Abstract
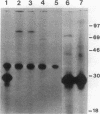
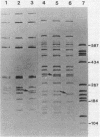
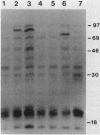
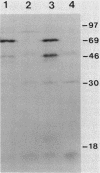
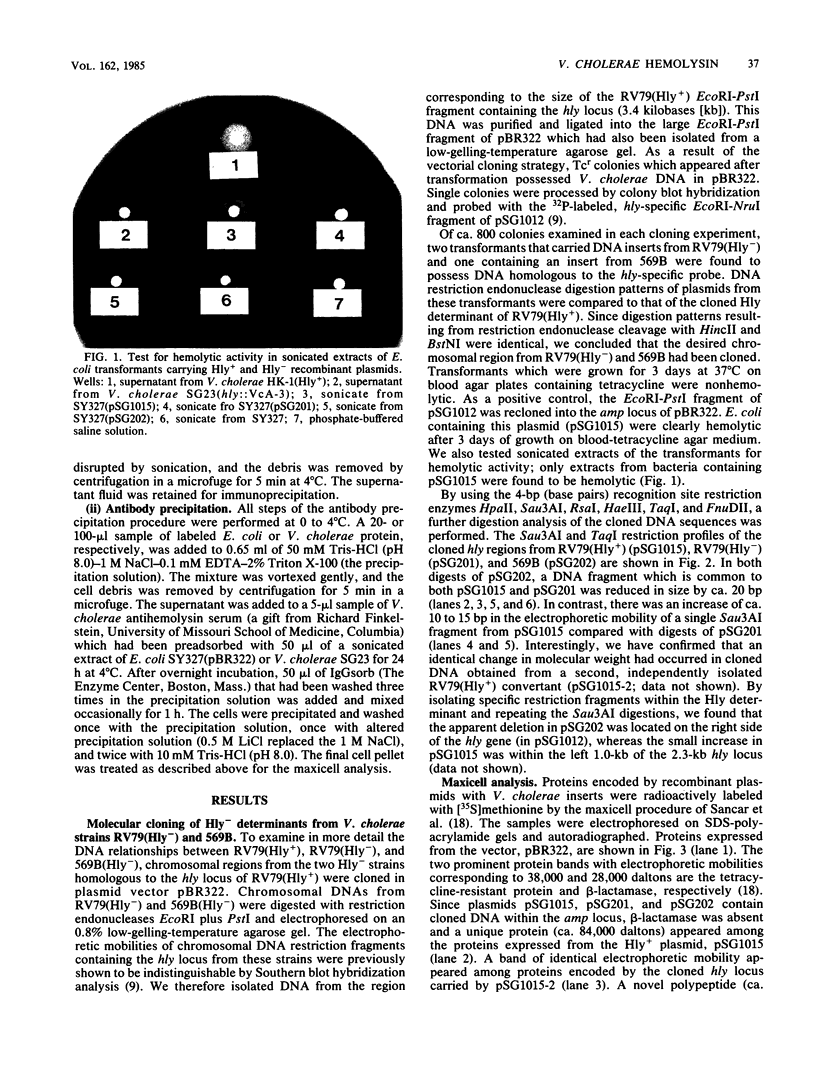
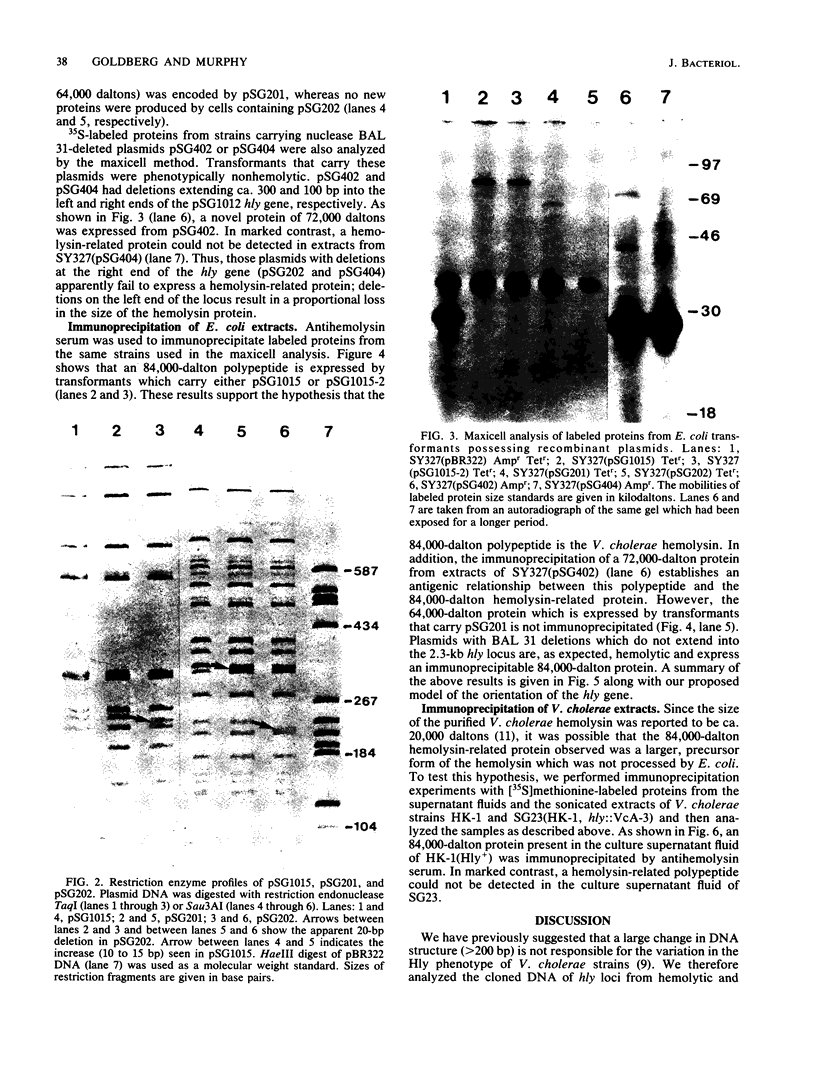
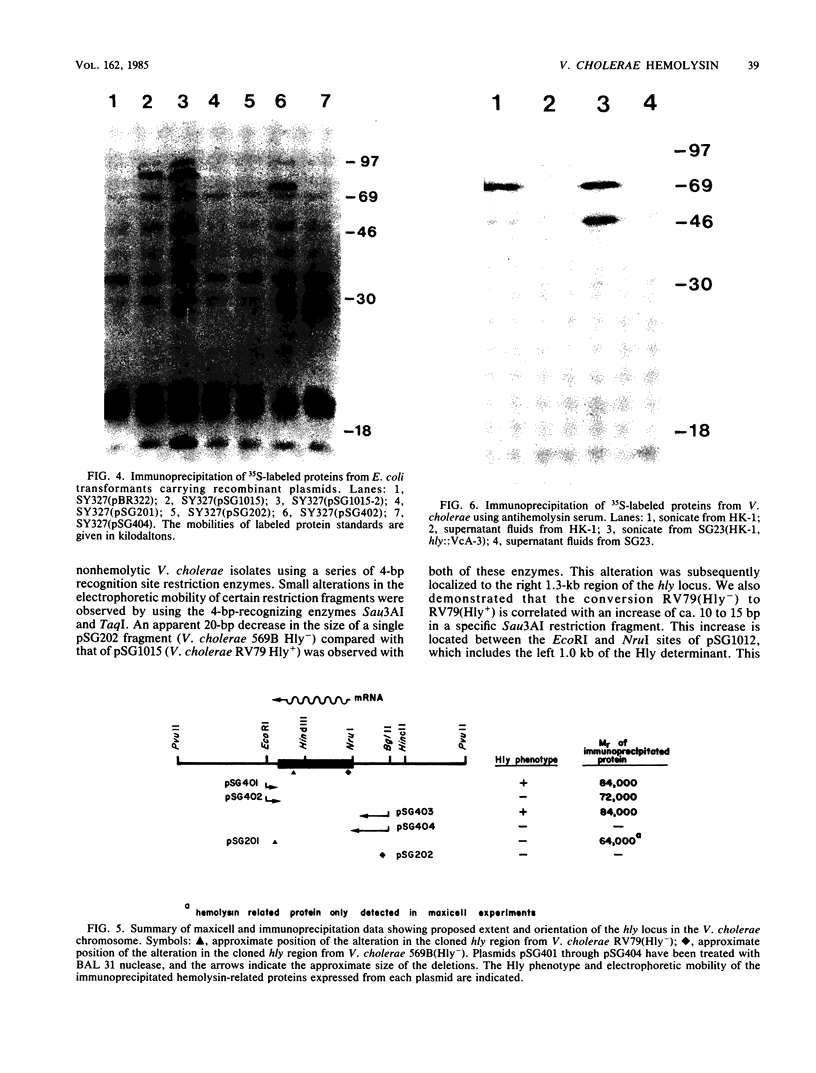
The Hly region from the chromosome of Vibrio cholerae El Tor strain RV79(Hly-) and the nonhemolytic classical strain 569B were cloned into plasmid vector pBR322. Escherichia coli K-12 transformants possessing these recombinant plasmids were nonhemolytic and were detected with a 32P-labeled hly-specific DNA probe. Restriction endonuclease Sau3AI digestions of the cloned hly loci of two independently obtained RV79(Hly+) convertants, when compared with the digests of cloned RV79(Hly-) loci, revealed that an apparent alteration (10 to 15 base pairs) had occurred. In contrast, an apparent 20-base-pair deletion was present in the cloned hly locus of the classical biotype V. cholerae strain 569B. Maxicell analysis and immunoprecipitation of labeled proteins of E. coli which are encoded by the cloned hly loci of RV79(Hly+) and from nuclease BAL 31-deleted plasmids, as well as immunoprecipitation of [35S]methionine-labeled V. cholerae proteins, suggest that the hemolysin is an 84,000-dalton polypeptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Dujaili A. H., Harris D. M. Pseudomonas aeruginosa infection in hospital: a comparison between 'infective' and 'environmental' strains. J Hyg (Lond) 1975 Oct;75(2):195–201. doi: 10.1017/s0022172400047227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Richardson S. H. Biochemistry of Vibrio cholerae virulence. 3. Nutritional requirements for toxin production and the effects of pH on toxin elaboration in chemically defined media. Infect Immun. 1973 Apr;7(4):567–572. doi: 10.1128/iai.7.4.567-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MOOR C. E. A NON-HAEMOLYTIC EL TOR VIBRIO AS THE CAUSE OF AN OUTBREAK OF PARACHOLERA IN WEST NEW GUINEA. THE EL TOR PROBLEM AND PANDEMIC PARACHOLERA IN THE WEST PACIFIC. Trop Geogr Med. 1963 Jun;15:97–107. [PubMed] [Google Scholar]
- Fairweather N., Kennedy S., Foster T. J., Kehoe M., Dougan G. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun. 1983 Sep;41(3):1112–1117. doi: 10.1128/iai.41.3.1112-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. L., Murphy J. R. Molecular cloning of the hemolysin determinant from Vibrio cholerae El Tor. J Bacteriol. 1984 Oct;160(1):239–244. doi: 10.1128/jb.160.1.239-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979 Dec;26(3):1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Falkow S. Nucleotide sequence homology between the heat-labile enterotoxin gene of Escherichia coli and Vibrio cholerae deoxyribonucleic acid. J Bacteriol. 1980 Oct;144(1):444–446. doi: 10.1128/jb.144.1.444-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Richardson S. H., Romig W. R. Production of Bacteriophage-Associated Materials by Vibrio cholerae: Possible Correlation with Pathogenicity. Infect Immun. 1970 Apr;1(4):417–420. doi: 10.1128/iai.1.4.417-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier J. G., Jones S. J., Cleary P. Small DNA deletions creating avirulence in Streptococcus pyogenes. Science. 1984 Aug 31;225(4665):935–938. doi: 10.1126/science.6089334. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., van den Bosch J. F., MacLaren D. M., de Graaff J. Hemolysin plasmid coding for the virulence of a nephropathogenic Escherichia coli strain. Infect Immun. 1982 Jan;35(1):32–37. doi: 10.1128/iai.35.1.32-37.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. O., Young J. R., Majiwa P. A. Genomic rearrangements correlated with antigenic variation in Trypanosoma brucei. Nature. 1979 Dec 20;282(5741):847–849. doi: 10.1038/282847a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Al-Omani M., Honda T., Takeda Y., Miwatani T. Non-O1 Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984 Jul;45(1):192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science. 1977 Apr 8;196(4286):170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]