Abstract
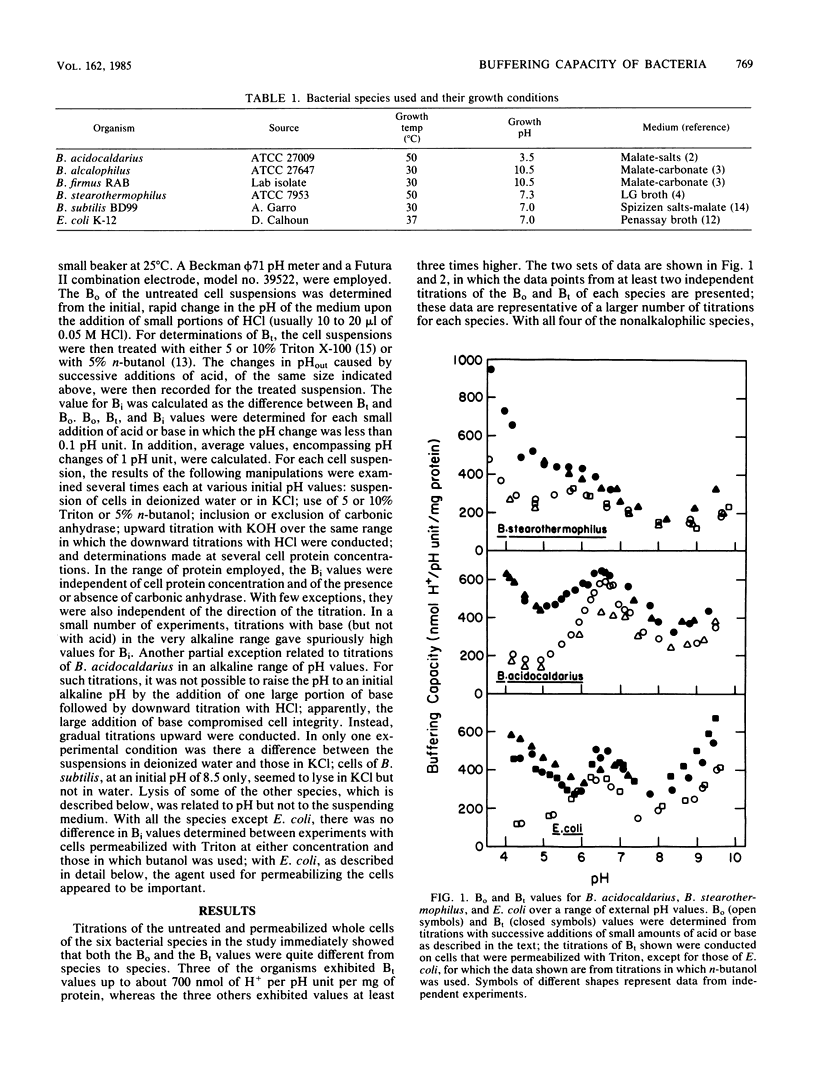
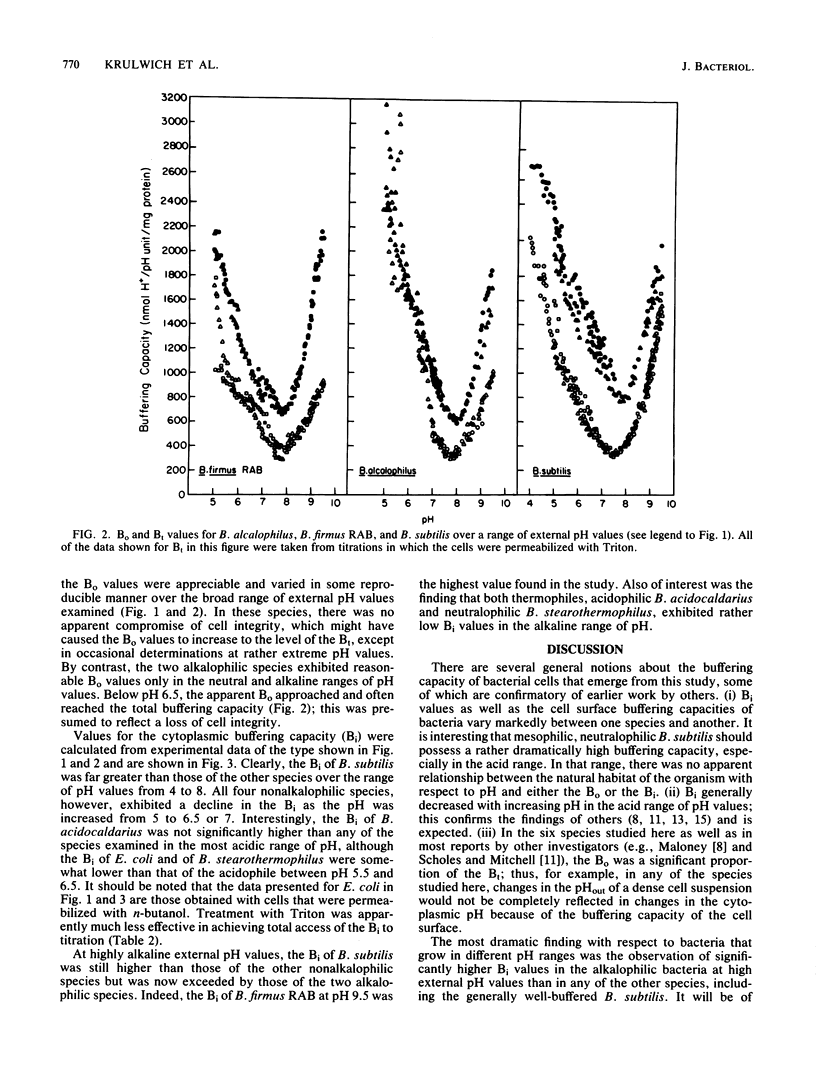
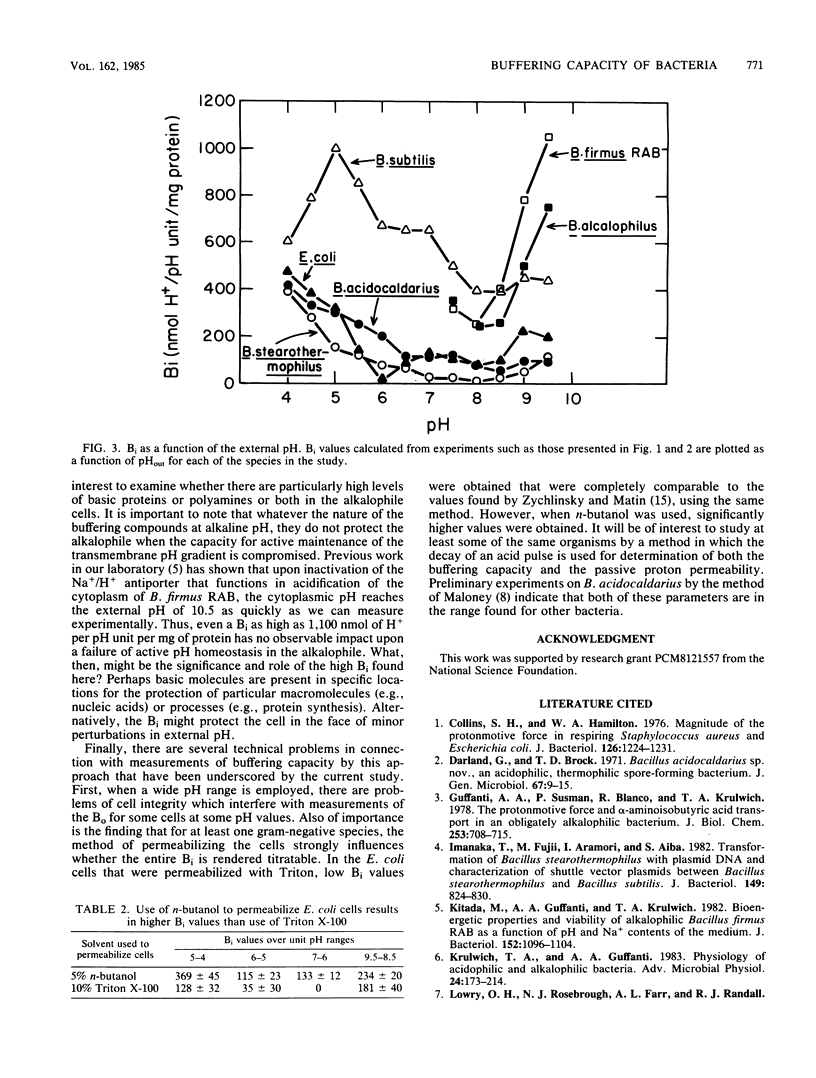
Cytoplasmic buffering capacities and buffering by whole cells were examined in six bacterial species: Bacillus acidocaldarius, Bacillus stearothermophilus, Escherichia coli, Bacillus subtilis, Bacillus alcalophilus, and Bacillus firmus RAB. Acid-base titrations were conducted on whole cells and cells permeabilized with Triton X-100 or n-butanol. In all of the species examined, the buffering capacity of intact cells was generally a significant proportion of the total buffering capacity, but the magnitude of the buffering capacity varied from species to species. Over the entire range of pH values from 4 to 9.5, B. subtilis exhibited a cytoplasmic buffering capacity that was much higher than that of B. stearothermophilus, B. acidocaldarius, or E. coli. The latter three species had comparable cytoplasmic buffering capacities at pH 4 to 9.5, as long as optimal conditions for cell permeabilization were employed. All of the nonalkalophiles exhibited a decrease in cytoplasmic buffering capacity as the external pH increased from pH 5 to 7. At alkaline pH values, the two thermophiles in the study had particularly low cytoplasmic buffering capacities, and the two alkalophilic bacteria had appreciably higher cytoplasmic buffering capacities than any of the other species studied. Cytoplasmic buffering capacities as high as 1,100 nmol of H+ per pH unit per mg of protein were observed in alkalophilic B. firmus RAB. Since previous studies have shown that immediate cytoplasmic alkalinization occurs upon loss of the active mechanisms for pH homeostasis in the alkalophiles, the very high buffering capacities apparently offer no global protection of internal pH. Perhaps, the high buffering capacities reflect protective mechanisms for specific macromolecules or process rather than part of the mechanisms for bulk pH homeostasis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins S. H., Hamilton W. A. Magnitude of the protonmotive force in respiring Staphylococcus aureus and Escherichia coli. J Bacteriol. 1976 Jun;126(3):1224–1231. doi: 10.1128/jb.126.3.1224-1231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aramori I., Aiba S. Transformation of Bacillus stearothermophilus with plasmid DNA and characterization of shuttle vector plasmids between Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1982 Mar;149(3):824–830. doi: 10.1128/jb.149.3.824-830.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Guffanti A. A., Krulwich T. A. Bioenergetic properties and viability of alkalophilic Bacillus firmus RAB as a function of pH and Na+ contents of the incubation medium. J Bacteriol. 1982 Dec;152(3):1096–1104. doi: 10.1128/jb.152.3.1096-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A. Physiology of acidophilic and alkalophilic bacteria. Adv Microb Physiol. 1983;24:173–214. doi: 10.1016/s0065-2911(08)60386-0. [DOI] [PubMed] [Google Scholar]
- Maloney P. C. Membrane H+ conductance of Streptococcus lactis. J Bacteriol. 1979 Oct;140(1):197–205. doi: 10.1128/jb.140.1.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Slayman C. L. Control of intracellular pH. Predominant role of oxidative metabolism, not proton transport, in the eukaryotic microorganism Neurospora. J Gen Physiol. 1982 Sep;80(3):377–402. doi: 10.1085/jgp.80.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. H., Moyer A. J. Penicillin: I. Methods of assay. J Bacteriol. 1944 Feb;47(2):199–209. doi: 10.1128/jb.47.2.199-209.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J Bioenerg. 1970 Jun;1(1):61–72. doi: 10.1007/BF01516089. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky E., Matin A. Cytoplasmic pH homeostasis in an acidophilic bacterium, Thiobacillus acidophilus. J Bacteriol. 1983 Dec;156(3):1352–1355. doi: 10.1128/jb.156.3.1352-1355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



