Abstract
One hundred and fifty years after the discovery of Neanderthals, it is held that this morphologically and genetically distinct human species does not differ from modern Homo sapiens in its craniofacial ontogenetic trajectory after the early post-natal period. This is striking given the evident morphological differences between these species, since it implies that all of the major differences are established by the early post-natal period and carried into adulthood through identical trajectories, despite the extent to which mechanical and spatial factors are thought to influence craniofacial ontogeny. Here, we present statistical and morphological analyses demonstrating that the spatio-temporal processes responsible for craniofacial ontogenetic transformations differ. The findings emphasize that pre-natal as well as post-natal ontogeny are both important in establishing the cranial morphological differences between adult Neanderthals and modern humans.
Keywords: human evolution, post-natal growth and development, geometric morphometrics, Procrustes form space
1. Introduction
How differences in ontogeny generate adult variation has been addressed in many different vertebrate groups, including primates (Falsetti & Cole 1992; Gomez 1992; Shea 1992), domestic dogs (Canis familiaris; Wayne 1986), rodents (Voss & Marcus 1992; Cardini & O'Higgins 2005), birds (Bjorklund 1996), reptiles (Monteiro & Soares 1997; O'Keefe et al. 1999) and fishes (Strauss & Fuiman 1985; Klingenberg & Ekau 1996; Zelditch et al. 2000). Klingenberg (1998) notes that one of the most striking generalities to emerge from these studies is that early development is more flexible than later (post-hatching/post-natal) growth. Further, during post-natal ontogeny, ‘changes in directions of growth trajectories tend to be fairly subtle, compared with ontogenetic scaling and lateral transposition’. He concludes that ‘there is more evolutionary flexibility for changes in early ontogeny (leading to lateral transposition) or for extension and truncation of conserved trajectories than for alterations of the relative growth rates of individual traits that are necessary to change the directions of trajectories’.
Certainly, this holds for several extant primates (reviewed in Cobb & O'Higgins 2004), but while comparing Neanderthals and modern humans, there is debate as to whether or not they show any post-natal divergence of craniofacial ontogenetic trajectories (Ponce de León & Zollikofer 2001; Ackerman & Krovitz 2002; Krovitz 2003). Thus, it is striking that 150 years after the discovery of the first Neanderthal, there is still no clear answer to the basic question of whether or not the post-natal changes in craniofacial form of Neanderthals parallel those of modern humans. In essence, the question is whether the differences between Neanderthals and our own species arise pre-natally and are simply carried into adulthood through parallel ontogenetic trajectories in size and shape (Ponce de León & Zollikofer 2001; Ackerman & Krovitz 2002) or further significant differences in form arise post-natally (Krovitz 2003).
From the broader perspective of evolution, natural selection has generated ontogenies that produce phenotypes adapted to their environmental conditions. Thus, the extent to which two related species share post-natal ontogenetic trajectories (i.e. grow, develop and scale similarly), at least in part, reflects the evolutionary mechanisms by which diversification occurs. Additionally, post-natal craniofacial ontogeny in being modulated by interaction with the external environment can be informative with respect to the immediate (e.g. biomechanical–functional; Rubin & Lanyon 1987) environment of the developing organism. Classically (Gould 1977; Alberch et al. 1979; Shea 1983; Rosas 1997; O'Higgins & Jones 1998; Zelditch et al. 2001, 2003; Strand-Viðarsdóttir et al. 2002; Bastir & Rosas 2004; Cobb & O'Higgins 2004; Mitteroecker et al. 2005), students of post-natal ontogeny seek explanations for adult form in both the extent to which form is determined at birth and the extent to which subsequent ontogeny generates new or additional aspects.
Neanderthals are very large-brained with large and projecting (prognathic) faces: their mandibles lack a chin; the mental foramen is located below the first molar; and there is often a space behind the third molar (retromolar space, commonly considered a Neanderthal autapomorphy; but see Franciscus & Trinkaus 1995; Trinkaus 2003; Rosas & Bastir 2004; Rosas et al. in press). In contrast, while modern humans are large-brained (although on average a little lesser than Neanderthals), they have small, less projecting (orthognathic) faces and their more gracile mandibles show a projecting chin, a human autapomorphy (Schwartz & Tattersall 2000; Lieberman et al. 2002; Rosas & Bastir 2004). While these differences are accepted as part of a species-specific suite of characters in adults, it is much less clear how they are formed during ontogeny.
Recent research on craniofacial ontogeny has led Ponce de León & Zollikofer (2001) to suggest that these basic differences and more subtle differences in overall proportions are already established in early post-natal ontogeny and are probably mainly due to pre-natal ontogenetic differences. Their geometric morphometric analysis was interpreted as showing that post-natal ontogenetic shape changes in Neanderthals did not differ from those of modern humans. Thus, parallel ontogenetic trajectories of shape transformation simply transform the post-natal cranium of both species in the same way. However, in their study no attempt was made to statistically falsify the hypothesis of parallel ontogenies. Ponce de León & Zollikofer (2001) also suggested that ontogenetic allometry, i.e. the post-natal covariation of shape with size, was identical in modern humans and Neanderthals.
Using Euclidean distance matrix analysis (Lele & Richtsmeier 1991; Richtsmeier 2002), Krovitz (2003) also noted the early presence of species-specific differences between Neanderthals and modern humans. Additionally, she found morphological differences that became further accentuated during post-natal ontogeny, implying divergent post-natal trajectories.
Thus, our current understanding of how the adult differences between Neanderthals and modern humans arise is unsatisfactory; do both human species grow similarly or differently? Is post-natal ontogeny an important contributor to differences in adult morphology? The current study addresses these questions using a substantial dataset of modern humans and a fair proportion (30, of which 10 are subadult) of all known Neanderthal mandibles.
From a consideration of what is known about the regulation of facial ontogeny, there is good reason to expect divergence of trajectories between these species. First, facial growth terminates rather late in ontogeny (Buschang et al. 1983; Bastir et al. 2006b). Therefore, it is unlikely that the retromolar space is present at early stages, as suggested by Ponce de León & Zollikofer (2001). Second, preliminary palaeohistological analysis has suggested different growth field distributions in mandibles of Homo heidelbergensis and modern humans (Martínez-Maza & Rosas 2002; Rosas et al. in press; Rosas & Martínez-Maza in press). These Middle Pleistocene hominids are ancestors to Neanderthals and differ considerably from modern humans (Rosas & Bastir 2004; Rosas et al. 2006). Since different growth field distributions imply different growth trajectories (O'Higgins & Jones 1998; O'Higgins et al. 2001), there is evidence of differences from modern humans in these putative Neanderthal ancestors. If Neanderthals shared their bone remodelling pattern (and thus ontogenetic trajectories) with modern humans, then this would require convergence. The unlikely alternative is that histological analysis of bone surface topography (Bromage 1989; O'Higgins & Jones 1998; Martínez-Maza & Rosas 2002; Rosas et al. in press; Rosas & Martínez-Maza in revision) is not informative with regard to the generation of adult form. Third, since many components of the facial skeleton scale with body size (Pirinen et al. 1994; Bastir 2004) and Neanderthals have different body proportions to those of modern humans (Ruff et al. 1997; Carretero et al. 2004), we might expect divergent facial ontogenetic trajectories. This is because it has been shown that the formation of the retromolar space depends strongly on allometric factors (Franciscus & Trinkaus 1995; Rosas & Bastir 2004). Finally, differences in basicranial architecture between Neanderthals and modern humans (Lieberman 1998; Spoor et al. 1999; McBratney & Lieberman 2003; Bastir et al. 2006a,b) probably have some knock-on effects on facial ontogeny, since it has been suggested that the anterior parts of basicranium and, particularly, the lateral parts form a template upon which the face grows (Enlow & Azuma 1975; Enlow 1990; Bastir & Rosas 2005; Bastir et al. 2006a; Bastir & Rosas 2006).
Thus, in the present study, we aim to compare the post-natal ontogenetic changes in the facial skeleton of Neanderthals and modern humans. An earlier study (Bastir et al. 2006b) has confirmed the hypothesis (Enlow 1990) that mandibular growth terminates late in facial ontogeny. Consequently, it is influenced by and reflects the spatial and mechanical consequences of the development of earlier maturing structures. Mandibular ontogeny can therefore be expected to be a sensitive indicator of facial ontogeny as a whole. The null hypothesis under test in this paper is that mandibular ontogeny in size and shape is identical between modern humans and Neanderthals.
2. Material and methods
Thirty three-dimensional landmarks (table 1) were digitized on original fossils or casts, where originals were not available (table 2) with a MicroScribe 3DX digitizer. The comparative sample of modern humans (N=141) consisted of two European samples from the nineteenth and twentieth centuries (Spitalfields, UK; Coimbra, Portugal; Rosas & Bastir 2002; Bastir & Rosas 2004). Further details of the samples, data acquisition and missing data treatment have been described elsewhere (Rosas & Bastir 2002; Bastir & Rosas 2004). Measurement error was evaluated by repeated measurements of the same specimens. The average deviation of the mean coordinate value was very small (0.47% of mean coordinate value; Rosas & Bastir 2002; Bastir & Rosas 2004). Where mandibles are damaged and landmarks are missing, these were estimated by mirroring the opposite side if present or by replacement with age- and species-specific mean data. To minimize the number of estimated landmarks, we avoided fossils with excessive damage, especially where this is bilateral.
Table 1.
Three-dimensional mandibular landmarks (l, left; r, right; Rosas & Bastir 2004).
| count | landmark |
|---|---|
| 1 | r. canine |
| 2 | r. mental foramen |
| 3 | r. inferior basal border |
| 4 | r. pre-angular notch |
| 5 | r. gonion |
| 6 | r. ramus flexure |
| 7 | r. condylion |
| 8 | r. mandibular notch |
| 9 | r. coronoid process |
| 10 | r. anterior ramus |
| 11 | r. posterior alveolar process limit |
| 12 | r. mandibular foramen |
| 13 | l. canine |
| 14 | l. mental foramen |
| 15 | l. inferior basal border |
| 16 | l. pre-angular notch |
| 17 | l. gonion |
| 18 | l. ramus flexure |
| 19 | l. condylion |
| 20 | l. mandibular notch |
| 21 | l. coronoid process |
| 22 | l. anterior ramus |
| 23 | l. posterior alveolar process limit |
| 24 | l. mandibular foramen |
| 25 | infradentale |
| 26 | B-point |
| 27 | menton |
| 28 | gnathion |
| 29 | foramen genioglossum |
| 30 | internal infradentale |
Table 2.
Hominid fossils, sex, ages and conservation status.
| fossil | sex | age (years or dental) | conservation | literature | |
|---|---|---|---|---|---|
| 1 | Amud 7 | unknown | 10 months | left hemimandible, right corpus behind m1+ramus missing | Schwartz & Tattersal (2002) |
| 2 | Archi | unknown | 2–3 years | complete corpus | Schwartz & Tattersal (2002) |
| 3 | Gibraltar 2 | unknown | 5 years | complete | Schwartz & Tattersal (2002) |
| 4 | Peche de l'Aze | unknown | 4 years | right hemimandible+symphysis | Schwartz & Tattersal (2002) |
| 5 | TeshikTash | male | M1 | Complete | Schwartz & Tattersal (2002) |
| 6 | Krapina C | unknown | M2 | right hemimandible+symphysis (condyle missing) | Radovcic et al. (1988) |
| 7 | Hortus4 | unknown | M2 | left hemimandible+right corpus, upper and posterior ramus missing | Schwartz & Tattersal (2002) |
| 8 | EhringsdorfG | unknown | M2 | left hemimandible+symphysis | Wolpoff (1999) |
| 9 | ZaskalnayaVI-72 | unknown | M2 | complete | personal observation |
| 10 | Le Fate II | unknown | M2 | Left hemimandible | Giacobini et al. (1984) |
| 13 | Krapina J | male | adult | complete mandible | Wolpoff (1999) |
| 14 | Vindija 206 | unknown | adult | right corpus, symphysis and anterior part of ramus | Wolpoff (1999) |
| 15 | Vindija 207 | unknown | adult | right ramus and adjacent part of corpus | Wolpoff (1999) |
| 16 | Vindija 265 | unknown | adult | left hemimandible | Wolpoff (1999) |
| 17 | Spy 1 | male | adult | nearly complete except both posterior rami missing | Schwartz & Tattersal (2002) |
| 18 | La Chapelle | male | adult | complete, no coronoids, no alveolar process | Schwartz & Tattersal (2002) |
| 19 | Aubesier 11 | unknown | adult | right hemimandible, no alveolar process, no coronoid | Lebel et al. (2001) |
| 20 | La Ferassie1 | male | adult | Complete | Schwartz & Tattersal (2002) |
| 21 | St Cesaire | male | adult | right ramus (coronoid missing), corpus, symphysis | Wolpoff (1999) |
| 22 | La Quina 9 | male | adult | both hemimandibles (ext. symphysis missing) | Wolpoff (1999) |
| 23 | Regourdou 1 | unknown | adult | complete, except left ramus (right gonial region missing) | Schwartz & Tattersal (2002) |
| 24 | Amud 1 | male | adult | complete, but asymmetric, left hemimandible better preserved | Schwartz & Tattersal (2002) |
| 25 | Tabun C1 | female | adult | complete, but distorted, right hemimandible undistorted | Schwartz & Tattersal (2002) |
| 26 | Kebara | male | adult | nearly complete except upper ramus region | Schwartz & Tattersal (2002) |
| 27 | Bañolas | female | adult | complete mandible | Lalueza et al. (1993) |
| 28 | Zafarraya | unknown | adult | complete mandible | Schwartz & Tattersal (2002) |
| 29 | Monte Circeo 2 | unknown | adult | corpora, right anterior ramus | Schwartz & Tattersal (2002) |
| 30 | Sidron 1 | male | adult | almost complete | Rosas & Aguirre (1999) |
| 32 | Sidron 2 | male | adult | left hemimandible | Rosas & Aguirre (1999) |
| 33 | Sidron 3 | female | adult | left hemimandible | Rosas et al. (2006) |
We used generalized Procrustes analysis (Rohlf & Slice 1990; Bookstein 1991) and principal components analysis (PCA; O'Higgins & Jones 1998; O'Higgins 2000) to obtain data on shape (Procrustes shape coordinates) and size (centroid size) that were subsequently analysed by various statistical methods.
First, a PCA in shape space was carried out of mandibular data from both groups combined. The scatters of the ontogenetic series on combinations of PCs and plots of PCs versus centroid size were examined for evidence of divergence of trajectories and linearity as a prerequisite to linear modelling and comparison of these trajectories. Second, a PCA in Procrustes form space (size–shape space) of the data from both groups combined was carried out and plots were examined in the same way. This latter method uses Procrustes registration, but reintroduces ln-CS to the data matrix (which now comprises shape coordinates and ln-CS) before principal components analysis is carried out (Mitteroecker et al. 2004). Ln-CS usually has the largest variance of any column of this matrix, and the first principal component of the size–shape (=form) distribution is often closely aligned with size. In this circumstance, this method is well suited to compare the ontogenetic scaling trajectories (Mitteroecker et al. 2004, 2005). These analyses were supplemented by multivariate regression of shape on centroid size within each group.
These analyses led to the finding that in each group, the first PC in shape space almost exclusively represents mandibular shape ontogeny. Subsequently, the angle between this PC from PCA of shape coordinates in each species was computed and its significance was assessed using a permutation test, in which species membership was randomly permuted and the angle recalculated. One thousand permutations were carried out and the estimated angle between the species was compared with the distribution of permuted angles to assess its significance. For this test, we retained the full, but unequally sized, samples of modern humans and Neanderthals, because reducing the modern human sample size to match that of Neanderthals would simply reduce the power of the test and equality of sample sizes is not a requirement of the test.
For comparing multivariate ontogenetic shape scaling (allometry), MANCOVA was employed, regressing shape (PC scores) on centroid size. The significance of the interaction between centroid size and species gives an indication of the likelihood that scaling differences exist between the species (Rosas & Bastir 2002).
3. Results
The principal components analyses indicate that the ontogenetic trajectories of each species are approximately linear in both Procrustes shape and form spaces. After common generalized Procrustes analysis and separate PCA of each species, the angle between the first PCs of each species is estimated to be 44° (computed from the dot product of the eigenvectors). A permutation test with 1000 runs indicates that this angle is significant with a p-value of less than 0.01. Thus, there is strong evidence of divergent ontogenetic shape changes between Neanderthals and modern humans.
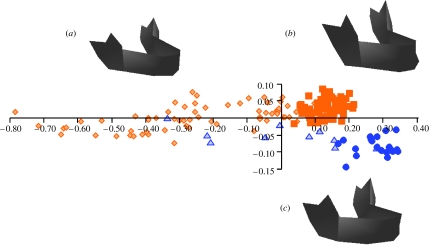
Subsequent analyses focus on ontogenetic scaling (allometry). The first comprises a PCA in Procrustes form space of the two species combined, from which PC1 explains 89% of the variance in size and shape; PC2, 3.2%. These are plotted in figure 1. PC1 represents ‘common’ aspects of ontogenetic shape change in both species with smaller, younger individuals towards its negative pole. For the same ontogenetic stage (assessed by dental development), there is a marked tendency for Neanderthals to have more positive scores on PC1. This means that, in general, juvenile Neanderthals express the aspects of form variation represented by PC1 that are typical of later stages in modern humans; Neanderthals being somewhat ‘overgrown’ relative to modern humans (see below).
Figure 1.
Ontogenetic form trajectories along PC1 and PC2 for modern humans (orange; subadults, light diamonds; adults, dark squares) and Neanderthals (blue; subadults, light triangles; adults, dark circles). The scatterplot shows divergent post-natal ontogenetic shape trajectories. The rendered surface models show the associated morphological changes from (a) juveniles at scores on PC1: -0.35 and PC2: 0 to (b) adult modern humans (scores on PC1: 0.15 and PC2: 0.025) and (c) Neanderthals (scores on PC1: 0.3 and PC2: −0.1). Note the strong vertical expansion, the relatively narrower and higher rami and chin formation in adult humans. These shape changes contrast with Neanderthals, who show much lower and broader rami, and very strong forward growth of the corpus and even more so at the dental arcade. These ontogenetic changes produce both the retromolar space and a sloping chinless symphysis in adults.
PC2 (figure 1a–c) represents some of the differences that develop between Neanderthals and modern humans, such that smaller, younger Neanderthals (central, −0.3, scores on PC1) overlay the human sample on PC2 while adults (rightmost scores on PC1) are widely separated. Thus, the trajectories diverge on the plot of PC1 versus PC2 (figure 1). A slight divergence is also present on PC3 and no further divergence was evident on inspection of other PCs except PC8, on which the juvenile Neanderthals are distinguished from the juvenile humans. On PC8, the scores for Neanderthals correlate r=0.6 and p=0.001 with centroid size, but those for humans show no significant correlation (r=−0.14 and p=0.1). Anatomically, PC8 represents mediolateral broadening of the precanine dental arcade and corpus and variation in the relative height of the coronoid. Note, however, that while this PC represents 0.3% of total size and shape variance, it accounts for approximately 1.5% of total shape variance. This is further evidence of the differences in post-natal ontogenetic scaling.
Multivariate analyses of covariance of all PC scores show a highly significant effect of both principal factors (species: Wilks' λ=0.18, F=4.74, p<0.0001 and centroid size: Wilks' λ=0.06, F=15.76, p<0.0001) as well as of their interaction (Wilks' λ=0.15, F=5.64, p<0.0001). These results indicate highly significantly different allometric scaling patterns in Neanderthals and modern humans.
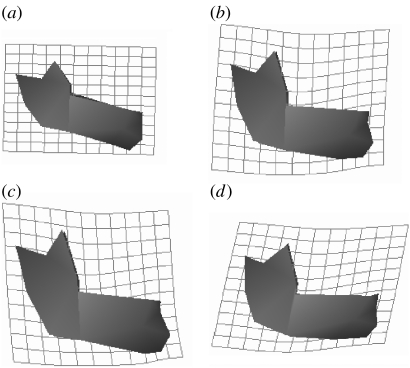
The inset images of figure 1a–c indicate the ontogenetic shape changes in both species represented by the first two PCs. The common aspects of facial ontogeny represented by PC1 and divergent aspects represented by PC2 are shown with overlain transformation grids in the mid-sagittal plane computed using a triplet of thin plate splines in figure 2. These grids therefore accurately represent ontogenetic deformations of the symphyseal region, through which they pass and interpolated deformations between the two hemimandibles elsewhere. The deformation consists of vertical increase in posterior facial height, leading to an increasingly rectangular spatial relationship between the corpus and the ramus. The surface rendered models in figure 1 and the TPS grids in figure 2c,d (humans, Neanderthals, respectively) further underline the post-natal ontogenetic differences between the species.
Figure 2.
The deformation grids in (a) and (b) show the aspects of post-natal growth and development in humans and Neanderthals as represented by PC1 alone. Both species increase their relative vertical facial height and show an increased angulation between the ramus and the corpus. In (c), the final stage of human ontogeny is shown, which is characterized by a strong supero–inferior expansion of the mandible and a retraction of the dental arcade with respect to the lower part of the symphysis (forming the human chin). In (d), shown are the increasingly forward growth in Neanderthal facial ontogeny, produced by a forward shift of the alveolar process, which is responsible for the typical Neanderthal retromolar space and the sloping symphysis.
In Neanderthals, mandibular ontogeny manifests relative forward and downward growth displacement of the molar region of the alveolar process producing the retromolar space and elevation of the anterior dental arcade, together with the anterior part of the mandibular corpus. Ontogenetic shape changes are much less pronounced in the posterior ramus and the inferior border of the corpus than in the anterior ramus and alveolar process. In contrast, modern humans are characterized by a strong vertical component of facial ontogenetic shape change considerably increasing the relative height of the ramus and by projection of the chin. The strong vertical component also produces a relatively narrow posterior mandible when compared with Neanderthals (not shown but evident in frontal perspective).
These different aspects of post-natal growth and development produce the typically enlarged, prognathic Neanderthal faces and the reduced, orthognathic faces in modern humans. Thus, morphological differences between the species are accentuated in post-natal ontogeny. The null hypothesis of identical ontogenetic shape change is rejected.
4. Discussion
A key question in evolutionary biology addressing morphological variation is how ontogenetic processes contribute to the generation of adult form. Until recently, a common view has been that the morphological diversification is principally grounded in pre-natal ontogenetic modification, whereas post-natal ontogenies are rather similar among mammals except for heterochronic modifications (see Klingenberg 1998 for a recent review). With the rise in geometric morphometrics (Bookstein 1991; Rohlf & Marcus 1993), providing more sensitive tools for detailed shape analyses, this picture has changed (O'Higgins & Jones 1998; O'Higgins et al. 2001).
The aim of this paper was to use three-dimensional geometric morphometric methods to compare the post-natal facial ontogeny by examining mandibles from Neanderthals and modern humans. Recent studies addressing this issue have produced ambiguous results (Ponce de León & Zollikofer 2001; Krovitz 2003).
Our study suggests that there exist similarities between these species in the ways their mandibles change shape and scale during post-natal ontogeny. These principally relate to forward and downward expansion of the face (Baume et al. 1983; Buschang et al. 1983; Bastir et al. 2006a,b). In both species, post-natal ontogeny produces drastic changes in the spatial relation between the corpus and the ramus owing to the vertical growth occurring at the anterior and posterior face (Bastir & Rosas 2004, 2005), and this fits with the results of Ponce de León & Zollikofer (2001).
The clear differences in the mandibular ontogeny of Neanderthals and humans probably relate to differences in the anterior face; that is, in the ethmomaxillary complex, which is tightly integrated with the mandible (Bastir et al. 2005). The Neanderthals show strong forward facial expansion, while in modern humans, expansion is more in the inferior direction (figure 2c,d). Such localized differences are compatible with the interpretations of Krovitz (2003). With respect to the alveolar region, it is possible that ontogenetic differences in the maturation of the teeth may also be involved in the observed divergence of shape trajectories (Boughner & Dean 2004; Macchiarelli et al. 2006).
Pertinent results were reported earlier in a study comparing the adult scaling relationships of different mandibular modules (Rosas & Bastir 2004). Neanderthals showed stronger antero–posterior variation in the ‘supra-nerve unit’ than did modern humans, indicating that variation among adults reflects the facial projection of this species (Bastir et al. 2005), while in modern humans, adult variation is related to facial height (Bastir & Rosas 2004). The results of the present study suggest that these earlier findings probably have a basis in contrasting patterns of facial ontogeny.
The differences between the adults of the human species in this study arise in large part through differences in pre-natal development and clearly in smaller part through differences in post-natal ontogeny. In addition to the shape and scaling trajectory differences identified in this paper, the post-natal facial ontogeny of Neanderthals is also probably characterized by higher rates of growth and shape transformation at very early developmental stages (Ponce de León & Zollikofer 2001; Ramirez Rozzi & Bermudez de Castro 2004). Plots of dental ages (not presented) show that Amud 7 is at the large extreme of the distribution of human material with comparable dental development. Older juvenile Neanderthals (Gibraltar, Teshik Tash, but particularly those approximating adolescence, such as Krapina C, Zaskalnaya, Ehringsdorf G) possess progressively larger sizes for their dental ages than their modern human dental counterparts. This is compatible with relatively increased developmental rates and possibly even implies the presence of an adolescent growth spurt (Bogin 1999) in Neanderthals.
Therefore, our study indicates that the facial ontogeny of both species is consistently different at all stages and that differences in spatio–temporal aspects of post-natal ontogeny contribute to the establishment of differences in adult form. Future studies should address the proximate causes for these differences. For example, palaeo-histological evidence for differences in growth field topography (Martínez-Maza & Rosas 2002; Rosas et al. 2006; Rosas & Martínez-Maza in press) could clarify the contribution of different growth patterns and rates to adult morphological differences. Future studies in Neanderthals should particularly investigate the junction of the corpus and the ramus (area of retromolar space) and the anterior corpus region, which is where most of the ontogenetic differences seem to occur.
Acknowledgments
We are grateful to Chris Stringer and Robert Kruszynski (NHM, London), and Vadim Stepanchuk (University of Kiew) for permission to study fossil material in their curation. We thank Javier Fortea, Marco de la Rasilla and the El Sidrón excavation team from the University of Oviedo, and Cayetana Martínez-Maza and Antonio García Tabernero (MNCN, Madrid), and Sam Cobb, Andrea Cardini and Kornelius Kupczik (HYMS, University of York) for their discussions. We are also particularly grateful for the constructive comments of two anonymous reviewers. M.B. was funded by a postdoctoral fellowship of the MEC (Spanish Ministry of Education and Science) at HYMS. Comparative data has been collected by a SYNTHESYS grant to M.B. at the NHM, London. This research was also undertaken with the support of research projects CGL-2006-02131 (MEC) and MRTN-CT-2005-019564 (EVAN).
References
- Ackermann R.R, Krovitz G.E. Common patterns of facial ontogeny in the hominid lineage. Anat. Rec. 2002;269:142–147. doi: 10.1002/ar.10119. doi:10.1002/ar.10119 [DOI] [PubMed] [Google Scholar]
- Alberch P, Gould S.J, Oster G.F, Wake D. Size and shape in ontogeny and phylogeny. Paleobiology. 1979;5:296–317. [Google Scholar]
- Bastir M. 2004 A geometric morphometric analysis of integrative morphology and variation in human skulls with implications for the Atapuerca-SH hominids and the evolution of Neandertals. Structural and systemic factors of morphology in the hominid craniofacial system. Department of Anthropology. Doctoral dissertation, Autonoma University of Madrid, Madrid.
- Bastir M, Rosas A. Facial heights: evolutionary relevance of postnatal ontogeny for facial orientation and skull morphology in humans and chimpanzees. J. Hum. Evol. 2004;47:359–381. doi: 10.1016/j.jhevol.2004.08.009. doi:10.1016/j.jhevol.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. The hierarchical nature of morphological integration and modularity in the human posterior face. Am. J. Phys. Anthropol. 2005;128:26–34. doi: 10.1002/ajpa.20191. doi:10.1002/ajpa.20191 [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Correlated variation between the lateral basicranium and the face: a geometric morphometric study in different human groups. Arch. Oral Biol. 2006;51:814–824. doi: 10.1016/j.archoralbio.2006.03.009. doi:10.1016/j.archoralbio.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Sheets D.H. The morphological integration of the hominoid skull: a partial least squares and PC analysis with morphogenetic implications for European Mid-Pleistocene mandibles. In: Slice D, editor. Modern morphometrics in physical anthropology. Kluwer Academic/Plenum Publishers; New York, NY: 2005. pp. 265–284. [Google Scholar]
- Bastir M, O'Higgins P, Rosas A. Human evolution: relationships between the basicranium and the face. Ann. Hum. Biol. 2006a;32:790. [Google Scholar]
- Bastir M, Rosas A, O'Higgins P. Craniofacial levels and the morphological maturation of the human skull. J. Anat. 2006b;209:637–654. doi: 10.1111/j.1469-7580.2006.00644.x. doi:10.1111/j.1469-7580.2006.00644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baume R, Buschang P, Weinstein S. Stature, head height, and growth of the vertical face. Am. J. Othod. 1983;83:477–484. [PubMed] [Google Scholar]
- Bjorklund M. Similarity of growth among great tits (Parus major) and blue tits (P. caeruleus) Biol. J. Linn. Soc. 1996;58:343–355. doi:10.1006/bijl.1996.0040 [Google Scholar]
- Bogin B. Evolutionary perspective on human growth. Annu. Rev. Anthropol. 1999;28:109–153. doi: 10.1146/annurev.anthro.28.1.109. doi:10.1146/annurev.anthro.28.1.109 [DOI] [PubMed] [Google Scholar]
- Bookstein F.L. Cambridge University Press; Cambridge, UK: 1991. Morphometric tools for landmark data. [Google Scholar]
- Boughner J.C, Dean M.C. Does space in the jaw influence the timing of molar crown initiation? A model using baboons (Papio anubis) and great apes (Pan troglodytes, Pan paniscus) J. Hum. Evol. 2004;46:253–275. doi: 10.1016/j.jhevol.2003.11.007. doi:10.1016/j.jhevol.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Bromage T.G. Ontogeny of the early hominid face. J. Hum. Evol. 1989;18:751–773. doi:10.1016/0047-2484(89)90088-2 [Google Scholar]
- Buschang P, Baume R, Nass G. A craniofacial growth maturity gradient for males and females between 4 and 16 years of age. Am. J. Phys. Anthropol. 1983;61:373–381. doi: 10.1002/ajpa.1330610312. doi:10.1002/ajpa.1330610312 [DOI] [PubMed] [Google Scholar]
- Cardini A, O'Higgins P. Post-natal ontogeny of the mandible and ventral cranium in Marmota species (Rodentia, Sciuridae): allometry and phylogeny. Zoomorphology. 2005;124:189–203. doi:10.1007/s00435-005-0008-3 [Google Scholar]
- Carretero J.M, Arsuaga J.-L, Martínez I, Quam R.M, Lorenzo C, Gracia A, Ortega A.I. Los humanos de la Sima de los Huesos (Sierra de Atapuerca) y la evolucion del cuerp en el genero Homo. In: Baquedano E, editor. Homenaje a Emiliano Aguirre. Museo Arqueologico Regional; Alcala de Henares, Spain: 2004. pp. 120–136. [Google Scholar]
- Cobb S, O'Higgins P. Hominins do not share a common postnatal facial ontogenetic shape trajectory. J. Exp. Zool. B: Mol. Dev. Evol. 2004;302B:302–321. doi: 10.1002/jez.b.21005. doi:10.1002/jez.b.21005 [DOI] [PubMed] [Google Scholar]
- Enlow D.H. 3rd edn. Saunders Company; Philadelphia, PA: 1990. Facial growth. [Google Scholar]
- Enlow D.H, Azuma M. Functional growth boundaries in the human and mammalian face. In: Bergsma D, Langman J, Paul N.W, editors. Morphogenesis and malformation of the face and the brain. Alan R. Riss; New York, NY: 1975. pp. 217–230. [PubMed] [Google Scholar]
- Falsetti A.B, Cole T.M. Relative growth of the postcranial skeleton in callitrichines. J. Hum. Evol. 1992;23:79–92. doi:10.1016/0047-2484(92)90044-A [Google Scholar]
- Franciscus R.G, Trinkaus E. Determinants of retromolar space presence in Pleistocene Homo mandibles. J. Hum. Evol. 1995;28:577–595. doi:10.1006/jhev.1995.1043 [Google Scholar]
- Giacobini G, Lumley M.-A.d, Yokoyama Y, Nguyen H.-V. Neanderthal child and adult remains from a Mousterian deposit in Northern Italy (Caverna delle fate, finale ligure) J. Hum. Evol. 1984;13:687–707. doi:10.1016/S0047-2484(84)80020-2 [Google Scholar]
- Gomez A.M. Primitive and derived patterns of relative growth among species of Lorisidae. J. Hum. Evol. 1992;23:219–233. doi:10.1016/S0047-2484(05)80001-6 [Google Scholar]
- Gould S.J. Harvard University Press; Cambridge, MA; London, UK: 1977. Ontogeny and phylogeny. [Google Scholar]
- Klingenberg C.P. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biol. Rev. 1998;73:79–123. doi: 10.1017/s000632319800512x. doi:10.1017/S000632319800512X [DOI] [PubMed] [Google Scholar]
- Klingenberg C.P, Ekau W. A combined morphometric and phylogenetic analysis of an ecomorphological trend: pelagization in Antarctic fishes (Perciformes: Nototheniidae) Biol. J. Linn. Soc. 1996;59:143–177. doi:10.1006/bijl.1996.0059 [Google Scholar]
- Krovitz G. Shape and growth differences between Neandertals and modern humans: grounds for a species level distinction? In: Thompson J.L, Krovitz G.E, Nelson A.J, editors. Patterns of growth and development in the genus Homo. Cambridge studies in biological and evolutionary anthropology. vol. 37. Cambridge University Press; Cambridge, UK; New York, NY: 2003. pp. 320–342. [Google Scholar]
- Lalueza C, Perez-Perez A, Turbon D. Microscopic study of the Banyoles mandible (Girona, Spain): diet, cultural activity and toothpick use. J. Hum. Evol. 1993;24:281–300. doi:10.1006/jhev.1993.1022 [Google Scholar]
- Lebel S, Trinkaus E, Faure M, Fernandez P, Guerin C, Richter D, Mercier N, Valladas H, Wagner G.A. Comparative morphology and paleobiology of Middle Pleistocene human remains from the Bau de l'Aubesier, Vaucluse, France. Proc. Natl Acad. Sci. USA. 2001;98:11 097–11 102. doi: 10.1073/pnas.181353998. doi:10.1073/pnas.181353998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele S, Richtsmeier J.T. Euclidean distance matrix analysis: a coordinate free approach for comparing biological shapes using landmark data. Am. J. Phys. Anthropol. 1991;86:415–427. doi: 10.1002/ajpa.1330860307. doi:10.1002/ajpa.1330860307 [DOI] [PubMed] [Google Scholar]
- Lieberman D.E. Sphenoid shortening and the evolution of modern human cranial shape. Nature. 1998;393:158–162. doi: 10.1038/30227. doi:10.1038/30227 [DOI] [PubMed] [Google Scholar]
- Lieberman D.E, McBratney B.M, Krovitz G. The evolution and development of cranial form in Homo sapiens. Proc. Natl Acad. Sci. USA. 2002;99:1134–1139. doi: 10.1073/pnas.022440799. doi:10.1073/pnas.022440799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchiarelli R, Bondioli L, Debenath A, Mazurier A, Tournepiche J, Birch W, Dean C. How Neanderthal molar teeth grew. Nature. 2006;444:748–775. doi: 10.1038/nature05314. doi:10.1038/nature05314 [DOI] [PubMed] [Google Scholar]
- Martínez-Maza C, Rosas A. Bone remodeling in the Atapuerca-SH mandibles. Implications for growth patterns in middle pleistocene hominids. Am. J. Phys. Anthropol. 2002;115:107–108. [Google Scholar]
- McBratney B, Lieberman D. Postnatal ontogeny of facial position in Homo sapiens and Pan troglodytes. In: Thomson J.L, Krovitz G, Nelson A, editors. Patterns of growth and development in the genus Homo. Cambridge University Press; Cambridge, UK: 2003. pp. 45–72. [Google Scholar]
- Mitteroecker P, Gunz P, Bernhard M, Schaefer K, Bookstein F.L. Comparison of cranial ontogenetic trajectories among great apes and humans. J. Hum. Evol. 2004;46:679–698. doi: 10.1016/j.jhevol.2004.03.006. doi:10.1016/j.jhevol.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P, Bookstein F.L. Heterochrony and geometric morphometrics: a comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evol. Dev. 2005;7:244–258. doi: 10.1111/j.1525-142X.2005.05027.x. doi:10.1111/j.1525-142X.2005.05027.x [DOI] [PubMed] [Google Scholar]
- Monteiro L.R, Soares M. Allometric analysis of the ontogenetic variation and evolution of the skull in Caiman spix, 1825 (Crocodylia: Alligatoridae) Herpetologica. 1997;53:62–69. [Google Scholar]
- O'Higgins P. The study of morphological variation in the hominid fossil record: biology, landmarks and geometry. J. Anat. 2000;197:103–120. doi: 10.1046/j.1469-7580.2000.19710103.x. doi:10.1046/j.1469-7580.2000.19710103.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Higgins P, Jones N. Facial growth in Cercocebus torquatus: an application of three-dimensional geometric morphometric techniques to the study of morphological variation. J. Anat. 1998;193:251–272. doi: 10.1046/j.1469-7580.1998.19320251.x. doi:10.1046/j.1469-7580.1998.19320251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Higgins P, Chadfield P, Jones N. Facial growth and the ontogeny of morphological variation within and between the primates Cebus apella and Cercocebus torquatus. J. Zool. 2001;254:337–357. doi:10.1017/S095283690100084X [Google Scholar]
- O'Keefe F.R, Rieppel O, Sander P.M. Shape disassociation and inferred heterochrony in a clade of pachypleurosaurs (Reptilia, Sauropterygia) Paleobiology. 1999;25:504–517. [Google Scholar]
- Pirinen S, Majurin A, Lenko H.L, Koski K. Craniofacial features in patients with deficient and excessive growth hormone. J. Craniofac. Genet. Dev. Biol. 1994;14:144–152. [PubMed] [Google Scholar]
- Ponce de León M, Zollikofer C. Neandertal cranial ontogeny and its implications for late hominid diversity. Nature. 2001;412:534–538. doi: 10.1038/35087573. [DOI] [PubMed] [Google Scholar]
- Radovcic J, Smith F.H, Trinkaus E, Wolpoff M. Mladost & Croatian Natural History Museum; Zagreb, Yugoslavia: 1988. The Krapina hominids. An illustrated catalog of skeletal collection. [Google Scholar]
- Ramirez Rozzi F, Bermudez de Castro J. Surprisingly rapid growth in Neanderthals. Nature. 2004;428:936–939. doi: 10.1038/nature02428. doi:10.1038/nature02428 [DOI] [PubMed] [Google Scholar]
- Richtsmeier J.T. The promise of geometric morphometrics. Yearb. Phys. Anthropol. 2002;45:63–91. doi: 10.1002/ajpa.10174. doi:10.1002/ajpa.10174 [DOI] [PubMed] [Google Scholar]
- Rohlf F.J, Marcus L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993;8:129–132. doi: 10.1016/0169-5347(93)90024-J. doi:10.1016/0169-5347(93)90024-J [DOI] [PubMed] [Google Scholar]
- Rohlf F.J, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 1990;39:40–59. doi:10.2307/2992207 [Google Scholar]
- Rosas A. A gradient of size and shape for the Atapuerca sample and Middle Pleistocene hominid variability. J. Hum. Evol. 1997;33:319–331. doi: 10.1006/jhev.1997.0138. doi:10.1006/jhev.1997.0138 [DOI] [PubMed] [Google Scholar]
- Rosas A, Aguirre E. Restos humanos Neandertales de la cueva del Sidron, Piloña, Asturias, Nota preliminar. Estudios geologicos. 1999;55:181–190. [Google Scholar]
- Rosas A, Bastir M. Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am. J. Phys. Anthropol. 2002;117:236–245. doi: 10.1002/ajpa.10023. doi:10.1002/ajpa.10023 [DOI] [PubMed] [Google Scholar]
- Rosas A, Bastir M. Geometric morphometric analysis of allometric variation in the mandibular morphology from the hominids of Atapuerca, Sima de los Huesos Site. Anat. Rec. 2004;278A:551–560. doi: 10.1002/ar.a.20049. doi:10.1002/ar.a.20049 [DOI] [PubMed] [Google Scholar]
- Rosas, A & Martínez-Maza, C. In press. Bone remodelling pattern of Homo heidelbergensis mandibles. J. Hum. Evol [DOI] [PubMed]
- Rosas A, et al. Paleobiology and comparative morphology of a late Neandertal sample from El Sidrón, Asturias, Spain. Proc. Natl Acad. Sci. USA. 2006;103:19 266–19 271. doi: 10.1073/pnas.0609662104. doi:10.1073/pnas.0609662104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas, A., Bastir, M., Martínez-Maza, C., Garcia-Tabernero, A. & Lalueza-Fox, C. In press. Inquiries into Neanderthal cranio-facial development and evolution: ‘accretion’ vs ‘organismic’ models. In Neanderthals revisited: new approaches and perspectives (eds K. Harvati & T. Harrison). New York, NY: Springer.
- Rubin C.T, Lanyon L.E. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J. Orthop. Res. 1987;5:300–310. doi: 10.1002/jor.1100050217. doi:10.1002/jor.1100050217 [DOI] [PubMed] [Google Scholar]
- Ruff C.B, Trinkaus E, Holliday T.W. Body mass and encephalization in Pleistocene Homo. Nature. 1997;387:173–176. doi: 10.1038/387173a0. doi:10.1038/387173a0 [DOI] [PubMed] [Google Scholar]
- Schwartz J.H, Tattersall I. The human chin revisited: what is it and who has it? J. Hum. Evol. 2000;38:367–409. doi: 10.1006/jhev.1999.0339. doi:10.1006/jhev.1999.0339 [DOI] [PubMed] [Google Scholar]
- Schwartz J, Tattersal I. Wiley-Liss; New York, NY: 2002. The human fossil record. Terminology and craniodental morphology of genus Homo (Europe) [Google Scholar]
- Shea B.T. Allometry and heterochrony in the African apes. Am. J. Phys. Anthropol. 1983;62:275–289. doi: 10.1002/ajpa.1330620307. doi:10.1002/ajpa.1330620307 [DOI] [PubMed] [Google Scholar]
- Shea B.T. Ontogenetic scaling of skeletal proportions in the talapoin monkey. J. Hum. Evol. 1992;23:283–307. doi:10.1016/S0047-2484(05)80004-1 [Google Scholar]
- Spoor F, O'Higgins P, Dean C, Lieberman D.E. Anterior sphenoid in modern humans. Nature. 1999;397:572. doi: 10.1038/17505. doi:10.1038/17505 [DOI] [PubMed] [Google Scholar]
- Strand-Viðarsdóttir U, O'Higgins P, Stringer C. A geometric morphometric study of regional differences in the ontogeny of the modern human facial skeleton. J. Anat. 2002;201:211–229. doi: 10.1046/j.1469-7580.2002.00092.x. doi:10.1046/j.1469-7580.2002.00092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R.E, Fuiman L.A. Quantitative comparisons of body form and allometry in larval and adult Pacific sculpins (Teleostei, Cottidae) Can. J. Zool. 1985;63:1582–1589. [Google Scholar]
- Trinkaus E. Neandertal faces were not long; modern human faces are short. Proc. Natl Acad. Sci. USA. 2003;100:8142–8145. doi: 10.1073/pnas.1433023100. doi:10.1073/pnas.1433023100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss R.S, Marcus L.F. Morphological evolution in muroid rodents II. Craniometric factor divergence in seven neotropical genera, with experimental results from Zygodontomys. Evolution. 1992;46:1918–1934. doi: 10.1111/j.1558-5646.1992.tb01178.x. doi:10.2307/2410040 [DOI] [PubMed] [Google Scholar]
- Wayne R.K. Cranial morphology of domestic and wild canids—the influence of development on morphological change. Evolution. 1986;40:243–261. doi: 10.1111/j.1558-5646.1986.tb00467.x. doi:10.2307/2408805 [DOI] [PubMed] [Google Scholar]
- Wolpoff M. McGraw-Hill; Boston, MA: 1999. Paleoanthropology. [Google Scholar]
- Zelditch M.L, Sheets H.D, Fink W.L. Spatiotemporal reorganization of growth rates in the evolution of ontogeny. Evolution. 2000;54:1363–1371. doi: 10.1111/j.0014-3820.2000.tb00568.x. doi:10.1554/0014-3820(2000)054[1363:SROGRI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zelditch M.L, Sheets D.H, Fink W.L. The spatial complexity and evolutionary dynamics of growth. In: Zelditch M.L, editor. Beyond heterochrony. Wiley Liss; New York, NY: 2001. pp. 145–194. [Google Scholar]
- Zelditch M.L, Lundrigan B.L, David Sheets H, Garland T. Do precocial mammals develop at a faster rate? A comparison of rates of skull development in Sigmodon fulviventer and Mus musculus domesticus. J. Evol. Biol. 2003;16:708–720. doi: 10.1046/j.1420-9101.2003.00568.x. doi:10.1046/j.1420-9101.2003.00568.x [DOI] [PubMed] [Google Scholar]