Abstract
A class of Azotobacter chroococcum mutants induced by Tn1 that were defective in normal aerobic nitrogen fixation when grown on sugars (Fos-) were corrected by provision of alpha-ketoglutarate or glutamate. In a representative mutant, Fos252, rates of evolution of 14CO2 from [14C]acetate or [14C]glucose were 5% of the parental values, although uptake and incorporation were normal for both substrates. The results suggest that a lesion affects the entry of substrates into the tricarboxylic acid cycle. The activity of citrate synthase in Fos252 in vitro was 5% that of the parents. The citrate synthase (gltA) gene from Escherichia coli was cloned into broad-host-range vectors and mobilized into Fos252. The plasmids restored parental citrate synthase activities to Fos252 and complemented the inability to fix N2 in air. The data indicate that a mutation causing an intrinsic limitation in respiratory capacity abolishes normal aerobic N2 fixation, which is consistent with the hypothesis of respiratory protection for nitrogenase in Azotobacter species.
Full text
PDF
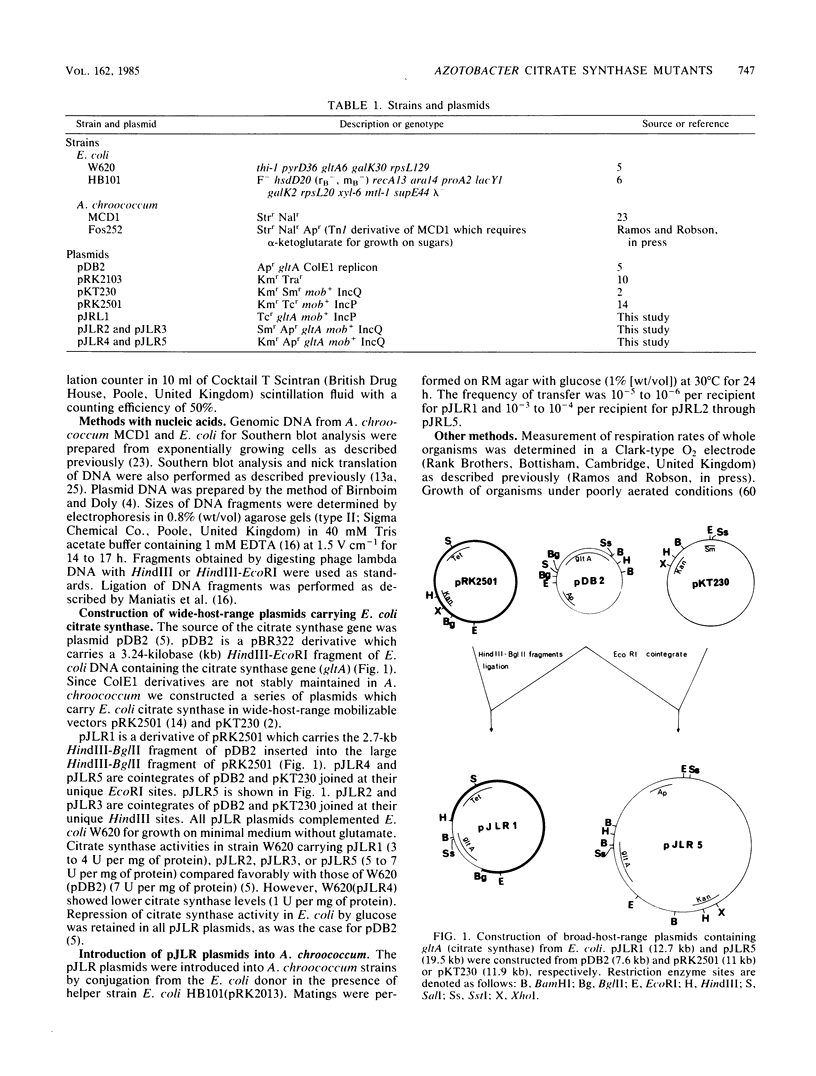
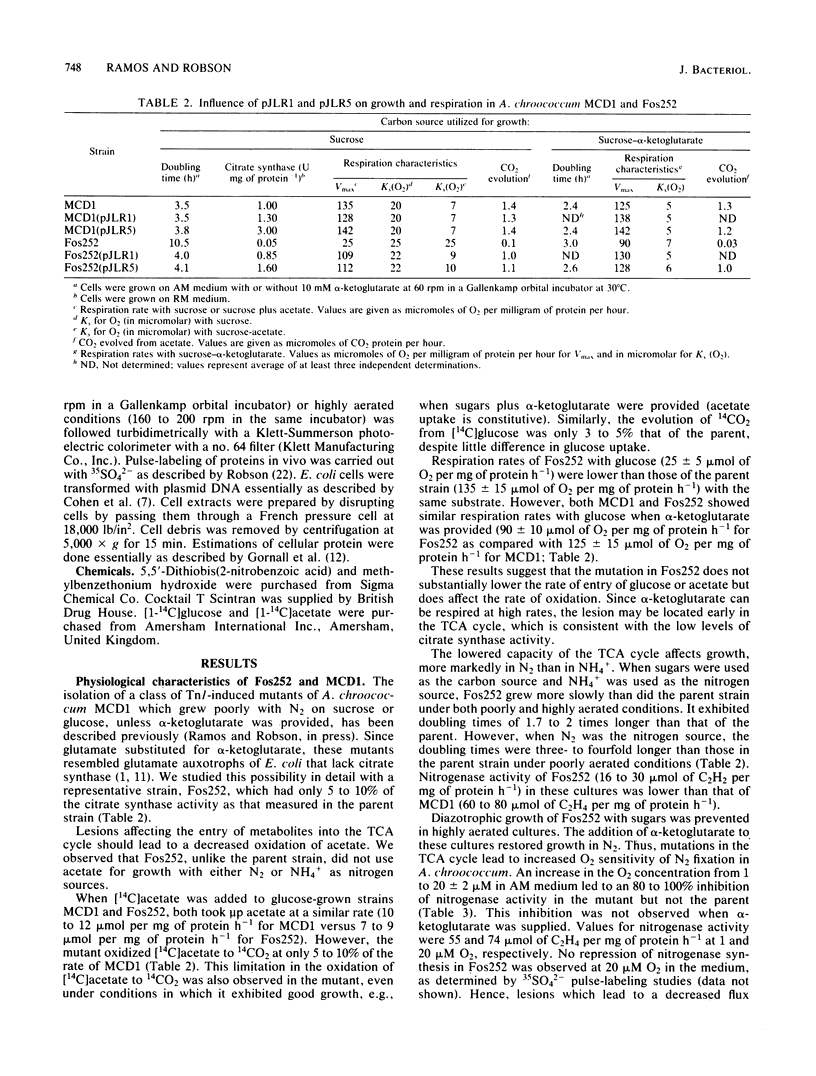
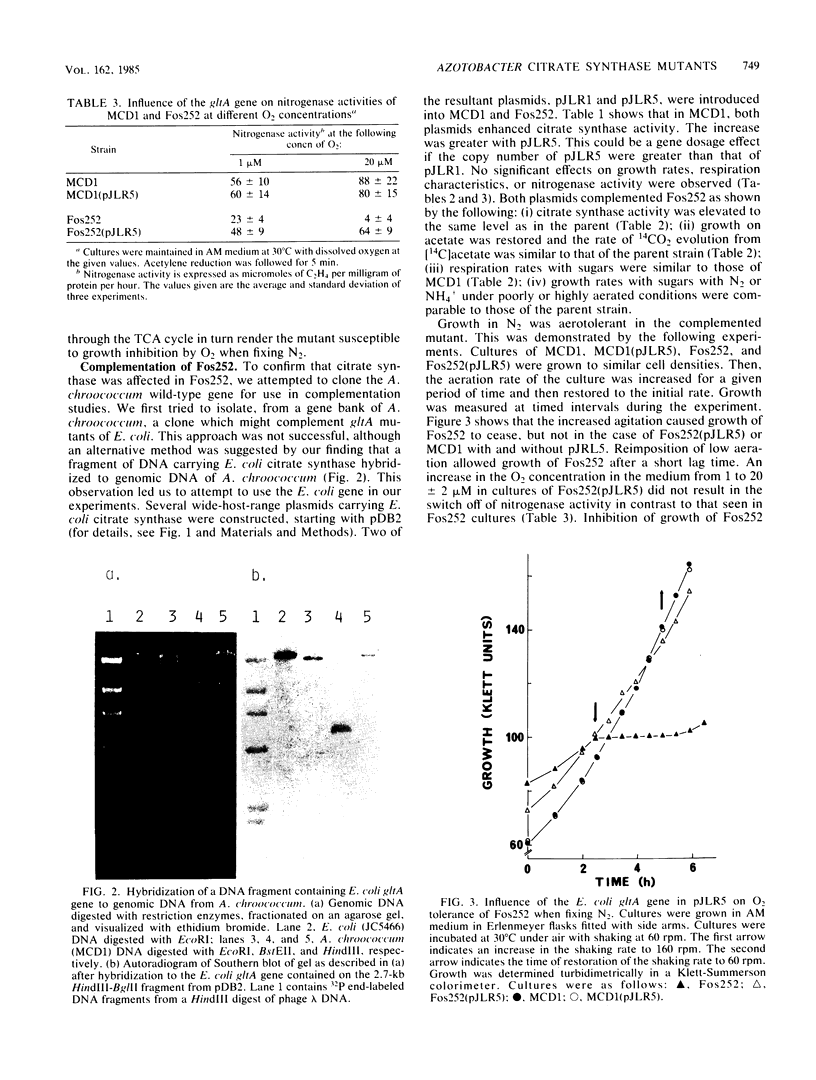


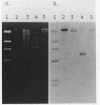
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWORTH J. M., KORNBERG H. L., NOTHMANN D. L. LOCATION OF THE STRUCTURAL GENE FOR CITRATE SYNTHASE ON THE CHROMOSOME OF ESCHERICHIA COLI K12. J Mol Biol. 1965 Mar;11:654–657. doi: 10.1016/s0022-2836(65)80021-3. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Timmis K. N. Host: vector systems for gene cloning in Pseudomonas. Curr Top Microbiol Immunol. 1982;96:47–67. doi: 10.1007/978-3-642-68315-2_4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxham D. P., Herbert C. J., Ner S. S., Drabble W. T. Citrate synthase activity in Escherichia coli harbouring hybrid plasmids containing the gltA gene. J Gen Microbiol. 1983 Jun;129(6):1889–1897. doi: 10.1099/00221287-129-6-1889. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., GILVARG C. The role of the tricarboxylic acid cycle in acetate oxidation in Escherichia coli. J Biol Chem. 1956 Sep;222(1):307–319. [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker H., Veeger C. Involvement of the cytoplasmic membrane in nitrogen fixation by Azotobacter vinelandii. Eur J Biochem. 1977 Jul 1;77(1):1–10. doi: 10.1111/j.1432-1033.1977.tb11634.x. [DOI] [PubMed] [Google Scholar]
- Jones R., Woodley P., Robson R. Cloning and organisation of some genes for nitrogen fixation from Azotobacter chroococcum and their expression in Klebsiella pneumoniae. Mol Gen Genet. 1984;197(2):318–327. doi: 10.1007/BF00330980. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Lakshmi T. M., Helling R. B. Selection for citrate synthase deficiency in icd mutants of Escherichia coli. J Bacteriol. 1976 Jul;127(1):76–83. doi: 10.1128/jb.127.1.76-83.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON J. W., WILSON P. W., BURRIS R. H. Direct demonstration of ammonia as an intermediate in nitrogen fixation by Azotobacter. J Biol Chem. 1953 Sep;204(1):445–451. [PubMed] [Google Scholar]
- Post E., Kleiner D., Oelze J. Whole cell respiration and nitrogenase activities in Azotobacter vinelandii growing in oxygen controlled continuous culture. Arch Microbiol. 1983 Jan;134(1):68–72. doi: 10.1007/BF00429410. [DOI] [PubMed] [Google Scholar]
- Robson R. L. Characterization of an oxygen-stable nitrogenase complex isolated from Azotobacter chroococcum. Biochem J. 1979 Sep 1;181(3):569–575. doi: 10.1042/bj1810569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Chesshyre J. A., Wheeler C., Jones R., Woodley P. R., Postgate J. R. Genome size and complexity in Azotobacter chroococcum. J Gen Microbiol. 1984 Jul;130(7):1603–1612. doi: 10.1099/00221287-130-7-1603. [DOI] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]