Abstract
Intracellular application of the sulfonylurea tolbutamide during whole-cell patch-clamp recordings stimulated exocytosis >5-fold when applied at a cytoplasmic Ca2+ concentration of 0.17 μM. This effect was not detectable in the complete absence of cytoplasmic Ca2+ and when exocytosis was elicited by guanosine 5′-O-(3-thiotriphosphate) (GTPγS). The stimulatory action could be antagonized by the sulfonamide diazoxide, by the Cl−-channel blocker 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), by intracellular application of the antibody JSB1 [originally raised against a 170-kDa multidrug resistance (mdr) protein], and by tamoxifen (an inhibitor of the mdr- and volume-regulated Cl− channels). Immunocytochemistry and Western blot analyses revealed that JSB1 recognizes a 65-kDa protein in the secretory granules. This protein exhibited no detectable binding of sulfonylureas and is distinct from the 140-kDa sulfonylurea high-affinity sulfonylurea receptors also present in the granules. We conclude that (i) tolbutamide stimulates Ca2+-dependent exocytosis secondary to its binding to a 140-kDa high-affinity sulfonylurea receptor in the secretory granules; and (ii) a granular 65-kDa mdr-like protein mediates the action. The processes thus initiated culminate in the activation of a granular Cl− conductance. We speculate that the activation of granular Cl− fluxes promotes exocytosis (possibly by providing the energy required for membrane fusion) by inducing water uptake and an increased intragranular hydrostatic pressure.
Keywords: insulin, sulfonyl urea receptor, ABC protein
Sulfonylureas have been used in the treatment of non-insulin-dependent diabetes mellitus (NIDDM or type-2 diabetes) for more than 30 years (1), but it is only recently that the molecular mechanisms of their action have been elucidated. It is now clear that the principal effect of the sulfonylureas is to stimulate insulin secretion from the pancreatic β cells (2). Patch-clamp experiments have revealed that this stimulation is a consequence of their ability to selectively inhibit ATP-sensitive K+ channels (KATP channels) in the β cell plasma membrane (3). It has recently been demonstrated that the KATP channel is composed of at least two components: a sulfonylurea receptor (SUR) and an inward rectifier potassium channel protein (KIR6.2; ref. 4). The binding of the sulfonylureas to SUR1, the β cell variety of SUR, results in the closure of the KATP channels. Since these channels are responsible for the maintenance of a repolarized (negative) membrane potential in the β cell, addition of sulfonylureas results in membrane depolarization that culminates in Ca2+ entry through voltage-gated L-type Ca2+ channels and the onset of Ca2+-dependent insulin secretion (5). Surprisingly, the majority (90%) of the high-affinity sulfonylurea binding sites in the β cell are intracellular and appear to associate with the insulin-containing secretory granules (6, 7), far away from the KATP channels in the plasma membrane. The role of these intracellular sulfonylurea receptors is not known. Studies on pancreatic zymogen granules have suggested the presence of ATP- and sulfonylurea-sensitive K+ and Cl− conductances in such membranes (8, 9). Sulfonylureas modulate these conductances reciprocally, which can be envisaged to result in a net accumulation of electrolytes, water uptake, and increased hydrostatic pressure inside the granules. It has been suggested that an increased hydrostatic pressure may promote membrane fusion (10). This model is not uncontested (cf. ref. 11), but recent measurements using atomic force microscopy provide evidence that conditions resulting in exocytosis are indeed associated with moderate (15–20%) expansion of zymogen granules (12).
We have previously reported that sulfonylureas potentiate depolarization-evoked and Ca2+-dependent exocytosis in pancreatic β cells (13). This effect is exerted distally to the elevation of the cytoplasmic free Ca2+ concentration ([Ca2+]i) and is observed at sulfonylurea concentrations only slightly higher than those required to block the KATP channels. Here we have characterized the participating mechanisms in greater detail, utilizing the standard whole-cell configuration (14), which allows application of drugs directly onto the intracellular SURs. We show that the effects of sulfonylureas on exocytosis in pancreatic β cells are secondary to their binding to 140-kDa high-affinity SURs in the granular membrane. Their action is mediated by a granular 65-kDa mdr-like protein and culminates in the activation of a 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS)-sensitive mechanism, possibly a granular Cl− conductance. The resultant accumulation of electrolytes within the granules leads to the uptake of water and thus increases the intragranular hydrostatic pressure. This may facilitate exocytosis by providing the energy required for the fusion process.
MATERIALS AND METHODS
Preparation and Culture of β Cells.
Mouse pancreatic β cells were isolated from NMRI-mice (Alab, Sweden, or Bomholtgaard, Denmark) or (for the Western blot experiments) ob/ob mice taken from the animal house at the Karolinska Institute. The animals were stunned by a blow against the head and killed by cervical dislocation. The pancreas was quickly removed and pancreatic islets were isolated by collagenase digestion. For immunostaining and the electrophysiological experiments, islets were dispersed into single cells by shaking in Ca2+-free solution (15). The resultant cell suspension was plated on Corning Petri dishes or 22-mm glass coverslips. The cells were maintained in tissue culture for up to 2 days in RPMI 1640 medium containing 5 mM glucose, 10% (vol/vol) fetal calf serum, 100 μg/ml streptomycin, and 100 international units/ml penicillin.
Electrophysiology.
Patch electrodes were made from borosilicate glass capillaries coated with Sylgard close to the tips and fire-polished. The pipette resistance ranged between 2 and 4 MΩ when the pipettes were filled with the intracellular solutions specified below. The experiments were conducted using the standard whole-cell configuration (14). Intracellular receptors are thereby directly exposed to the sulfonylurea included in the pipette solution dialyzing the cell interior.
Exocytosis was detected as changes in cell capacitance as previously described (16, 17) or by using the “captrack” function of an EPC9 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany). Exocytosis was elicited either by infusion, through the recording electrode, of a Ca2+–EGTA buffer with a free Ca2+ concentration of 0.17 μM or the stable GTP analogue guanosine 5′-O-(3-thiotriphosphate) (GTPγS) (18) or by photorelease of Ca2+ from a caged precursor to stimulate secretion (19). All experiments were carried out at 32°C.
Amperometry.
Electrochemical detection of insulin secretion (amperometry) was carried out as described by Smith et al. (20). Briefly, the cells were loaded overnight with 0.6 mM 5-hydroxytryptamine (5-HT; serotonin) added to the culture medium. Amperometric currents were detected at +650 mV by using ProCFE carbon fibers (Axon instruments, Burlingame, CA) attached to an EPC-9 amplifier (HEKA Electronics). The carbon fibers were calibrated prior to each experiment by adding 1 μM 5-HT to the extracellular medium. Because the sensitivity of the carbon fibers varied considerably, data are presented as the equivalent increase in the extracellular concentration of 5-HT.
Solutions.
The standard extracellular medium consisted of (in mM) 138 NaCl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2, 5 d-glucose, and 5 Hepes (pH 7.4 with NaOH). When exocytosis was elicited by addition of Ca2+ through the recording electrode, the pipette-filling solution contained (mM) 125 potassium glutamate, 10 KCl, 10 NaCl, 1 MgCl2, 5 Hepes, 10 EGTA (pH 7.15 with KOH), 3 Mg-ATP and 0 or 5 CaCl2. The resulting free Ca2+ concentrations were estimated by using the binding constants of Martell and Smith (21), as 0 and 0.17 μM, respectively; the former Ca2+ concentration being unable to elicit exocytosis in itself. Cyclic AMP (0.1 mM) and GTPγS were included in the pipette solution as indicated in the text or the legends to the figures.
In the experiments involving photorelease of caged Ca2+, the pipette solution contained (in mM) 110 potassium glutamate, 10 KCl, 10 NaCl, 1 MgCl2, 25 Hepes, 3 Mg-ATP, 3 o-nitrophenyl EGTA (NP-EGTA; Molecular Probes), and 1.5 CaCl2 (pH 7.1 with KOH); calculated initial [Ca2+]i: <0.2 μM. The antibody JSB1 (Boehringer Mannheim), raised against the multidrug resistance P-glycoprotein 22, was added to a final concentration of 5 μg/ml. Nonimmunized mouse IgG served as the control. It was ascertained, by using dextran-conjugated fura-2 with a molecular mass of 100 kDa (Molecular Probes), that wash-in of the antibody is completed within 4–5 min. When using the infusion protocol, it is important that a high intracellular concentration of the test compound is attained shortly after establishment of the whole-cell configuration. All pharmacological agents were therefore included in the pipette-filling solution at maximally effective concentrations (usually 100 μM).
Photorelease of Caged Ca2+ and Measurements of [Ca2+]i.
Photolysis of the Ca2+/NP-EGTA complex was effected by brief (<2-ms) flashes of ultraviolet (UV) light produced by a XF-10 photolysis apparatus (HiTech Scientific, Salisbury, U.K.). The resulting increases in [Ca2+]i were measured by dual-wavelength spectrofluorimetry using 10 μM of the low-affinity Ca2+ indicator BTC (a benzothiazole coumarin derivative from Molecular Probes; Kd was estimated to 70 μM) and the hardware and software of Ionoptix (Milton, MA) as described elsewhere (19, 23). Judging from the increases in [Ca2+]i obtained after UV-irradiation, the efficiency of liberation was ≈30% for a 2-ms flash.
Immunofluorescence.
Cells were fixed in 4% formaldehyde and stained with JSB1 antibody as recommended by the supplier. An Oregon green-conjugated goat anti-mouse antibody (Molecular Probes) was used to label the cells with the fluorophore. Confocal images were obtained with a Leica TCS-NT confocal microscope (Leica Lasertechnik, Heidelberg, Germany) equipped with a PL APO 100×/1.4–0.7 (oil) objective and a FITC filter set (excitation at 488 nm and detection of emission at 515–545 nm). Images were reconstructed from a three-dimensional stack of images throughout the cell.
Subcellular Fractionation.
β cells from ob/ob mice were fractionated by using a sucrose density gradient as described (24). Fractions enriched in plasma membrane or insulin granules were identified by using an antibody against the Na+/K+-ATPase α subunit (plasma membrane) and by determination of insulin content (granules). As expected, the granular fraction exhibited 20-fold higher insulin content than the plasma membrane fraction and no detectable Na+/K+-ATPase.
SDS/Page and Western Blot Analyses.
Electrophoresis and blotting procedures were performed essentially as described earlier (9). Briefly, proteins (20 μg per lane) were separated by SDS/PAGE on 7.5% acrylamide Laemmli minigels and transferred overnight onto polyvinylidene difluoride (PVDF) membranes (DuPont/NEN). Blots were incubated overnight with primary antibodies against mdr1 [5 μg/ml JSB1 or 0.5 μg/ml C219 (Alexis or Boehringer Mannheim)]. After incubation with horseradish-peroxidase (HRP)-conjugated secondary antibody (1:6000 dilution, Amersham-Buchler, Germany) for 60 min, blots were developed in enhanced chemiluminescence reagents and signals were visualized on x-ray films. The epitopes of C219 are located at N-terminal residues 568–574 and C-terminal amino acids 1213–1219. These epitopes are highly conserved amino acid sequences found in all P-glycoprotein isoforms characterized so far (25). JSB1 was originally raised against a colchicine-selected mutant of the Chinese hamster ovary cell line (26) and has been shown to be mdr1-specific by binding to a cytosolic domain of mdr1 (27), probably at the C-terminal amino acids 1028–1035 (28).
Photoaffinity Labeling.
Photoaffinity labeling was performed according to Kramer et al. (29). Plasma membranes (54 μg) and granular membranes (43 μg) isolated from ob/ob mouse islets were equilibrated with 20 nM [3H]glibenclamide in the absence or presence of 1 μM unlabeled glibenclamide for 1 h and then irradiated with UV light (312 nm) for 2 min. For separation of unbound radioactivity, samples were diluted 9-fold with homogenization buffer and centrifuged for 60 min at 100,000 × g. The membrane pellets were resuspended in sample buffer containing 1% SDS and 1% 2-mercaptoethanol (pH 9), boiled for 3 min, and separated by SDS/PAGE on 7.5% acrylamide minigels. The lanes were cut into 3-mm pieces and digested in Soluene-350 (Packard BioScience, The Netherlands), and radioactivities were measured in a liquid scintillation counter.
Data Analysis.
Exocytosis is quoted as the rate of capacitance increase (in fF/s). In the infusion experiments, the steady-state increase observed during the first ≈60 s after establishment of the whole-cell configuration was measured. In the experiments involving photorelease of caged Ca2+, the average rate of capacitance increase over the initial 100 ms, when endocytosis is negligible, after flash photolysis was determined. Data are presented as mean values ± SEM of indicated (n) number of experiments. Statistical significances were evaluated with Student’s t test for unpaired data.
RESULTS
Intracellularly Applied Tolbutamide Stimulates Ca2+-Dependent Exocytosis.
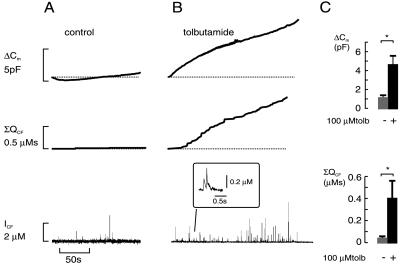
Fig. 1 shows the increase in cell capacitance (reflecting exocytosis) occurring during intracellular dialysis with a Ca2+-EGTA buffer with 0.17 μM [Ca2+]i. At this low [Ca2+]i, which is just above the resting concentration, exocytosis was minimal and no increase in cell capacitance occurred (Fig. 1A, Top). Inclusion of tolbutamide (0.1 mM) in this pipette solution (which dialyzes the cell interior during standard whole-cell recordings within ≈10 s) accelerated exocytosis, and there was a time-dependent increase in cell capacitance (Fig. 1B, Top). On average, the rate of capacitance increase measured over the first 60 s after the establishment of the whole-cell configuration increased by a factor of 10 in the presence of tolbutamide compared with that seen in the absence of the sulfonylurea; from 2 ± 2 fF/s under control conditions (n = 13) to 18 ± 4 fF/s (n = 18) in the presence of tolbutamide (P < 0.001).
Figure 1.
Intracellular tolbutamide stimulates exocytosis in β cells. Parallel recordings of exocytosis as an increase in cell capacitance (ΔCm) and as the amperometric current detected with a carbon fiber electrode (ICF). Exocytosis was triggered by inclusion of a Ca2+-EGTA buffer with a free [Ca2+]i of 0.17 μM in the absence (A) and presence (B) of 0.1 mM tolbutamide, respectively. Note the parallel increase in cell capacitance and the cumulative amperometric charge (ΣQCF). (Inset) Expanded portion of the amperometric recording in the presence of tolbutamide. (C) Mean increase in cell capacitance (Upper) and cumulative amperometric charge (ΣQCF, Lower) occurring over the initial 4 min (i.e., the time required to reach a new steady-state level) in the absence (n = 4) and presence (n = 10) of 0.1 mM tolbutamide; ∗, P < 0.05.
To ascertain that the increase in cell capacitance elicited by tolbutamide does indeed reflect stimulation of insulin secretion, we combined capacitance measurements with carbon fiber amperometry (Fig. 1 A and B, Middle). The slow increase in cell capacitance (Cm) observed under control conditions correlated with the appearance of few amperometric events (trace labeled ICF) and the time integral of the amperometric recording (ΣQCF) was flat. In the presence of tolbutamide, however, numerous amperometric transients were observed. Consistent with what has been described previously by others (20), the amplitude of these spikes was highly variable, possibly reflecting uneven loading of the granules with 5-hydroxytryptamine or the granules being of different size. The time course of ΣQCF roughly paralleled that of the capacitance increase (compare Top and Middle traces in Fig. 1B), and the extent of stimulation was essentially the same (Fig. 1C).
Tolbutamide failed to stimulate exocytosis when it was applied in the complete absence of cytoplasmic Ca2+, and the rate of capacitance increase measured during the first 60 s amounted to 3 ± 1 fF/s (n = 6) under control conditions and 2 ± 2 fF/s (n = 7) in the presence of 0.1 mM tolbutamide. The same negative result was obtained when exocytosis was elicited by infusion of the stable GTP analogue GTPγS (40 μM; cf. ref. 18). The rate of capacitance increase observed in the presence of GTPγS amounted to 5 ± 2 fF/s (n = 16) under control conditions and 6 ± 2 fF/s (n = 17) when tolbutamide had been included in the pipette solution. Collectively these data indicate that the increase in cell capacitance evoked by intracellular tolbutamide reflects a true stimulation of insulin secretion and that tolbutamide potentiates Ca2+-dependent exocytosis but that it is not an initiator of exocytosis.
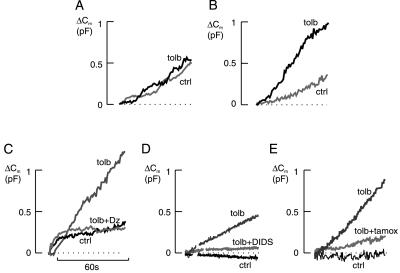
Tolbutamide was unable to stimulate exocytosis in β cells preincubated in 10 mM glucose and 10 μM forskolin (Fig. 2A; cf. refs. 30 and 31). However, it remained stimulatory in cells of the same preparation that had not been subjected to the preincubation protocol (Fig. 2B). In cells preincubated with glucose and forskolin, exocytosis elicited by 0.17 μM [Ca2+]i amounted to 10 ± 5 fF/s (n = 6) and 10 ± 2 fF/s (n = 6) in the absence and presence of 0.1 mM tolbutamide, respectively. The corresponding values in cells exposed to 5 mM glucose alone were 6 ± 2 fF/s (n = 6) and 18 ± 4 fF/s (n = 6; P < 0.05).
Figure 2.
(A) Failure of tolbutamide (tolb) to stimulate exocytosis in cells preincubated with high glucose and forskolin (cf. ref. 31). ctrl, Control. In all cases, secretion was evoked by intracellular dialysis with 0.17 μM [Ca2+]i. (B) Stimulation of exocytosis by tolbutamide in the same preparation of β cells but preincubated with 5 mM glucose only. (C) Change in cell capacitance (Cm) taking place under control conditions and in the presence of tolbutamide (0.1 mM) alone or in combination with diazoxide (dz) (0.1 mM) as indicated. (D) Failure of tolbutamide to stimulate exocytosis in the presence of the Cl−-channel blocker DIDS. Tolbutamide (0.1 mM) and DIDS (0.2 mM) were added as indicated. (E) Stimulation of exocytosis by tolbutamide (0.1 mM) is antagonized by tamoxifen (tamox) (0.1 mM). Traces shown are representative of 5–11 separate experiments.
Pharmacological Modulation of Tolbutamide-Induced Exocytosis.
The inhibition of the plasma membrane KATP channels by tolbutamide can be antagonized by diazoxide (3). As shown in Fig. 2C, the stimulatory action of intracellular tolbutamide on exocytosis could likewise be counteracted by diazoxide. The rate of capacitance increase fell from about 20 ± 2 fF/s (n = 7) in the presence of tolbutamide alone to 4 ± 2 fF/s (n = 5) in the simultaneous presence of tolbutamide and diazoxide. The latter value is similar to that obtained under control conditions (0.17 μM [Ca2+]i in the absence of any drugs, see above). When applied in the presence of 0.17 μM [Ca2+]i alone, diazoxide exerted a small (30%) inhibitory action that did not reach statistical significance (not shown).
DIDS-sensitive Cl− channels have been described in zymogen granules (32). We therefore explored whether such channels participate in sulfonylurea-stimulated exocytosis by inclusion of this Cl−-channel blocker in the pipette solution. Indeed, DIDS abolished the ability of tolbutamide to stimulated exocytosis when applied intracellularly at a concentration of 0.2 mM (Fig. 2D, compare Fig. 1). The rates of exocytosis measured in the presence of the Cl−-channel blocker during the first 60 s after establishing the whole-cell configuration amounted to 1 ± 1 fF/s (n = 6) and 1 ± 1 fF/s (n = 6) in the absence and presence of tolbutamide (0.1 mM), respectively.
We next tested for the possible involvement of an mdr-like protein by using tamoxifen, an inhibitor of this protein in a variety of systems (33, 34). As illustrated in Fig. 2E, inclusion of tamoxifen (0.1 mM) in the pipette solution abolished the stimulatory action of tolbutamide. The rate of exocytosis measured in the presence of both tamoxifen and tolbutamide amounted to 7 ± 1 fF/s (n = 8). This rate is lower than that obtained in the presence of tolbutamide alone [13 ± 1 fF/s (n = 11); P < 0.01] but similar to that seen under control conditions [7 ± 1 fF/s (n = 9)].
We have previously reported that tolbutamide remains capable of stimulating exocytosis when intracellular K+ is replaced with Cs+ (13). By contrast, tolbutamide is without stimulatory action when N-methyl-d-glucamine+ (NMDG+) substituted for intracellular K+ (not shown). This observation argues that a nonselective ion channel permeable to both K+ and Cs+, but not to NMDG+, participates in the process. Interestingly, cyclic AMP remained capable of potentiating secretion under these experimental conditions, suggesting that its stimulatory actions (17) involve mechanisms that are at least in part distinct from those activated by tolbutamide.
The Antibody JSB1 Abolishes Action of Tolbutamide on Exocytosis.
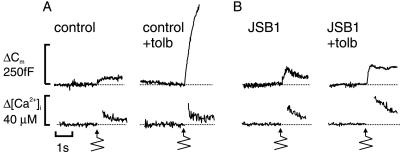
The effects of tamoxifen pointed to the involvement of an mdr-like protein in the stimulatory effect of tolbutamide on exocytosis. The monoclonal antibody JSB1, raised against a 170-kDa multidrug resistance P-glycoprotein (22), interferes with a Cl− conductance in zymogen granules and thereby prevents their swelling (9). Fig. 3 contains an experiment in which cells were infused with this antibody. Here exocytosis was elicited by photorelease of Ca2+ from the caged precursor Ca2+/NP-EGTA. Liberation of Ca2+ was effected >4 min after establishment of the whole-cell configuration to allow wash-in of the antibody (see Materials and Methods). Tolbutamide remained capable of stimulating exocytosis under these experimental conditions (Fig. 3A). The initial exocytotic rate, measured during the first 100 ms, amounted to 286 ± 53 fF/s (n = 11) and 798 ± 112 fF/s (n = 13; P < 0.0005) in the absence and presence of tolbutamide, respectively. The stimulation of exocytosis produced by tolbutamide is not the result of [Ca2+]i being higher and measured increases in [Ca2+]i averaged 48 ± 9 μM (n = 11) and 45 ± 7 μM (n = 13) in the absence and presence of drug. Tolbutamide remained equally stimulatory when the cells were dialyzed with nonimmunized mouse IgG (5 μg/ml; data not shown). However, tolbutamide failed to stimulate exocytosis after the wash-in of the antibody JSB1 (5 μg/ml; Fig. 3B). The rate of capacitance increase amounted to 338 ± 78 fF/s (n = 9) in the presence of the antibody but absence of tolbutamide. The rate of exocytosis fell to 200 ± 88 fF/s (n = 9; not significantly different from the control value) when tolbutamide was applied in the presence of the antibody. Again, [Ca2+]i was the same in the absence and presence of tolbutamide and averaged 42 ± 9 μM (n = 9) and 49 ± 3 μM (n = 9), respectively. These results suggest that JSB1 selectively interferes with the ability of tolbutamide to enhance exocytosis while not affecting Ca2+-induced secretion.
Figure 3.
The antibody JSB1 antagonizes the stimulatory action of tolbutamide on Ca2+-dependent exocytosis. (A) Increases in cytoplasmic free Ca2+ (Δ[Ca2+]i) and cell capacitance (ΔCm) occurring in response to photorelease of Ca2+ from the caged complex Ca2+/NP-EGTA (bottom arrows) under control conditions and in the presence of 0.1 mM tolbutamide. (B) As in A but 5 μg/ml antibody JSB1 had been included in the pipette solution. Traces shown are representative of 9–11 experiments.
JSB1 Recognizes a 65-kDa Granule Membrane Protein That Does Not Bind the Sulfonylurea Glibenclamide.
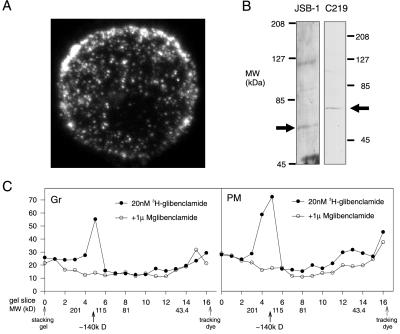
We next utilized immunofluorescence confocal microscopy to visualize the distribution of JSB1 binding within the β cell (Fig. 4A). Binding of JSB1 exhibited a punctuate distribution which was similar to that of the insulin-containing granules. To characterize the protein recognized by the antibody, we carried out Western blot analyses of isolated granule membranes from mouse islets with the mdr1-specific monoclonal antibodies JSB1 (5 μg/ml) and C219 (0.5 μg/ml). Both JSB1 and C219 recognized a 65-kDa protein (Fig. 4B). For comparison, we blotted purified plasma membranes from mouse islets (not shown). In these membranes, C219 labeled a protein with a molecular mass of ≈160 kDa, close to molecular mass of full-size multidrug resistance P-glycoprotein but different from the 65-kDa protein detected in the granular membranes. These data suggest that whereas full-length P-glycoprotein is expressed in the plasma membrane of islet cells, the secretory granules contain a truncated form of the protein with a molecular mass of 65 kDa. Glibenclamide is traditionally used for binding experiments because its affinity is ≈1000-fold higher than that of tolbutamide. We have shown previously that it affects exocytosis in the same way as tolbutamide (13). In rat pancreatic zymogen granule membranes, a 65-kDa protein was specifically labeled by the sulfonylurea [3H]glibenclamide, though with low affinity (Kd ≈ 6 μM) (35). However, 1 nM [3H]glibenclamide was completely displaced by 1 μM unlabeled glibenclamide in both plasma and granular membranes prepared from mouse islets, suggesting that it reflects binding of glibenclamide to sites with higher affinity for the sulfonylurea (not shown). Photoaffinity labeling experiments with [3H]glibenclamide (Fig. 4C) revealed that the sulfonylureas associate with a protein with a molecular mass of ≈140 kDa in both the plasma membrane and the secretory granule fractions. This corresponds to the approximate molecular mass of the cloned sulfonylurea receptor (4) but twice the molecular mass of the protein recognized by JSB1 in the granules. The protein recognized by JSB1 and C219 is therefore likely to be distinct from the cloned 140-kDa SUR1. This conclusion is in keeping with the finding that JSB1, when included in the pipette solution, was without effect on both the amplitude of the KATP conductance and the ability of tolbutamide to block whole-cell KATP currents in β cells (not shown). Results obtained with HEK293 cells transfected with the KIR6.2/SUR1 complex also reinforce this idea. Although SUR1 was present in the plasma membrane at 7–8 times higher density than in mouse β cells (as determined by the amplitude of whole-cell KATP currents), there was no detectable increase in JSB1-labeling as compared with nontransfected cells (not shown).
Figure 4.
Binding of JSB1 and C219 in pancreatic β cells. (A) Distribution of JSB1 binding in a pancreatic β cell revealed by confocal immunocytochemistry. Note the punctuate distribution. (×2,000.) (B) Western blot analyses of β cell granular membranes from mouse islets with the mdr1-specific monoclonal antibodies JSB1 and C219. Islet granule membrane proteins (20 μg) were probed with 5 μg/ml JSB1 or 0.5 μg/ml C219. Arrows indicate the position of the 65-kDa granular membrane protein cross-reacting with the anti-mdr1 antibodies. (C) Photoaffinity labeling of plasma membrane (PM) and secretory granules (Gr) from mouse islets with [3H]glibenclamide. The positions of the molecular mass markers (MW), the stacking gel, and the tracking dye are indicated. In both experiments, a peak of specifically bound radioactivity was detected at ≈140 kDa. Because of the scarcity of material, the experiments were conducted only once (C) or twice (B). However, identical results were obtained when membranes from Ins1 insulinoma cells were used (n = 2, not shown).
DISCUSSION
In this study we confirm that high-affinity SURs are present in both the plasma membrane and the secretory granules of insulin-secreting pancreatic β cells. Whereas the role of these receptors in forming the plasma membrane KATP channels is well established, the significance of the granular receptors remains elusive. Here we demonstrate that intracellularly applied tolbutamide accelerates exocytosis in voltage-clamped β cells. This effect occurs independently of plasma membrane KATP-channel closure and opening of voltage-gated Ca2+ channels. The data complement our previous observations made with intact pancreatic β cells in the perforated patch whole-cell configuration (13).
Relationship Between SUR and Stimulation of Exocytosis by Tolbutamide.
It is tempting to attribute the ability of tolbutamide to enhance exocytosis to the presence of high-affinity sulfonylurea-binding sites on the secretory granules. Sulfonylurea- and ATP-sensitive K+ and Cl− conductances have been found in isolated pancreatic zymogen granules and have been implicated in the release process in acinar cells (8). The granular K+ and Cl− channels are reciprocally controlled by a 65-kDa protein (9) that is recognized by the monoclonal antibodies JSB1 and C219. Here we demonstrate that JSB1 abolishes the stimulatory action of tolbutamide on exocytosis and detects a 65-kDa granular protein in pancreatic β cells. However, unlike the situation in acinar cells (35), the sulfonylurea does not exhibit any detectable high-affinity binding to the protein recognized by JSB1 in the pancreatic β cell. The previous findings that JSB1 binds to secretory granules in mast cells (36) and pancreatic acinar cells, taken together with the present observation made in insulin-secreting cells, suggest that mdr-like proteins play a general role in the control of exocytosis.
Comparison with Previous Work.
The view that tolbutamide is capable of stimulating exocytosis in the β cell by a distal effect is not uncontested (30, 31). Recently it was reported, however, that tolbutamide stimulates insulin secretion from min6 cells by a protein kinase C-dependent mechanism (37). The failure of others to reproduce our findings we attribute to differences in the experimental protocols. Whereas our cells were exposed to a medium containing 5 mM glucose during the experiments, the β cell used in the studies in which no late effect of tolbutamide on exocytosis was detected had been preincubated with high glucose and forskolin. Indeed, using the same protocol as that employed in the study of Mariot et al. (31), we were likewise unable to detect any stimulatory action of tolbutamide (Fig. 2 A and B). Such a protocol results in low cytosolic ADP levels. This is significant, since the stimulatory action of tolbutamide on calcium-induced exocytosis appears to counteract an ADP-mediated inhibitory action (S.B. and E.R., unpublished results).
Model for the Regulation of Exocytosis by Sulfonylureas.
The nature of the interaction between the protein(s) identified by JSB1 in insulin-secreting β cells and SUR1 remains unestablished, but it seems possible that they associate to form a functional complex that regulates ion fluxes in the secretory granules (see Fig. 5). In this context it is pertinent that Kramer et al. (29) have detected high-affinity binding of the sulfonylurea glimepiride to a 65-kDa protein in pancreatic β cells. In the same study when identical procedures were used, glibenclamide bound to a 140-kDa protein. Labeling of the 140-kDa protein by [3H]glibenclamide was inhibited by unlabeled glimepiride and, vice versa, glibenclamide inhibited labeling of the 65-kDa protein by [3H]glimepiride. This finding raises the interesting possibility that there exists a complex between the 140-kDa SUR and the 65-kDa mdr1-like protein. Tolbutamide and diazoxide can be envisaged to act by stabilizing or destabilizing the formation of this complex, respectively. In this context it may be relevant that a Cl− conductance that is activated by the sulfonylurea glibenclamide and also by cell swelling has been identified in the plasma membrane of insulin-secreting cells (38). Such swelling-induced currents have been documented in a number of systems (33, 34) and are typically controlled by mdr-like proteins. It is pertinent that the pharmacology of these currents (block by DIDS and tamoxifen) is similar to that we observe for the effects of tolbutamide on exocytosis.
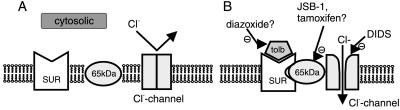
Figure 5.
Model for the stimulatory action of tolbutamide on exocytosis. (A) Under control conditions, the high-affinity SUR and mdr-like protein tend to be unassociated. (B) Tolbutamide (tolb) binds to SUR, facilitating its association with the mdr protein. When the SUR/mdr-complex has formed, it couples to the Cl− channel and increases the open probability. In addition, the participation of nonselective cation-permeable channels is suggested by the failure of tolbutamide to stimulate exocytosis after replacement of K+/Cs+ with NMDG+ (not included in model). Diazoxide acts by reversing the effect of tolbutamide on SUR. JSB1 and tamoxifen prevent the coupling between the SUR/mdr-complex and the Cl− channel and thereby abolish the ability of tolbutamide to increase channel activity. DIDS blocks the Cl− channel by direct interaction with the channel protein.
How do granular ion fluxes stimulate exocytosis in β cells? One possibility is that the 65-kDa mdr1-like protein, perhaps in a complex with SUR (see above), functions as part of the exocytotic machinery. Such an idea would be consistent with the recent demonstration that the Cl− channel CFTR, another member of the ABC class of proteins, to which mdr1 belongs, can functionally interact with the exocytotic proteins syntaxin and munc18 (39). Interaction between the 65-kDa protein and the exocytotic machinery might also explain why the action of tolbutamide on exocytosis is restricted to the Ca2+-dependent pathway of release, whereas GTPγS-induced release was unaffected. Measurements of cell capacitance have revealed that these two pathways of secretion involve both common and distinct processes and that the two pathways diverge at a late stage (18). Biochemical experiments have further indicated that Ca2+-induced secretion involves SNARE-proteins such as syntaxin (40), cellubrevin, VAMP-2 (41), and synaptotagmin (42), whereas GTPγS-evoked secretion is resistant to maneuvers that interfere with the function of these proteins. However, the observation that JSB1 abolishes tolbutamide-stimulated exocytosis without affecting Ca2+-induced secretion argues that this interpretation is too simplistic. The finding that the Cl−-channel blocker DIDS, the KATP-channel activator diazoxide, and removal of cytoplasmic cations all suppress tolbutamide-stimulated exocytosis instead argues that the modulation of exocytosis is secondary to changes of granular ion fluxes. It can be speculated that the 65-kDa granular protein represents a regulatory link between the exocytotic machinery and granular ion channels. The latter possibly include DIDS-sensitive Cl− channels and nonselective cation-conducting channels as suggested by the failure of tolbutamide to be stimulatory after the replacement of K+/Cs+ with NMDG+. We acknowledge that DIDS and tamoxifen are rather unspecific and inhibit KATP channels when applied at the concentrations used in this study. However, if closure of KATP channels were their chief mode of action, then they would be expected to stimulate (i.e., exert a tolbutamide-like effect) rather than inhibit exocytosis. Further support for the involvement of Cl− channels comes from recent immunostaining experiments suggesting the presence of ClC-2 Cl− channels on the secretory granules (S.B., unpublished results). We propose that tolbutamide activates Cl− channels after its association with the SUR/mdr-complex. The influx of Cl− leads to the net uptake of electrolytes and water into the granules. This in turn results in an increased intragranular hydrostatic pressure, which then promotes exocytosis, perhaps by providing the energy required for the fusion of the granular membrane with the plasma membrane (10). We point out that such a model is perfectly consistent with the experimental finding that the action of tolbutamide is exerted at the final stages of exocytosis and that it enhances rather than initiates Ca2+-dependent secretion.
Acknowledgments
Financial support was obtained from the Swedish Medical Research Council (Grants 8647, 12812, and 13147), the Novo Nordisk Research Committee, the Swedish Diabetes Association, the Magnus Bergvalls Stiftelse, the Åke Wibergs Stiftelse, the Deutsche Forschungsgemeinschaft (Grant DFG Th345/6–1), the Juvenile Diabetes Foundation/Wallenberg Foundation Diabetes Research Award, Albert Påhlssons Stiftelse, Kungliga Fysiografiska Sällskapet vid Lunds Universitet, and the Swedish Association for Medical Research.
ABBREVIATIONS
- mdr
multidrug resistance
- [Ca2+]i
cytoplasmic free Ca2+-concentration
- GTPγS
guanosine 5′-O-(3-thiotriphosphate)
- DIDS
4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid
- SUR
sulfonylurea receptor
- NP-EGTA
o-nitrophenyl EGTA
- NMDG+
N-methyl-d-glucamine+
References
- 1.Henquin J C. Diabetologia. 1992;35:907–912. doi: 10.1007/BF00401417. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft S J, Ashcroft F M. Biochim Biophys Acta. 1992;1175:45–59. doi: 10.1016/0167-4889(92)90008-y. [DOI] [PubMed] [Google Scholar]
- 3.Trube G, Rorsman P, Ohno-Shosaku T. Pflügers Arch. 1986;407:493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Bryan L, Nichols C G, Wechsler S W, Clement J P, 4th, Boyd A E, III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson D A. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 5.Ashcroft F M, Rorsman P. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 6.Carpentier J L, Sawano F, Ravazzola M, Malaisse W J. Diabetologia. 1986;29:259–261. doi: 10.1007/BF00454887. [DOI] [PubMed] [Google Scholar]
- 7.Ozanne S E, Guest P C, Hutton J C, Hales C N. Diabetologia. 1995;38:277–282. doi: 10.1007/BF00400631. [DOI] [PubMed] [Google Scholar]
- 8.Thévenod F, Chathadi K V, Jiang B, Hopfer U. J Membr Biol. 1992;129:253–266. doi: 10.1007/BF00232907. [DOI] [PubMed] [Google Scholar]
- 9.Thévenod F, Anderie I, Schulz I. J Biol Chem. 1994;269:24410–24417. [PubMed] [Google Scholar]
- 10.Woodbury D J. Mol Membr Biol. 1995;12:165–171. doi: 10.3109/09687689509027504. [DOI] [PubMed] [Google Scholar]
- 11.Breckenridge L J, Almers W. Proc Natl Acad Sci USA. 1987;84:1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jena B P, Schneider S W, Geibel J P, Webster P, Oberleithner H. Proc Natl Acad Sci USA. 1997;94:13317–13322. doi: 10.1073/pnas.94.24.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliasson L, Renström E, Ämmälä C, Berggren P-O, Bertorello A M, Bokvist K, Chibalin A, Deeney J T, Flatt P R, Gäbel J, et al. Science. 1996;271:813–815. doi: 10.1126/science.271.5250.813. [DOI] [PubMed] [Google Scholar]
- 14.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 15.Rorsman P, Trube G. J Physiol (London) 1986;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ämmälä C, Eliasson L, Bokvist K, Larsson O, Ashcroft F M, Rorsman P. J Physiol (London) 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renström E, Eliasson L, Rorsman P. J Physiol (London) 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proks P, Eliasson L, Ämmälä C, Rorsman P, Ashcroft F M. J Physiol (London) 1996;496:255–264. doi: 10.1113/jphysiol.1996.sp021682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renström E, Eliasson L, Bokvist K, Rorsman P. J Physiol (London) 1996;494:41–52. doi: 10.1113/jphysiol.1996.sp021474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith P A, Duchen M R, Ashcroft F M. Pflügers Arch. 1995;430:808–818. doi: 10.1007/BF00386180. [DOI] [PubMed] [Google Scholar]
- 21.Martell A E, Smith R M. Critical Stability Constants. New York: Plenum; 1974. , Vols. 1 and 2. [Google Scholar]
- 22.Scheper R J, Bulte J W, Brakkee J G, Quak J J, van der Schoot E, Balm A J, Meijer C J, Broxterman H J, Kuiper C M, Lankelma J, et al. Int J Cancer. 1988;42:389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- 23.Bokvist K, Eliasson L, Ämmälä C, Renström E, Rorsman P. EMBO J. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chibalin A V, Katz A I, Berggren P-O, Bertorello A M. Am J Physiol. 1997;273:C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- 25.Georges E, Bradley G, Gariepy J, Ling V. Proc Natl Acad Sci USA. 1990;87:152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheper R J, Bulte J W, Brakkee J G, Quak J J, van der Schoot E, Balm A J, Meijer C J, Broxterman H J, Kuiper C M, Lankelma J, Pinedo H M. Int J Cancer. 1988;15:389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- 27.Schinkel A H, Roelofs E M, Borst P. Cancer Res. 1991;51:2628–2635. [PubMed] [Google Scholar]
- 28.Rao V V, Anthony D C, Piwnica-Worms D. J Histochem Cytochem. 1995;43:1187–1192. doi: 10.1177/43.12.8537634. [DOI] [PubMed] [Google Scholar]
- 29.Kramer W, Oekonomopulos R, Pünter J, Summ H-D. FEBS Lett. 1988;229:355–359. doi: 10.1016/0014-5793(88)81155-4. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Barrado M J, Jonas J C, Gilon P, Henquin J C. Eur J Pharmacol. 1996;298:279–286. doi: 10.1016/0014-2999(95)00806-3. [DOI] [PubMed] [Google Scholar]
- 31.Mariot P, Gilon P, Nenquin M, Henquin J C. Diabetes. 1998;47:365–373. doi: 10.2337/diabetes.47.3.365. [DOI] [PubMed] [Google Scholar]
- 32.DeLisle R C, Hopfer U. Am J Physiol. 1986;250:G489–G496. doi: 10.1152/ajpgi.1986.250.4.G489. [DOI] [PubMed] [Google Scholar]
- 33.Ehring G R, Osipchuk Y V, Cahalan M D. J Gen Physiol. 1994;104:1129–1161. doi: 10.1085/jgp.104.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valverde M A, Bond T D, Hardy S P, Taylor J C, Higgins C F, Altamirano J, Alvarez-Leefmans F J. EMBO J. 1996;15:4460–4468. [PMC free article] [PubMed] [Google Scholar]
- 35.Braun M, Anderie I, Thévenod F. FEBS Lett. 1997;411:255–259. doi: 10.1016/s0014-5793(97)00711-4. [DOI] [PubMed] [Google Scholar]
- 36.Crivellato E, Travan L, Candussio L, Klugman F B, Decorti G. Histochem J. 1997;29:193–198. doi: 10.1023/a:1026445724699. [DOI] [PubMed] [Google Scholar]
- 37.Tian Y A, Johnson G, Ashcroft S J. Diabetes. 1998;47:1722–1726. doi: 10.2337/diabetes.47.11.1722. [DOI] [PubMed] [Google Scholar]
- 38.Kinard T A, Satin L S. Diabetes. 1995;44:1461–1466. doi: 10.2337/diab.44.12.1461. [DOI] [PubMed] [Google Scholar]
- 39.Naren A P, Nelson D J, Xie W, Jovov B, Pevsner J U, Bennett M K, Benos D J, Quick M W, Kirk K L. Nature (London) 1997;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- 40.Martin F, Salinas E, Vazquez J, Soria B, Reig J A. Biochem J. 1996;320:201–205. doi: 10.1042/bj3200201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regazzi R, Wollheim C B, Lang J, Theler J M, Rossetto O, Montecucco C, Sadoul K, Weller U, Palmer M, Thorens B. EMBO J. 1995;15:6951–6959. doi: 10.1002/j.1460-2075.1995.tb07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang J, Fukuda M, Zhang H, Mikoshiba K, Wollheim C B. EMBO J. 1997;16:5837–5846. doi: 10.1093/emboj/16.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]