Abstract
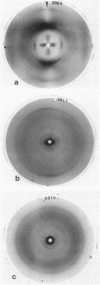
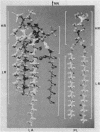
The conformational properties of the isolated S form of Salmonella sp. lipopolysaccharide (LPS), of Re mutant LPS, and of free lipid A were investigated by using X-ray diffraction and conformational energy calculations. The data obtained showed that LPS in a dried, in a hydrated, and probably also in an aqueous dispersion state is capable of forming bilayered lamellar arrangements similar to phospholipids. From the bilayer packing periodicities, a geometrical model of the extensions of the LPS regions lipid A, 2-keto-3-deoxyoctulosonic acid, and O-specific chain along the membrane normal could be calculated. Furthermore, the lipid A component was found to assume a remarkably high ordered conformation: its fatty acid chains were tightly packed in a dense hexagonal lattice with a center-to-center distance of 0.49 nm. The hydrophilic backbone of lipid A showed a strong tendency to form domains in the membrane, resulting in a more or less parallel arrangement of lipid A units. According to model calculations, the hydrophilic backbone of lipid A appears to be oriented approximately 45 degrees to the membrane surface, which would lead to a shed roof-like appearance of the surface structure in the indentations of which the 2-keto-3-deoxyoctulosonic acid moiety would fit. In contrast, the O-specific chains assume a low ordered, heavily coiled conformation. Comparison of these structural properties with those known for natural phospholipids in biological membranes indicates that the high state of order of the lipid A portion of LPS might be an important factor in the structural role and permeation barrier functions of LPS in the outer membrane of gram-negative bacteria.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnickel G., Labischinski H., Bradaczek H., Giesbrecht P. Conformational energy calculation on the peptide part of murein. Eur J Biochem. 1979 Mar 15;95(1):157–165. doi: 10.1111/j.1432-1033.1979.tb12950.x. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E. Evidence of bilayer structure and of membrane interactions from X-ray diffraction analysis. Biochim Biophys Acta. 1982 May 12;650(4):167–207. doi: 10.1016/0304-4157(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Brade H., Rietschel E. T. Alpha-2----4-interlinked 3-deoxy-D-manno-octulosonic acid disaccharide. A common constituent of enterobacterial lipopolysaccharides. Eur J Biochem. 1984 Dec 3;145(2):231–236. doi: 10.1111/j.1432-1033.1984.tb08543.x. [DOI] [PubMed] [Google Scholar]
- Burge R. E., Draper J. C. The structure of the cell wall of the Gram-negative bacterium Proteus vulgaris. 3. A lipopolysaccharide "unit membrane". J Mol Biol. 1967 Sep 14;28(2):205–210. doi: 10.1016/s0022-2836(67)80003-2. [DOI] [PubMed] [Google Scholar]
- Burnell E., van Alphen L., Verkleij A., de Kruijff B., Lugtenberg B. 31P nuclear magnetic resonance and freeze-fracture electron microscopy studies on Escherichia coli. III. The outer membrane. Biochim Biophys Acta. 1980 Apr 24;597(3):518–532. doi: 10.1016/0005-2736(80)90224-2. [DOI] [PubMed] [Google Scholar]
- Coughlin R. T., Haug A., McGroarty E. J. Physical properties of defined lipopolysaccharide salts. Biochemistry. 1983 Apr 12;22(8):2007–2013. doi: 10.1021/bi00277a042. [DOI] [PubMed] [Google Scholar]
- Davis J. H., Nichol C. P., Weeks G., Bloom M. Study of the cytoplasmic and outer membranes of Escherichia coli by deuterium magnetic resonance. Biochemistry. 1979 May 15;18(10):2103–2112. doi: 10.1021/bi00577a041. [DOI] [PubMed] [Google Scholar]
- EHRENBERG W., FRANKS A. Small-angle x-ray scattering. Nature. 1952 Dec 20;170(4338):1076–1077. doi: 10.1038/1701076a0. [DOI] [PubMed] [Google Scholar]
- Emmerling G., Henning U., Gulik-Krzywicki T. Order-disorder conformation transition of hydrocarbon chains in lipopolysaccharide from Escherichia coli. Eur J Biochem. 1977 Sep;78(2):503–509. doi: 10.1111/j.1432-1033.1977.tb11763.x. [DOI] [PubMed] [Google Scholar]
- Formanek H. Possible orientation of the fatty acid chains in lipopolysaccharide. Z Naturforsch C. 1982 May-Jun;37(5-6):428–440. doi: 10.1515/znc-1982-5-613. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Levine Y. K. Low-angle x-ray diffraction. Mol Biol Biochem Biophys. 1981;31:437–487. doi: 10.1007/978-3-642-81537-9_9. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gally H. U., Pluschke G., Overath P., Seelig J. Structure of Escherichia coli membranes. Fatty acyl chain order parameters of inner and outer membranes and derived liposomes. Biochemistry. 1980 Apr 15;19(8):1638–1643. doi: 10.1021/bi00549a018. [DOI] [PubMed] [Google Scholar]
- Gund P., Andose J. D., Rhodes J. B., Smith G. M. Three-dimensional molecular modeling and drug design. Science. 1980 Jun 27;208(4451):1425–1431. doi: 10.1126/science.6104357. [DOI] [PubMed] [Google Scholar]
- Howard J. C., Ali A., Scheraga H. A., Momany F. A. Investigation of the conformations of four tetrapeptides by nuclear magnetic resonance and circular dichroism spectroscopy, and conformational energy calculations. Macromolecules. 1975 Sep-Oct;8(5):607–622. doi: 10.1021/ma60047a008. [DOI] [PubMed] [Google Scholar]
- Janoff A. S., Haug A., McGroarty E. J. Relationship of growth temperature and thermotropic lipid phase changes in cytoplasmic and outer membranes from Escherichia coli K12. Biochim Biophys Acta. 1979 Jul 19;555(1):56–66. doi: 10.1016/0005-2736(79)90071-3. [DOI] [PubMed] [Google Scholar]
- Leps B., Barnickel G., Bradaczek H. Structural studies on the bacterial cell wall peptidoglycan pseudomurein. I. Conformational energy calculations on the glycan strands in C1 conformation and comparison with murein. J Theor Biol. 1984 Mar 7;107(1):85–114. doi: 10.1016/s0022-5193(84)80123-x. [DOI] [PubMed] [Google Scholar]
- Leps B., Labischinski H., Barnickel G., Bradaczek H., Giesbrecht P. A new proposal for the primary and secondary structure of the glycan moiety of pseudomurein. Conformational energy calculations on the glycan strands with talosaminuronic acid in 1C conformation and comparison with murein. Eur J Biochem. 1984 Oct 15;144(2):279–286. doi: 10.1111/j.1432-1033.1984.tb08461.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Mitsui T. X-ray diffraction studies of membranes. Adv Biophys. 1978;10:97–135. [PubMed] [Google Scholar]
- Nakayama H., Mitsui T., Nishihara M., Kito M. Relation between growth temperature of E. coli and phase transition temperatures of its cytoplasmic and outer membranes. Biochim Biophys Acta. 1980 Sep 2;601(1):1–10. doi: 10.1016/0005-2736(80)90508-8. [DOI] [PubMed] [Google Scholar]
- Nichol C. P., Davis J. H., Weeks G., Bloom M. Quantitative study of the fluidity of Escherichia coli membranes using deuterium magnetic resonance. Biochemistry. 1980 Feb 5;19(3):451–457. doi: 10.1021/bi00544a008. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Biosynthesis of cell wall lipopolysaccharide in gram-negative enteric bacteria. Adv Enzymol Relat Areas Mol Biol. 1968;31:77–124. doi: 10.1002/9780470122761.ch3. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Permeability of the outer membrane of bacteria. Angew Chem Int Ed Engl. 1979 May;18(5):337–350. doi: 10.1002/anie.197903373. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Takeuchi Y., Ohnishi S. I., Nakae T. Outer membrane of Salmonella typhimurium. Electron spin resonance studies. Biochim Biophys Acta. 1977 Feb 14;465(1):152–164. doi: 10.1016/0005-2736(77)90363-7. [DOI] [PubMed] [Google Scholar]
- Némethy G., Scheraga H. A. Protein folding. Q Rev Biophys. 1977 Aug;10(3):239–252. doi: 10.1017/s0033583500002936. [DOI] [PubMed] [Google Scholar]
- Pincus M. R., Burgess A. W., Scheraga H. A. Conformational energy calculations of enzyme-substrate complexes of lysozyme. I. Energy minimization of monosaccharide and oligosaccharide inhibitors and substrates of lysozyme. Biopolymers. 1976 Dec;15(12NA-NA-770103-770104):2485–2521. doi: 10.1002/bip.1976.360151212. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hasin M., Razin S. The outer membrane of Proteus mirabilis. II. The extractable lipid fraction and electron-paramagnetic resonance analysis of the outer and cytoplasmic membranes. Biochim Biophys Acta. 1975 Feb 14;375(3):395–405. doi: 10.1016/0005-2736(75)90355-7. [DOI] [PubMed] [Google Scholar]
- Rottem S., Leive L. Effect of variations in lipopolysaccharide on the fluidity of the outer membrane of Escherichia coli. J Biol Chem. 1977 Mar 25;252(6):2077–2081. [PubMed] [Google Scholar]
- Scheraga H. A. Recent progress in the theoretical treatment of protein folding. Biopolymers. 1983 Jan;22(1):1–14. doi: 10.1002/bip.360220104. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Graham J. A., Nath K. The morphologic structure of isolated bacterial lipopolysaccharide. J Mol Biol. 1967 Apr 14;25(1):15–21. doi: 10.1016/0022-2836(67)90275-6. [DOI] [PubMed] [Google Scholar]
- Strain S. M., Fesik S. W., Armitage I. M. Structure and metal-binding properties of lipopolysaccharides from heptoseless mutants of Escherichia coli studied by 13C and 31P nuclear magnetic resonance. J Biol Chem. 1983 Nov 25;258(22):13466–13477. [PubMed] [Google Scholar]
- Takeuchi Y., Nikaido H. Persistence of segregated phospholipid domains in phospholipid--lipopolysaccharide mixed bilayers: studies with spin-labeled phospholipids. Biochemistry. 1981 Feb 3;20(3):523–529. doi: 10.1021/bi00506a013. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Ueki T., Mitsui T., Nikaido H. X-ray diffraction studies of outer membranes of Salmonella typhimurium. J Biochem. 1979 Jan;85(1):173–182. doi: 10.1093/oxfordjournals.jbchem.a132307. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Van Alphen L., Lugtenberg B., Rietschel E. T., Mombers C. Architecture of the outer membrane of Escherichia coli K12. Phase transitions of the bacteriophage K3 receptor complex. Eur J Biochem. 1979 Nov;101(2):571–579. doi: 10.1111/j.1432-1033.1979.tb19752.x. [DOI] [PubMed] [Google Scholar]
- Wawra H., Buschmann H., Formanek H., Formanek S. Strukturuntersuchung mit Röntgenbeugungsmethoden an Lipopolysacchariden von Salmonella minnesota Mutanten S SF 1111 und R 595 SF 1167. Z Naturforsch C. 1979 Mar-Apr;34(3-4):171–178. [PubMed] [Google Scholar]
- Wilkins M. H., Blaurock A. E., Engelman D. M. Bilayer structure in membranes. Nat New Biol. 1971 Mar 17;230(11):72–76. doi: 10.1038/newbio230072a0. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Verkleij A., Burnell E., Lugtenberg B. 31P nuclear magnetic resonance and freeze-fracture electron microscopy studies on Escherichia coli. II. Lipopolysaccharide and lipopolysaccharide-phospholipid complexes. Biochim Biophys Acta. 1980 Apr 24;597(3):502–517. doi: 10.1016/0005-2736(80)90223-0. [DOI] [PubMed] [Google Scholar]