Abstract
Cannibalism has been documented as a possible disease transmission route in several species, including humans. However, the dynamics resulting from this type of disease transmission are not well understood. Using a theoretical model, we explore how cannibalism (i.e. killing and consumption of dead conspecifics) and intraspecific necrophagy (i.e. consumption of dead conspecifics) affect host–pathogen dynamics. We show that group cannibalism, i.e. shared consumption of victims, is a necessary condition for disease spread by cannibalism in the absence of alternative transmission modes. Thus, endemic diseases transmitted predominantly by cannibalism are likely to be rare, except in social organisms that share conspecific prey. These results are consistent with a review of the literature showing that diseases transmitted by cannibalism are infrequent in animals, even though both cannibalism and trophic transmission are very common.
Keywords: host–pathogen dynamics, vertical transmission, spongiform encephalopathy, social organization, prion disease, Kuru
1. Introduction
It is now realized that cannibalism is very widespread in the animal kingdom and is a major mortality factor in the biology of numerous species (Fox 1975; Polis 1981; Elgar & Crespi 1992; Van Schaik & Janson 2000). Cannibalism differs from other predator–prey interactions, in that both the prey and the predator belong to the same species, and this intraspecific predation can result in population dynamics that are very different from that found in systems without cannibalism (Claessen et al. 2004). In humans (but less so in animals), the term cannibalism has been applied to two somewhat different scenarios. In past human societies, individuals were either actively killed and consumed following an attack on neighbouring villages or tribes, or individuals were consumed as part of a funeral ceremony after death (Volhard 1968). We restrict our use of the term cannibalism to the former case (where there is active killing and consumption of living prey) and refer to the consumption of dead conspecifics as ‘intraspecific necrophagy’. As we will show below, the dynamics of these two scenarios are quite different and it is important to discriminate between them.
It is well established that cannibalism in the past has been a common practice in many human societies worldwide (Volhard 1968; White 1992; Defleur et al. 1999; Marlar et al. 2000; Lindenbaum 2004), in spite of some of the cases being exaggerated (Wendt 1989). The connection between cannibalism and disease has received considerable attention in the case of Kuru, a degenerative prion disease transmitted through cannibalism and necrophagy among the Fore people in Papua New Guinea (Lindenbaum 1979; Collinge et al. 2006). However, there has been no general investigation into the possible role that cannibalism might play in disease transmission in animals. In this study, we first review examples of disease transmission through cannibalism and then use a theoretical model to identify the condition under which a disease can successfully invade a host population if cannibalism is the predominant mode of transmission.
(a) Review of cannibalistic disease transmission
We carried out a literature review to determine instances in which cannibalism has been documented as a mechanism of disease transmission. Using Web of Science and Biological Abstracts, we carried out a search based on various combinations of the roots of the keywords ‘disease’, ‘cannibalism’, ‘intraspecific predation’ or ‘necrophagy’. We then noted whether a disease was recorded as being transmitted through cannibalism, if there were alternative transmission modes and, if so, whether cannibalism was the major mode of disease transmission in the system. This review revealed that cannibalism was documented as the predominant transmission mode in very few species (table 1), even though specific instances of cannibalistic transmission had been noted in many groups of organisms (mammals, reptiles, amphibians, fishes, insects and crustaceans), as well as in many pathogen types (including prions, viruses, bacteria and microsporidia). Indeed, cannibalism was implicated as the major transmission mode for only two cases: prion transmission in humans (Lindenbaum 1979) and transmission of the protozoan Sarcocystis in lizards (Matuschka & Bannert 1989). In all other reported cases of cannibalistic disease transmission, alternative disease transmission modes such as necrophagy or heterospecific trophic transmission were of greater importance (table 1).
Table 1.
Studies where disease transmission through cannibalism has been suggested. (Cases where cannibalism and/or necrophagy are recorded as the major transmission modes are marked by * and/or † symbols.)
| host | disease | other transmission modes | references | |
|---|---|---|---|---|
| mammals | humans | prion disease (Kuru)*† | necrophagy | Alpers (1979) and Lindenbaum (1979) |
| carnivores (multiple species) | nematodes (multiple species) | necrophagy | Pozio (2000) | |
| ferrets | bovine tuberculosis | necrophagy, direct contact | Qureshi et al. (2000) | |
| mice | malaria | necrophagy, insect vectors | Malagon et al. (1994) | |
| pigs | Aujeszky's disease | direct contact | Hahn et al. (1997) | |
| swine | Trichinella | necrophagy, other food | Hanbury et al. (1986) | |
| polar bears and walruses | Trichinella (multiple species) | necrophagy, trophic transmission | Forbes (2000) | |
| hamsters | scrapie | Prusiner et al. (1985) | ||
| reptiles | lizards | sarcocysts* | coprophagy | Matuschka & Bannert (1989) |
| amphibians | frogs | iridovirus | necrophagy, water | Pearman et al. (2004) |
| salamanders | iridovirus, bacteria (multiple species) | water, direct contact | Pfennig et al. (1991, 1998) | |
| spadefoot toads | bacteria (multiple species) | water | Pfennig (2000) | |
| insects | meal moths | bacteria†, granulosus virus† | necrophagy, free-living virus stages | Knell et al. (1996, 1998) and Boots (1998) |
| noctuid moths (multiple species) | nuclear polyhedrosis virus | necrophagy | Dhandapani et al. (1993) and Chapman et al. (1999) | |
| bugs | Chagas disease | coprophagy | Schaub et al. (1989) | |
| parasitoid wasps | Microsporidia | maternal transmission | Geden et al. (1995) | |
| crustaceans | prawns | stained prawn disease | water | Bower et al. (1996) |
| shrimp | white spot syndrome | water | Wu et al. (2001) | |
| amphipods | Microsporidia† | unknown | MacNeil et al. (2003) | |
| fishes | multiple species | helminthes, nematodes (multiple species) | trophic transmission | De Buron & Maillard (1987), Koie (2000) and McCormick & Nickol (2004) |
| Arctic chars | tapeworms (multiple species) | trophic transmission | Hammar (2000) |
(b) Model of disease transmission through cannibalism
To investigate the characteristics of diseases transmitted by cannibalism, we combined a classical susceptible–infected model with a model of a cannibalistic predator–prey system. Throughout, we refer to the predator in a cannibalistic interaction as the ‘cannibal’ and the prey as the ‘victim’. We considered the rate of change of susceptibles (S) to be a function of the following (successive terms in equation (1.1a)): birth rate of susceptibles; the increase in birth rate due to resources gained by cannibalism; loss of susceptibles due to cannibalism; loss of susceptibles to the infected class through cannibalistic transmission; and loss of susceptibles by death. The rate of change of infecteds (I) was considered to be a function of the following (successive terms in equation (1.1b)): increase in infecteds due to cannibalistic transmission; loss of infecteds due to cannibalism; and death of infecteds,
| (1.1a) |
| (1.1b) |
where I is the number of infected and infectious individuals; S is the number of healthy and susceptible individuals; N=I+S; b is the birth rate, i.e. number of offspring born per individual per unit time; e is the number of individuals produced for each victim consumed, a dimensionless quantity with e<1 owing to the energy loss during trophic transfer (i.e. a cannibal cannot produce more than one individual per victim consumed); a is the attack rate, i.e. number of victims killed and consumed per cannibal per victim available per unit time; g is the number of cannibals sharing one conspecific victim; δ is the transmission probability for a cannibalistic event; d is the disease-independent death rate per individual per unit time; and ν is the added death rate due to infection per individual per unit time. By analogy with predator–prey models, the term e is included to represent the increase in reproduction of the cannibal resulting from conspecific predation; the nutritional value gained from cannibalism can be substantial (Polis 1981) even for humans (Darnstreich & Moren 1974). The group size of cannibals sharing a conspecific, g, has several important implications. If g>1, cannibals have to ‘share’ a victim; this effectively reduces the nutritional energy gained per cannibal per victim consumed to e/g. Thus, the reproduction gained per cannibal is (e/g)aN, but g cannibals benefit per event. Thus, the total increase in reproduction per cannibal is given by eaN. Moreover, if individuals share one victim, this creates the possibility for one infected individual to simultaneously infect multiple (g) cannibals. Note that this formulation assumes that individuals hunt independently, but share the victim with g other individuals after an attack has occurred. An alternative scenario might be to assume that individuals hunt in groups (e.g. the cannibalistic population could be divided into N/g groups). However, our qualitative results hold true irrespective of the specific formulation of the ‘hunting’ mode. Thus, we restrict our analysis to explore the first scenario in detail.
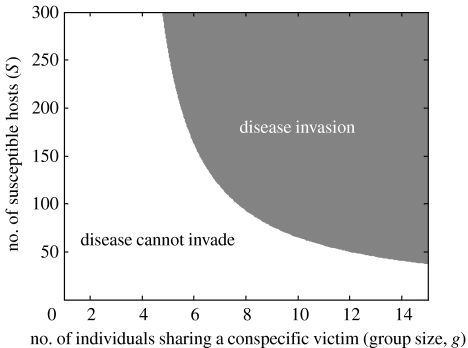
Using equations (1.1a) and (1.1b), it can be readily shown that a disease cannot become established through cannibalistic transmission if the cannibal–victim interaction is one-to-one. Assuming that the disease is rare, i.e. I is small and N≈S, disease spread requires that δagS>aS+d+ν. If g=1, this inequality is not possible (given that δ has to be less than 1). A disease can therefore only invade if g>(aS+d+v)/δaS>1, i.e. group cannibalism is a necessary but not sufficient condition for disease transmission by cannibalism (figure 1). Given group cannibalism, there will be a threshold population density S>(d+ν)/[a(δg−1)] for disease spread; this threshold decreases with higher attack rates and greater levels of group cannibalism (figure 1). Note that we assume that infected and healthy individuals are equally likely to be eaten. If infected individuals are more likely to be cannibalized, the threshold density will be lower, making it easier for the disease to invade (assuming all else equal). The number of secondary infections per existing infection (R0) of the disease is approximately R0=δagS/(aS+d+v), where S at equilibrium in the absence of the disease is =(b(d)/a(1−e) and is similar to that for a directly transmitted disease. The change in the total population size can be derived by adding equations (1.1a) and (1.1b),
| (1.2) |
Figure 1.
Threshold condition for successful invasion of a disease transmitted through cannibalism as a function of the number of susceptible hosts and group size. Parameter values are d=0.8, ν=0.5, a=0.01 and δ=0.3.
At equilibrium, dN/dt=0 and equation (1.2) gives a quadratic function for host population size with the solution
| (1.3) |
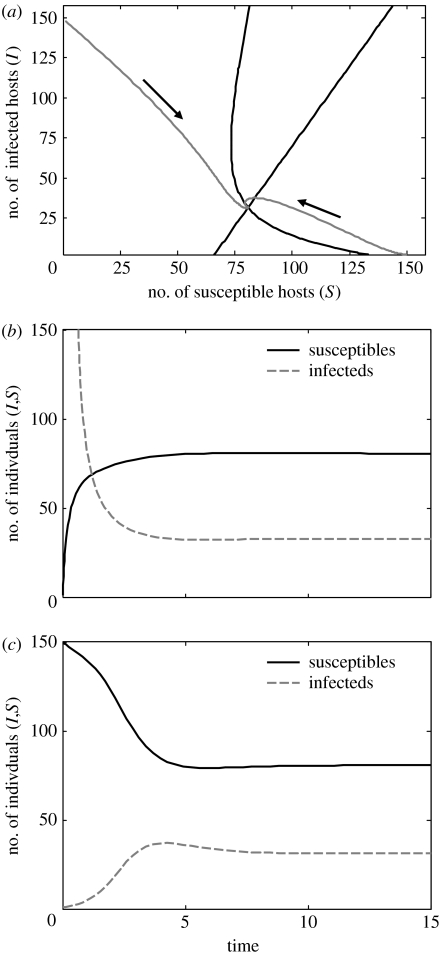
The equilibrium host population number, , can only be zero if . Given that the conversion efficiency, e<1, and all other parameters are positive, this latter term is always negative, and therefore of a cannibalistic host can never be equal to zero (assuming b>d). Thus, a disease that is solely transmitted through group cannibalism can never drive the host to extinction. Furthermore, the whole population can never become infected completely because the condition can also never be met (assuming all parameters are greater than zero). An example of the phase plot and time course of such a disease is shown in figure 2. Therefore, a cannibalistically transmitted disease will remain endemic in the host population if it can invade the host. Numerical simulations show that the equilibria are stable over a large parameter region (figure 2).
Figure 2.
Dynamics of susceptible and infected hosts. (a) Zero-growth isoclines of susceptible (curved black line) and infected (straight black line) hosts. The two grey lines with arrows indicate trajectories predicted by the model in equations (1.1a) and (1.1b) from two different initial conditions (S common and I rare, S rare and I common). Time course of these trajectories is displayed individually in (b) and (c). Parameter values are b=1.5, d=0.8, ν=0.5, a=0.01, δ=0.3, e=0.5 and g=10.
(c) Model of disease transmission through intraspecific necrophagy
In many species including humans, another form of cannibalism is the consumption of dead conspecifics, i.e. intraspecific necrophagy. In the animal kingdom, this is a common transmission route for many diseases (table 1). The dynamics of the system can be described as follows:
| (1.4a) |
| (1.4b) |
| (1.4c) |
| (1.4d) |
where DI and DS are the number of dead infecteds and dead susceptibles, respectively, and γ is the decay rate of dead individuals. Necrophagy differs from cannibalism (where individuals are actively killed), in that consumption and disease transmission are proportional to the number of dead individuals. Another difference between cannibalism and necrophagy arises because, in the latter, there is no density-dependent mortality due to consumption of conspecifics. In the case of necrophagy, the disease can spread even without group necrophagy if S>γ/aδ, although the threshold conditions are easier to meet with group feeding (S>γ/agδ). From equations (1.4a)–(1.4d), it can be shown that in the presence of the disease, the equilibrium density of susceptibles =γ/agδ. Similarly to the previous cases, this indicates that the population can never become completely infected, assuming that all parameters are greater than zero.
2. Discussion
We have shown that group cannibalism by multiple individuals on one victim is a necessary (albeit not always sufficient) precondition for disease spread through cannibalism. However, in the animal kingdom, cannibalism is generally a one-on-one interaction in which a larger and stronger individual kills and consumes a smaller and weaker conspecific (Polis 1981). Under these conditions, cannibalism is likely to be an ineffective mode of disease transmission. Our model is clearly simplified, in that it assumes there is no cannibalism-independent population regulation, that infection itself does not influence the probability of being cannibalistic or being cannibalized and that there is no age structure. However, intuitively, it is clear that in one-on-one cannibalism, the R0 of a cannibalistically transmitted disease has to be less than unity unless the infected individuals have some compensatory advantage: an infected individual can only transmit the disease to one other individual and this victim is killed in the transmission process. This is somewhat analogous to the case of vertical (maternal) transmission, in which one female parent leaves on average only one offspring, such that the spread of vertically transmitted parasites only occurs under special circumstances (e.g. where the infected individual has a higher fitness than the uninfected). This is consistent with our finding that diseases spread through cannibalism are rare in natural populations, and that cannibalistically transmitted parasites or pathogens usually have one or more alternative transmission modes. This rarity of cannibalistic transmission is all the more notable because trophic transmission itself (disease transmission from prey to predator) is a commonplace in parasites with life cycles involving alternate hosts, especially in the digenean trematodes and cestodes (Odening 1976), as well as in many nematodes (Anderson 2000). Moreover, it would seem that within-species trophic transmission via cannibalism would evolve more easily than cross-species trophic transmission because in the former case the host species and hence the internal environment for the pathogen would remain unchanged.
An interesting ‘exception that proves the rule’ is the case of cannibalistic transmission of several species of the protozoan Sarcocystis in lizards of the genus Gallotia. Sarcosystis species normally have alternate hosts, but in several species of Gallotia that live on the Canary Islands, parasitic transmission is directly through cannibalism (Matuschka & Bannert 1989). However, in the lizards, cannibalism is frequently partial, taking place by consumption of autotomized tails. Thus, one individual host can infect several cannibals over the course of its lifetime following tail regeneration. The process therefore has the dynamics of group cannibalism, even though there is no simultaneous consumption by a group.
There are otherwise only a few records of group cannibalism in which several smaller individuals kill and together consume one larger conspecific. This has been seen in social Hymenoptera and Isoptera (Polis 1981) and in some species where the mother serves as a food source for its offspring (Evans et al. 1995). Cases where several adults kill and consume an infant together have also been recorded in social mammals. This occurs in lions, for example, when a group of new males acquires another male's harem (Bertram 1975). It also occurs in chimpanzees where male groups commonly attack conspecifics (Arcadi & Wrangham 1999; Mitani et al. 2002; Wilson et al. 2004). Humans have also had the required social structure and social practices that promote disease spread by cannibalism. Historically, members of families or a village often shared captured individuals in ritualized meals (Volhard 1968; Sahlins 1983; Wendt 1989), and the group size of individuals sharing one victim was often very large. Additionally, some human societies practiced cannibalism across groups and necrophagy within the group (Volhard 1968; Conklin 2001). This seems to have been the case for the Fore people of Papua New Guinea, in which both intraspecific necrophagy and cross-group cannibalism were common (Lindenbaum 1979; Rumsey 1999). Thus, in the case of Kuru, necrophagy could maintain and spread the disease within a village, while cross-group cannibalism could be the mechanism that promoted the disease spread on a larger meta-population scale. Cannibalism in humans has been a common and widespread practice that dates back at least to the Neanderthals (Defleur et al. 1999; Marlar et al. 2000). Several authors have argued that cannibalism has been a part of the natural ecology of human societies owing to the substantial nutritional gain (Darnstreich & Moren 1974).
Our results suggest that the occurrence of a disease such as Kuru in humans is most probably the result of the frequent occurrence of group cannibalism and/or group necrophagy. Additionally, the persistence of Kuru into modern times may in part be explained by the pathogenic agent's resilience to cooking, although, in many of the cannibalistic rituals, raw human flesh was often consumed (Lindenbaum 1979). Because cannibalism is no longer a regular feature of human populations, it is not possible to assess the degree to which other diseases may have had this transmission mode. However, it has been speculated that the transmission and establishment of tapeworms in humans may have been aided by cannibalism (Hoberg et al. 2001), and it is quite conceivable that a number of blood-borne infections may have been regularly transmitted in the same way.
We conclude that although many pathogenic agents have the potential for transmission from prey to predator, diseases transmitted predominantly by cannibalism are rare because the epidemiological conditions necessary for its spread, especially group cannibalism, are rarely met in natural populations. However, we suspect that such transmission occurs in animals such as chimpanzees where cannibalism occurs by socially organized groups of males (Arcadi & Wrangham 1999; Mitani et al. 2002; Wilson et al. 2004). Cannibalistic transmission may therefore play an important role in the maintenance of blood-borne infections like simian immunodeficiency virus (Santiago et al. 2002) or different types of hepatitis (Weiner et al. 1995; Birkenmeyer et al. 1998) in these populations. Given the importance of social primates as disease reservoirs for humans (Wolfe et al. 1998), this possibility deserves further investigation.
Acknowledgments
We are thankful to Amy Dunham, Mike Boots and Henry Wilbur and two anonymous reviewers for their critical comments on earlier versions of the manuscript.
References
- Alpers M. Epidemiology and ecology of Kuru. In: Prusiner S.B, Hadlow W.J, editors. Slow transmissible diseases of the nervous system. vol. 1. Academic Press; New York: 1979. [Google Scholar]
- Anderson R.C. CABI publishing; Wallington, UK: 2000. Nematode parasites of vertebrates. [Google Scholar]
- Arcadi A.C, Wrangham R.W. Infanticide in chimpanzees: review of cases and a new within-group observation from the Kanyawara study group in Kibale National Park. Primates. 1999;40:337–351. doi:10.1007/BF02557557 [Google Scholar]
- Bertram B.C. Social factors influencing reproduction in wild lions. J. Zool. 1975;177:463–482. [Google Scholar]
- Birkenmeyer L.G, Desai S.M, Muerhoff A.S, Leary T.P, Simons J.N, Montes C.C, Mushahwar I.K. Isolation of a GB virus-related genome from a chimpanzee. J. Med. Virol. 1998;56:44–51. doi: 10.1002/(sici)1096-9071(199809)56:1<44::aid-jmv8>3.0.co;2-n. doi:10.1002/(SICI)1096-9071(199809)56:1<44::AID-JMV8>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Boots M. Cannibalism and the stage-dependent transmission of a viral pathogen of the Indian meal moth, Plodia interpunctella. Ecol. Entomol. 1998;23:118–122. doi:10.1046/j.1365-2311.1998.00115.x [Google Scholar]
- Bower S.M, Meyer G.R, Boutillier J.A. Stained prawn disease (SPD) of Pandalus platyceros in British Columbia, Canada, caused by a rickettsial infection. Dis. Aquat. Org. 1996;24:41–54. [Google Scholar]
- Chapman J.W, Williams T, Escribano A, Caballero P, Cave R.D, Goulson D. Age-related cannibalism and horizontal transmission of a nuclear polyhedrosis virus in larval Spodoptera frugiperda. Ecol. Entomol. 1999;24:268–275. doi:10.1046/j.1365-2311.1999.00224.x [Google Scholar]
- Claessen D, De Roos A.M, Persson L. Population dynamic theory of size-dependent cannibalism. Proc. R. Soc. B. 2004;271:333–340. doi: 10.1098/rspb.2003.2555. doi:10.1098/rspb.2003.2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Whitfield J, McKintosh E, Beck J, Mead S, Thomas D, Alpers M.P. Kuru in the 21st century—an acquired human prion disease with very long incubation periods. Lancet. 2006;2006:2068–2074. doi: 10.1016/S0140-6736(06)68930-7. doi:10.1016/S0140-6736(06)68930-7 [DOI] [PubMed] [Google Scholar]
- Conklin B.A. University of Texas Press; Austin, TX: 2001. Consuming grief. Compassionate cannibalism in an Amazonian society. [Google Scholar]
- Darnstreich M, Moren G. Does New Guinean cannibalism have a nutritional value. Human Ecol. 1974;2:1–12. doi:10.1007/BF01507342 [Google Scholar]
- De Buron I, Maillard C. Experimental transfer by ichthyophagy and cannibalism of adult helminths, parasite of fishes. Annales De Parasitologie Humaine Et Comparee. 1987;62:188–191. [Google Scholar]
- Defleur A, White T, Valensi P, Slimak L, Cregut-Bonnoure E. Neanderthal cannibalism at Moula-Guercy, Ardeche, France. Science. 1999;286:128–131. doi: 10.1126/science.286.5437.128. doi:10.1126/science.286.5437.128 [DOI] [PubMed] [Google Scholar]
- Dhandapani N, Jayaraj S, Rabindra R.J. Cannibalism on nuclear polyhedrosis-virus infected larvae by Heliothis armigera (Hubn) and its effect on viral-infection. Insect Sci. Appl. 1993;14:427–430. [Google Scholar]
- Elgar M.A, Crespi B.J, editors. Cannibalism: ecology and evolution among diverse taxa. Oxford University Press; New York, NY: 1992. [Google Scholar]
- Evans T.A, Wallis E.J, Elgar M.A. Making a meal of mother. Nature. 1995;376:299. doi:10.1038/376299a0 [Google Scholar]
- Forbes L.B. The occurrence and ecology of Trichinella in marine mammals. Vet. Parasitol. 2000;93:321–334. doi: 10.1016/s0304-4017(00)00349-6. doi:10.1016/S0304-4017(00)00349-6 [DOI] [PubMed] [Google Scholar]
- Fox L.R. Cannibalism in natural populations. Annu. Rev. Ecol. Syst. 1975;6:87–106. doi:10.1146/annurev.es.06.110175.000511 [Google Scholar]
- Geden C.J, Long S.J, Rutz D.A, Becnel J.J. Nosema disease of the parasitoid Muscidifurax raptor (Hymenoptera, Pteromalidae)—prevalence, patterns of transmission, management, and impact. Biol. Control. 1995;5:607–614. doi:10.1006/bcon.1995.1072 [Google Scholar]
- Hahn E.C, Page G.R, Hahn P.S, Gillis K.D, Romero C, Annelli J.A, Gibbs E.P.J. Mechanisms of transmission of Aujeszky's disease virus originating from feral swine in the USA. Vet. Microbiol. 1997;55:123–130. doi: 10.1016/s0378-1135(96)01309-0. doi:10.1016/S0378-1135(96)01309-0 [DOI] [PubMed] [Google Scholar]
- Hammar J. Cannibals and parasites: conflicting regulators of bimodality in high latitude Arctic char, Salvelinus alpinus. Oikos. 2000;88:33–47. doi:10.1034/j.1600-0706.2000.880105.x [Google Scholar]
- Hanbury R.D, Doby P.B, Miller H.O, Murrell K.D. Trichinosis in a herd of swine-Cannibalism as a major mode of transmission. J. Am. Vet. Med. Assoc. 1986;188:1155–1159. [PubMed] [Google Scholar]
- Hoberg E.P, Alkire N.L, De Queiroz A, Jones A. Out of Africa: origins of the taenia tapeworms in humans. Proc. R. Soc. B. 2001;268:781–787. doi: 10.1098/rspb.2000.1579. doi:10.1098/rspb.2000.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knell R.J, Begon M, Thompson D.J. Transmission of Plodia interpunctella granulosis virus does not conform to the mass action model. J. Anim. Ecol. 1998;67:592–599. doi:10.1046/j.1365-2656.1998.00219.x [Google Scholar]
- Knell R.J, Begon M, Thompson D.J. Transmission dynamics of Bacillus thuringiensis infecting Plodia interpunctella: a test of the mass action assumption with an insect pathogen. Proc. R. Soc. B. 1996;263:75–81. doi: 10.1098/rspb.1996.0013. doi:10.1098/rspb.1996.0013 [DOI] [PubMed] [Google Scholar]
- Koie M. Life cycle and seasonal dynamics of Cucullanus cirratus OF Miller, 1777 (Nematoda, Ascaridida, Seuratoidea, Cucullanidae) in Atlantic cod, Gadus morhua L. Can. J. Zool. 2000;78:182–190. doi:10.1139/cjz-78-2-182 [Google Scholar]
- Lindenbaum S. Mayfield Publishing Company; Mountain Few, CA: 1979. Kuru sorcery: disease and dangers in the New Guinean highlands. [Google Scholar]
- Lindenbaum S. Thinking about cannibalism. Annu. Rev. Anthropol. 2004;33:475–498. doi:10.1146/annurev.anthro.33.070203.143758 [Google Scholar]
- MacNeil C, Dick J.T.A, Hatcher M.J, Fielding N.J, Hume K.D, Dunn A.M. Parasite transmission and cannibalism in an amphipod (Crustacea) Int. J. Parasitol. 2003;33:795–798. doi: 10.1016/s0020-7519(03)00110-3. doi:10.1016/S0020-7519(03)00110-3 [DOI] [PubMed] [Google Scholar]
- Malagon F, Castillo L, Tapia J.L. Experimental transmission of murine malaria by cannibalism on mice infected by Plasmodium berghei yoelii. Acta Protozool. 1994;33:191–193. [Google Scholar]
- Marlar R.A, Leonard B.L, Billman B.R, Lambert P.M, Marlar J.E. Biochemical evidence of cannibalism at a prehistoric Puebloan site in southwestern Colorado. Nature. 2000;407:74–78. doi: 10.1038/35024064. doi:10.1038/35024064 [DOI] [PubMed] [Google Scholar]
- Matuschka F.R, Bannert B. Recognition of cyclic transmission of Sarcocystis stehlinii n-sp in the Gran Canarian giant lizard. J. Parasitol. 1989;75:383–387. doi:10.2307/3282593 [PubMed] [Google Scholar]
- McCormick A.L, Nickol B.B. Postcyclic transmission and its effect on the distribution of Paulisentis missouriensis (acanthocephala) in the definitive host Semotilus atromaculatus. J. Parasitol. 2004;90:103–107. doi: 10.1645/GE-3170. doi:10.1645/GE-3170 [DOI] [PubMed] [Google Scholar]
- Mitani J.C, Watts D.P, Muller M.N. Recent developments in the study of wild chimpanzee behavior. Evol. Anthropol.: Issues, News, and Rev. 2002;11:9–25. doi:10.1002/evan.10008 [Google Scholar]
- Odening K. Conception and terminology of hosts and parasitology. Adv. Parasitol. 1976;14:1–93. doi: 10.1016/s0065-308x(08)60513-8. [DOI] [PubMed] [Google Scholar]
- Pearman P.B, Garner T.W.J, Straub M, Greber U.F. Response of the Italian agile frog (Rana latastei) to a Ranavirus, frog virus 3: a model for viral emergence in naive populations. J. Wildl. Dis. 2004;40:660–669. doi: 10.7589/0090-3558-40.4.660. [DOI] [PubMed] [Google Scholar]
- Pfennig D.W. Effect of predator-prey phylogenetic similarity on fitness consequences of predation: a trade-off between nutrition and disease. Am. Nat. 2000;155:335–345. doi: 10.1086/303329. doi:10.1086/303329 [DOI] [PubMed] [Google Scholar]
- Pfennig D.W, Loeb M.L.G, Collins J.P. Pathogens as a factor limiting the spread of cannibalism in tiger salamanders. Oecologia. 1991;88:161–166. doi: 10.1007/BF00320806. doi:10.1007/BF00320806 [DOI] [PubMed] [Google Scholar]
- Pfennig D.W, Ho S.G, Hoffman E.A. Pathogen transmission as a selective force against cannibalism. Anim. Behav. 1998;55:1255–1261. doi: 10.1006/anbe.1997.9996. doi:10.1006/anbe.1997.9996 [DOI] [PubMed] [Google Scholar]
- Polis G.A. The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst. 1981;12:225–251. doi:10.1146/annurev.es.12.110181.001301 [Google Scholar]
- Pozio E. Factors affecting the flow among domestic, synanthropic and sylvatic cycles of Trichinella. Vet. Parasitol. 2000;93:241–262. doi: 10.1016/s0304-4017(00)00344-7. doi:10.1016/S0304-4017(00)00344-7 [DOI] [PubMed] [Google Scholar]
- Prusiner S.B, Cochran S.P, Alpers M.P. Transmission of scrapie in hamsters. J. Infect. Dis. 1985;152:971–978. doi: 10.1093/infdis/152.5.971. [DOI] [PubMed] [Google Scholar]
- Rumsey A. The white man as cannibal in the new Guinean highlands. In: Goldman L.R, editor. The anthropology of cannibalism. Bergin and Garvey; Westport, CT: 1999. p. 168. [Google Scholar]
- Qureshi T, Labes R.E, Lambeth M, Montgomery H, Griffin J.F.T, Mackintosh C.G. Transmission of Mycobacterium bovis from experimentally infected ferrets to non-infected ferrets (Mustela furo) N. Z. Vet. J. 2000;48:99–104. doi: 10.1080/00480169.2000.36173. [DOI] [PubMed] [Google Scholar]
- Sahlins M. Raw women, cooked men, and other “great things” of the Fiji Islands. In: Brown P, Tuzin D, editors. The ethnography of cannibalism. Society for Psychological Anthropology; Washington, DC: 1983. pp. 72–93. [Google Scholar]
- Santiago M.L, et al. SIVcpz in wild chimpanzees. Science. 2002;295:465. doi: 10.1126/science.295.5554.465. doi:10.1126/science.295.5554.465 [DOI] [PubMed] [Google Scholar]
- Schaub G.A, Boker C.A, Jensen C, Reduth D. Cannibalism and coprophagy are modes of transmission of Blastocrithidia triatomae (Trypanosomatidae) between triatomines. J. Protozool. 1989;36:171–175. doi: 10.1111/j.1550-7408.1989.tb01067.x. [DOI] [PubMed] [Google Scholar]
- Van Schaik C.P, Janson C.H. Cambridge University Press; Cambridge, UK: 2000. Infacticide by males and its implications. [Google Scholar]
- Volhard E. Strecker und Schroeder Verlag; Stuttgart, Germany: 1968. Kannibalismus. Studien zur Kulturkunde. [Google Scholar]
- Weiner A, Erickson A.L, Kansopon J, Crawford K, Muchmore E, Hughes A.L, Houghton M, Walker C.M. Persistent hepatitis-C virus-infection in a chimpanzee is associated with emergence of a cytotoxic T-lymphocyte escape variant. Proc. Natl Acad. Sci. USA. 1995;92:2755–2759. doi: 10.1073/pnas.92.7.2755. doi:10.1073/pnas.92.7.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt A. Eurpaeische Hochschulschriften: Reihe 19, Volkskunde, Ethnologie. Verlag Peter Lang GmbH; Frankfurt, Germany: 1989. Kannibalismus in Brasilien: Eine Analyse europaeischer Reiseberichte in Amerika-Darstellungen fuer die Zeit zwischen 1500 und 1654. [Google Scholar]
- White T.D. Princeton University Press; Princeton, NJ: 1992. Prehistoric cannibalism in Mancos 5MTUMR-2346. [Google Scholar]
- Wilson M.L, Wallauer W.R, Pusey A.E. New cases of intergroup violence among chimpanzees in Gombe National Park, Tanzania. Int. J. Primatol. 2004;25:523–549. doi:10.1023/B:IJOP.0000023574.38219.92 [Google Scholar]
- Wolfe N.D, Escalante A.A, Karesh W.B, Kilbourn A, Spielman A, Lal A.A. Wild primate populations in emerging infectious disease research: the missing link? Emerg. Infect. Dis. 1998;4:149–158. doi: 10.3201/eid0402.980202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.L, Namikoshi A, Nishizawa T, Mushiake K, Teruya K, Muroga K. Effects of shrimp density on transmission of in Penaeus japonicus by cannibalism and the waterborne route. Dis. Aquat. Org. 2001;47:129–135. doi: 10.3354/dao047129. [DOI] [PubMed] [Google Scholar]