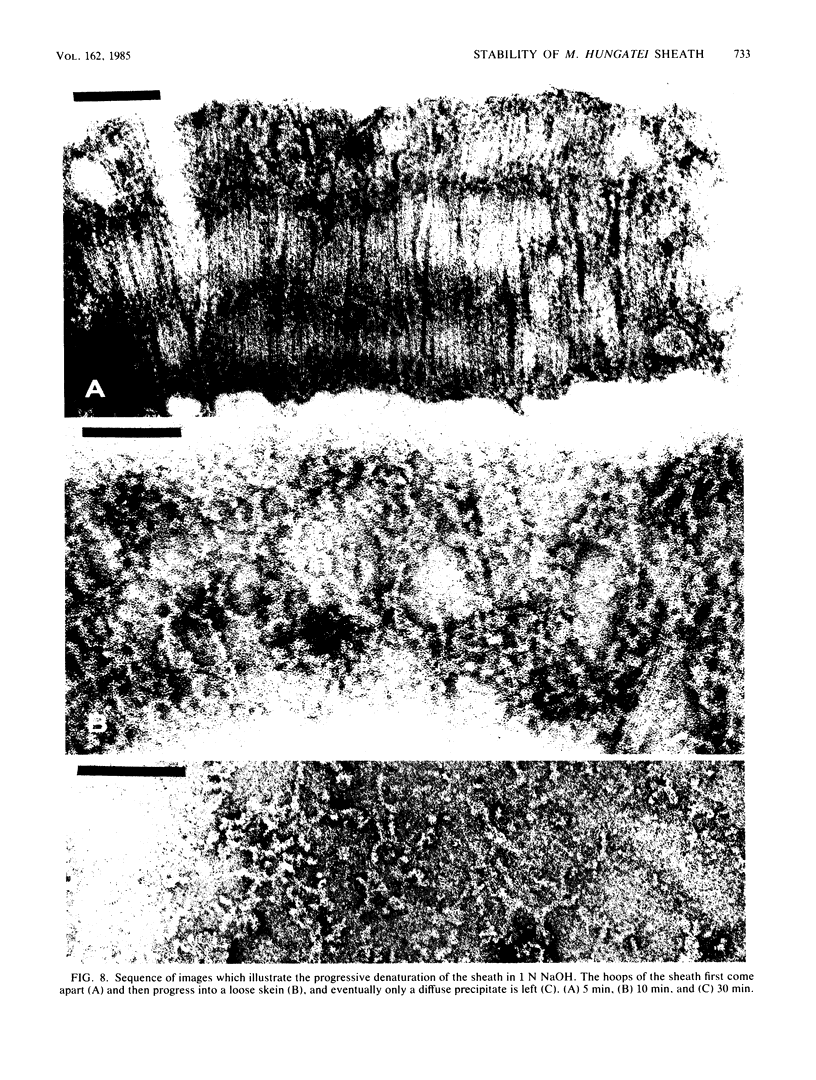
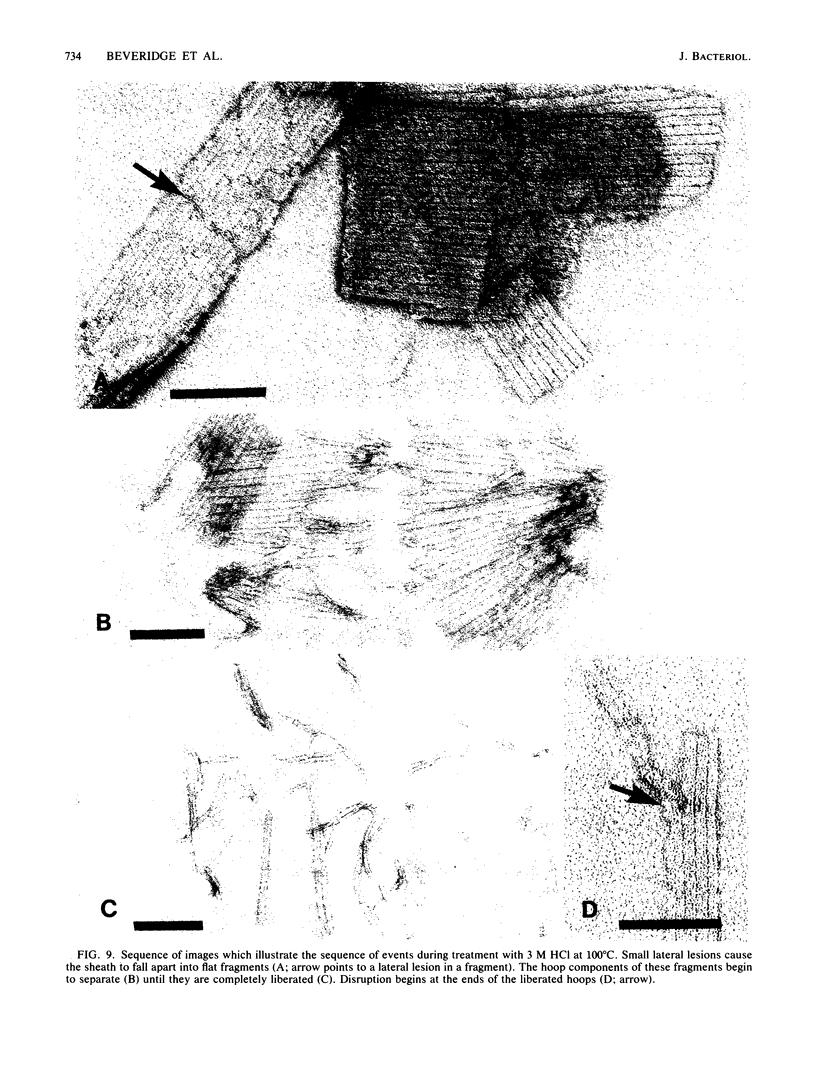
The proteinaceous sheath of Methanospirillum hungatei was isolated by lysing cells in 50 mM dithiothreitol, separating the sheath from other cellular material by discontinuous sucrose density centrifugation, and removing the "cell spacers" with dilute NaOH. The isolated sheath material consisted of hollow tubes which had a highly ordered surface array. The stability of the sheath to treatment with denaturants and to enzymatic digestion was examined by a turbidimetric assay in conjunction with electron microscopy and optical or electron diffraction. The sheath was resistant to a range of proteases and also was not digested by peptidoglycan-degrading enzymes, a lipase, a cellulase, a glucosidase, or Rhozyme (a mixture of galactosidases, acetylglucosaminidase, acetylgalactosaminidase, fucosidase, and mannosidases). In addition to being unaffected by common salts, thiol-reducing agents, and EDTA, the layer was resistant to powerful denaturants such as 6 M urea, 6 M guanidinium hydrochloride, 10 M LiSCN, cyanogen bromide, sodium periodate, and 1% sodium dodecyl sulfate. Strong bases, boiling 3 N HCl, and performic acid did attack the sheath; in these cases, the array was systematically disassembled in a progressive manner, which was followed by electron microscopy. The layer was slightly modified by N-bromosuccinimide in urea, but the array remained intact. The stability of the sheath was remarkable, not only as compared to other bacterial surface arrays, but also as compared to proteins generally, and possibly indicated the presence of covalent cross-links between protein subunits.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Darland G., Brock T. D., Samsonoff W., Conti S. F. A thermophilic, acidophilic mycoplasma isolated from a coal refuse pile. Science. 1970 Dec 25;170(3965):1416–1418. doi: 10.1126/science.170.3965.1416. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Green N. M., Wrigley N. G., Russell W. C., Martin S. R., McLachlan A. D. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 1983;2(8):1357–1365. doi: 10.1002/j.1460-2075.1983.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz H., Wiegers U., Adamietz P. Stimulation of proteinase K action by denaturing agents: application to the isolation of nucleic acids and the degradation of 'masked' proteins. Eur J Biochem. 1975 Aug 1;56(1):103–108. doi: 10.1111/j.1432-1033.1975.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Colvin J. R., Sprott G. D. Spontaneous protoplast formation in Methanobacterium bryantii. J Bacteriol. 1982 Jan;149(1):346–353. doi: 10.1128/jb.149.1.346-353.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O., König H. Chemical composition of the peptidoglycan-free cell walls of methanogenic bacteria. Arch Microbiol. 1978 Aug 1;118(2):141–152. doi: 10.1007/BF00415722. [DOI] [PubMed] [Google Scholar]
- Koval S. F., Murray R. G. The isolation of surface array proteins from bacteria. Can J Biochem Cell Biol. 1984 Nov;62(11):1181–1189. doi: 10.1139/o84-152. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Sprott G. D., Smith I. C. Novel complex polar lipids from the methanogenic archaebacterium Methanospirillum hungatei. Science. 1981 Mar 13;211(4487):1163–1164. doi: 10.1126/science.7466385. [DOI] [PubMed] [Google Scholar]
- Makula R. A., Singer M. E. Ether-containing lipids of methanogenic bacteria. Biochem Biophys Res Commun. 1978 May 30;82(2):716–722. doi: 10.1016/0006-291x(78)90933-6. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Colvin J. R., McKellar R. C. Spheroplasts of Methanospirillum hungatii formed upon treatment with dithiothreitol. Can J Microbiol. 1979 Jun;25(6):730–738. doi: 10.1139/m79-106. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., McKellar R. C. Composition and properties of the cell wall of Methanospirillum hungatii. Can J Microbiol. 1980 Feb;26(2):115–120. doi: 10.1139/m80-017. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., McKellar R. C., Shaw K. M., Giroux J., Martin W. G. Properties of malate dehydrogenase isolated from Methanospirillum hungatii. Can J Microbiol. 1979 Feb;25(2):192–200. doi: 10.1139/m79-030. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Shaw K. M., Jarrell K. F. Isolation and chemical composition of the cytoplasmic membrane of the archaebacterium Methanospirillum hungatei. J Biol Chem. 1983 Mar 25;258(6):4026–4031. [PubMed] [Google Scholar]
- Stewart M. The structure of chicken scale keratin. J Ultrastruct Res. 1977 Jul;60(1):27–33. doi: 10.1016/s0022-5320(77)80038-5. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Langworthy T. A. Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science. 1979 Jan 5;203(4375):51–53. doi: 10.1126/science.758677. [DOI] [PubMed] [Google Scholar]
- Walker I. D., Bridgen J. The keratin chains of avian scale tissue. Sequence heterogeneity and the number of scale keratin genes. Eur J Biochem. 1976 Aug 1;67(1):283–293. doi: 10.1111/j.1432-1033.1976.tb10660.x. [DOI] [PubMed] [Google Scholar]
- Weiss R. L. Subunit cell wall of Sulfolobus acidocaldarius. J Bacteriol. 1974 Apr;118(1):275–284. doi: 10.1128/jb.118.1.275-284.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Fox G. E. Archaebacteria. J Mol Evol. 1978 Aug 2;11(3):245–251. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Bowen V. G. Comparative ultrastructure of methanogenic bacteria. Can J Microbiol. 1975 Feb;21(2):121–129. doi: 10.1139/m75-019. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Bowen V. G. Fine structure of Methanospirillum hungatii. J Bacteriol. 1975 Jan;121(1):373–380. doi: 10.1128/jb.121.1.373-380.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]