Abstract
Rearing in darkness slows the time course of the visual cortical critical period, such that at 5 weeks of age normal cats are more plastic than dark-reared cats, while at 20 weeks dark-reared cats are more plastic (Mower, Dev. Brain Res., 58: 151-158, 1991). Thus, genes that are important for visual cortical plasticity should show differences in expression between normal and dark-reared visual cortex that are of opposite direction in young versus older animals. Previously, we showed by differential display PCR and northern blotting that mRNA for Munc13-3, a mammalian homologue of the C. elegans uncoordinated (unc) gene, shows such bidirectional regulation in cat visual cortex (Yang et al., J. Neurosci., 22: 8614-8618, 2002). Here, the analysis is extended to Munc13-3 protein in mouse visual cortex, which will provide the basis for gene manipulation analysis. In mice, Munc13-3 protein was elevated 2.3 fold in dark-reared compared to normal visual cortex at 3.5 weeks and 2.0 fold in normal compared to dark-reared visual cortex at 9.5 weeks. Analysis of variance of protein levels showed a significant interaction, indicating that the effect of dark rearing depended on age. This bidirectional regulation was restricted to visual cortex and did not occur in frontal cortex. Bidirectional regulation was also specific to Munc13-3 and was not found for other Munc13 family members. Munc13 proteins serve a central priming function in synaptic vesicle exocytosis at glutamatergic and GABAergic synapses and this work contributes to the growing evidence indicating a role of Munc13 genes in synaptic plasticity.
Keywords: Munc13, Visual Deprivation, Neuronal Plasticity
Introduction
The neonatal visual cortex is a highly plastic structure and its development is guided by visual experience during early postnatal life. Such environmental effects on visual cortical development are exemplified by rearing cats with monocular deprivation, a condition that leads to dramatic anatomical and physiological abnormalities (see Sherman and Spear, 1982; Daw, 1994 for reviews). In normal development, sensitivity to monocular deprivation is limited to a “critical period”, which in cats begins several weeks after birth, peaks at about 5-6 weeks, gradually declines to low levels at 5 months and disappears at about 1 year of age (Daw, 1994).
Rearing in total darkness extends this critical period and prolongs neuronal plasticity far beyond its normal age limits (Cynader and Mitchell, 1980; Mower et al., 1981). Dark rearing, therefore, provides a means to dissociate changes associated with the state of visual cortical plasticity from changes associated with the general maturation of the animal. It is an ideal paradigm for isolating changes in gene expression involved in controlling neuronal plasticity. Electrophysiological results indicate that the effect of dark rearing is to slow the entire time course of the critical period, such that at young ages (5 weeks) normal cats are more plastic than dark-reared cats, while at later ages (20 weeks) dark-reared cats are more plastic (Mower, 1991). This result has been independently verified by another laboratory (Beaver et al., 2001). Thus, a stringent criterion is that genes that are important for plasticity in visual cortex will show differences in expression between normal and dark-reared cats that are of opposite direction in young versus older animals.
Differential display PCR screening of normal and dark-reared cat visual cortex at 5 and 20 weeks has identified Munc13-3 as a gene which shows such bidirectional regulation (Yang et al., 2002). Munc13-3 is a member of a family of proteins (including Munc13-1 and Munc13-2), all of which play a role in synaptic vesicle priming in glutamatergic and GABAergic synapses (Augustin et al., 1999b; Brose et al., 1998; Varoqueaux et al., 2002). Bidirectional regulation of mRNA levels was specific to Munc13-3. Developmental changes in expression levels of several synaptic vesicle proteins are due to changes in posttranscriptional regulation (Daly and Ziff, 1997). It is therefore unclear if the bidirectional regulation of Munc13-3 mRNA by age and visual experience will extend to protein expression or if the specificity to Munc13-3 will be maintained. Additionally, mice, which allow mechanistic gene manipulation analysis, also show a critical period for visual cortical plasticity (Gordon and Stryker, 1996; Guire et al., 1999; Sawtell et al., 2003) and prolongation of the critical period by dark rearing (Fagiolini et al., 2003). The present study was undertaken to determine if bidirectional regulation due to age and visual experience extends to Munc13-3 protein in mouse visual cortex.
Experimental Procedures
Animals
Mice were reared in a normal 12 hour light/dark cycle or in complete darkness from birth to 3.5 or 9.5 weeks of age. The ages were based on published studies of the time course of the critical period in mouse visual cortex (Gordon and Stryker, 1996; Guire et al., 1999; Sawtell et al., 2003). Three independent groups of mice including all four ages and rearing conditions (normal 3.5 weeks, dark-reared 3.5 weeks, normal 9.5 weeks, dark-reared 9.5 weeks) were raised (12 animals in each group, 36 in total). For each age/rearing condition within each group, three animals were pooled to provide sufficient tissue and to reduce individual variability. Visual cortex (monocular and binocular regions), frontal cortex and in several normal 9.5 week mice cerebellum were dissected, immediately frozen, and stored at −80°C until used for western blots. Mice were killed by an overdose of sodium pentobarbital prior to tissue dissection (75 mg/kg, intraperitoneal injection). All procedures conformed to the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee.
Western Blot Analysis
Homogenates of visual cortex were prepared in the presence of a standard protease inhibitor cocktail (Sigma, St. Louis, MO). Centrifugation was done to yield a crude synaptosomal fraction (P2) and equal amounts of protein were loaded onto a 4-15% polyacrylamide SDS gel (Bio-Rad, Hercules, CA), separated by electrophoresis, and then electrophoretically transferred to a polyvinylidene fluoride membrane using a Transblot cell (Bio-Rad). After blotting, the membrane was stained with Ponceau S in 5% trichloroacetic acid to assess protein separation. Nonspecific binding sites were blocked with 5% non-fat milk in Tris buffered saline with 0.1% Tween 20 (TBST) for 60 minutes. Blots were then incubated with primary antisera specific for each Munc13 isoform at a dilution of 1:1000 at room temperature for 2 hr. The Munc13-1, Munc13-2 and Munc13-3 antibodies were provided by Drs. Nils Brose and Frederique Varoqueaux. Blots were extensively washed in TBST and incubated with appropriate horseradish peroxidase conjugated secondary antibodies at room temperature for 2 hr. Specific protein bands were visualized by enhanced chemiluminescence (ECL) detection reagents (Amersham Biosciences, Piscataway, NJ). For ECL visualization, an optimal exposure (0.5 to 5 min.) to BioMax MR film (Kodak, Rochester, NY) was selected for densitometric analysis. The blots were then stripped and reprobed with the other Munc13 antibodies and a mouse monoclonal antibody to β-actin (Sigma) to control for loading variations. Subsequent immunostainings were carried out as described above. All densitometric measures for Munc13 proteins were corrected against β-actin.
Results
Regional distribution of Munc13 protein expression
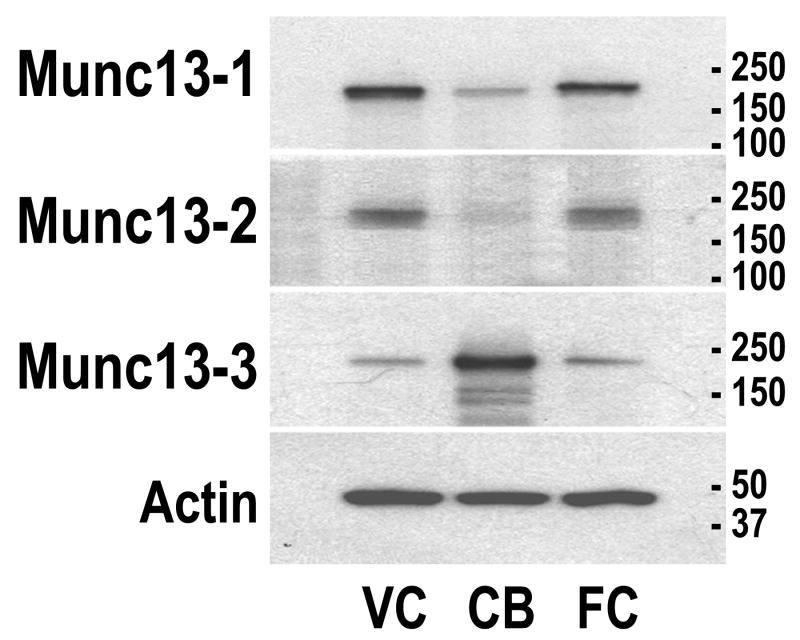
The expression of the three Munc13 proteins was investigated in two forebrain structures, visual cortex (the experimental structure of this study) and frontal cortex (the control structure), and the cerebellum (a reference hindbrain structure) (Fig. 1). Munc13-1 was expressed in all structures and more abundant in the cortex as compared to the cerebellum; Munc13-2 was expressed in the cortex as well, but was not detected in the cerebellum; Munc13-3 was expressed in all structures but was more abundant in the cerebellum compared to the cortex. These different regional patterns of expression of the Munc13 proteins are in agreement with the pattern of the mRNAs reported in the cat (Yang et al., 2002). Moreover, these expression patterns are in general agreement with the expression patterns of Munc13 proteins previously reported in the forebrain and hindbrain of the rat (Augustin et al., 1999a, 2001). The antibodies labeled bands with the appropriate size for all three Munc13 proteins, further indicating their specificity.
Figure 1.
Western blot analysis of regional distributions of Munc13 proteins in mouse brain. Samples from visual cortex (VC), frontal cortex (FC), and cerebellum (CB) were loaded and sequentially probed, stripped and reprobed with the Munc13 family member specific antibodies and with β-actin antibody to control for loading errors. Size standards in kDa are indicated on all western blots.
Bidirectional regulation of Munc13-3 by age and dark rearing in the visual cortex
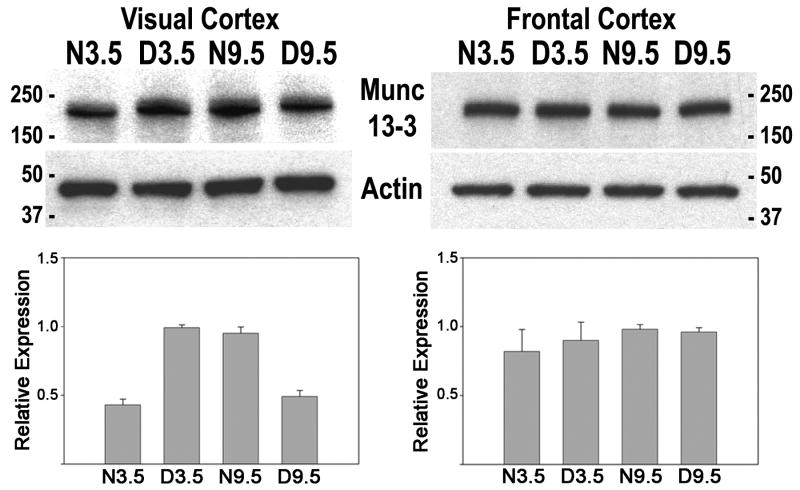
Figure 2 shows western blots of visual cortex for three independent groups of mice, each group having the four age/rearing conditions (normal 3.5, dark reared 3.5, normal 9.5, dark reared 9.5). Standardized densitometric analysis indicated Munc13-3 was elevated 2.3 fold in dark-reared compared to normal mouse visual cortex at 3.5 weeks and 2 fold in normal compared to dark-reared mouse visual cortex at 9.5 weeks. Analysis of variance showed a significant interaction (F[1,11]= 508.26, p<.001), indicating that the effect of dark rearing was age dependent. In normal development, Munc13-3 expression increased 2 fold between the peak of the critical period (3.5 weeks) and its nadir (9.5 weeks). Munc13-3 protein showed opposite direction regulation by age and dark rearing in the visual cortex of mice.
Figure 2.
Analysis of Munc13-3 protein expression in mouse visual cortex and frontal cortex. Mice were reared in a normal environment (N) or complete darkness (D) until an age of 3.5 (N3.5, D3.5) or 9.5 weeks (N9.5, D9.5). Also shown are the same blots stripped and reprobed with a β-actin antibody to control for loading variations. Size standards in kDa are presented for all blots. Beneath are densitometric results for Munc13-3 protein levels in mouse visual and frontal cortex (corrected against β-actin) from three independent groups of mice. For each of the three groups of mice, values for each age/rearing condition were normalized against the highest value in each group. Means and standard errors from the three groups for each age/rearing condition are plotted for visual and frontal cortex.
Bidirectional regulation of Munc13-3 expression is specific to visual cortex
To investigate whether bidirectional regulation of Munc13-3 is specific to visual cortex or is a generalized effect throughout neocortex, we analyzed the effects of age and dark rearing on Munc13-3 protein expression in mouse frontal cortex (Fig. 2). Levels of Munc13-3 expression were similar in all rearing/age conditions and analysis of variance of densitometric results indicated no statistically significant interaction due to age and dark rearing for Munc13-3 expression in frontal cortex. The bidirectional regulation of Munc13-3 expression was specific to visual cortex and did not occur in frontal cortex.
Bidirectional regulation is specific to Munc13-3
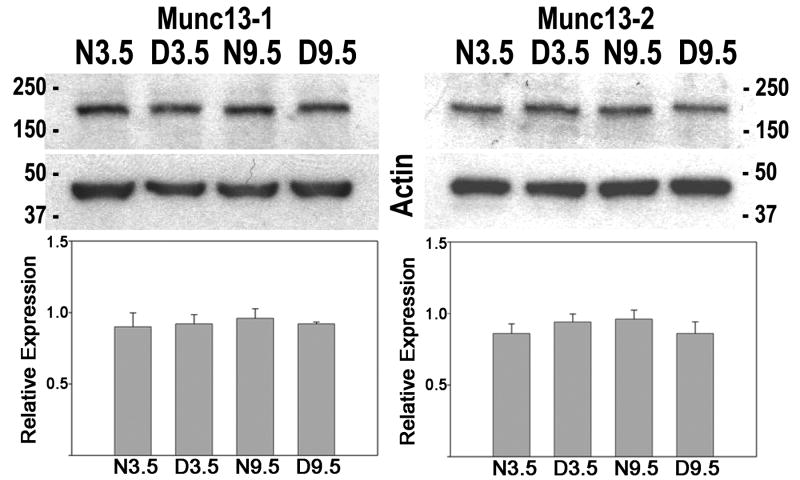
Since Munc13-3 is a member of a family of proteins that also includes Munc13-1 and Munc13-2, the question arises whether bidirectional regulation of the level of protein expression by age and dark rearing is specific to Munc13-3. Figure 3 shows western blot results for Munc13-1 and Munc13-2 in normal and dark-reared mouse visual cortex at 3.5 and 9.5 weeks. Levels of Munc13-1 and Munc13-2 expression were similar in all rearing/age conditions and analysis of variance of densitometric results indicated no statistically significant interaction due to age and dark rearing. Bidirectional regulation of expression was specific to Munc13-3 and did not occur with the other members of the gene family.
Figure 3.
Bidirectional regulation of expression was specific to Munc13-3. Western blot and densitometric results for Munc13-1 and Munc13-2 in mouse visual cortex are shown. Conventions as in figure 2.
Discussion
The present results indicate that Munc13-3 protein shows opposite direction differences in expression due to age and dark rearing in mouse visual cortex. Munc13-3 expression is greater in dark-reared than normal visual cortex at the peak of the critical period and is greater in normal than dark-reared visual cortex at the nadir of the critical period. Bidirectional regulation of the levels of visual cortical expression have also been demonstrated in rodent visual cortex for other synaptic plasticity related molecules, including Dab-1 (Yang et al., 2006) and cpg15 (Lee and Nedivi, 2002).
Is Munc13-3 a plasticity repressor?
Electrophysiologically, visual cortex is more plastic in normal than dark reared visual cortex at the peak of the critical period and is more plastic in dark-reared than normal visual cortex at the nadir of the critical period (Mower, 1991; Beaver et al. 2002). In a differential gene screen of normal and dark-reared cat visual cortex at the peak and nadir of the critical period, two different patterns of bidirectional regulation of gene expression were uncovered (Yang et al., 2001, 2002, 2006). One pattern, elevation in normal at the peak of the critical period and elevation in dark-reared at the nadir, is the pattern indicated by electrophysiological results (Mower, 1991; Beaver et al., 2001). This expression profile could represent genes that promote neuronal plasticity. Other genes show an opposite pattern of bidirectional regulation, elevation in dark-reared at the peak of the critical period and elevation in normal at the nadir of the critical period. Munc13-3 shows this pattern, which could represent genes that repress neuroplasticity.
What do these two complementary patterns of bidirectional regulation of gene expression due to age and dark rearing represent? The importance of a balance between activator and repressor genes has been documented in a number of systems such as tumorigenesis, axon guidance, synaptic growth and learning and memory (Abel et al., 1998). Visual cortical plasticity has historically been attributed to excitatory neuronal activation mechanisms, but recent evidence has indicated that repressor processes also play an important role. Chondroitin sulphate proteoglycans (CSPGs), a part of the extracellular matrix, have been implicated as inhibitory for visual cortical experience dependent plasticity. CSPGs organize at a time coincident with the end of the critical period, and more importantly, degradation of CSPGs in the adult restores ocular dominance plasticity (Pizzorusso et al., 2002). Similarly, Nogo is a myelin derived protein that impedes recovery after trauma, and in visual cortex, mutation of the Nogo receptor prolongs the critical period for monocular deprivation (McGee et al., 2005).
Inhibition mediated by γ-amino butyric acid (GABA) has also been implicated as a plasticity repressor. The GABA system in visual cortex [synthetic enzymes and cellular localization (Guo et al., 1997), receptors (Shaw et al., 1986), receptor subunit composition (Chen et al., 2001) and electrophysiological function (Komatsu, 1983)] develops with a prolonged time course during early postnatal life and reaches mature levels late in the critical period. GABA inhibition has been postulated to be responsible for the decline in plasticity (Komatsu, 1983; Artola and Singer, 1987) and to act as a “plasticity gate” (Kirkwood and Bear, 1994). More direct evidence for GABA inhibition as a plasticity repressor came from study of mice engineered to overexpress brain derived neurotrophic factor (BDNF). These animals show precocious maturation of the GABA synthetic enzyme glutamic acid decarboxlase 65 (GAD65) and also show a premature closure of the visual cortical critical period (Huang et al., 1999). Other evidence indicates a more complex role for GABA inhibition (Hensch et al., 1998; Fagiolini and Hensch, 2000; Iwai et al., 2003). GAD65 knockout mice show reduced plasticity throughout the critical period but subtle pharmacological stimulation of postsynaptic GABA receptors in GAD65 mutants restores plasticity. These findings have been interpreted to mean that a threshold of inhibition, in conjunction with excitation, is necessary to activate plasticity mechanisms (Hensch and Fagiolini, 2005). The combined results of these BDNF and GAD65 gene manipulation results suggest that inhibition plays a role both in activating plasticity mechanisms when they are low and in repressing plasticity mechanisms when they are high. It appears that an immature threshold level of GABA inhibition cooperates with excitation to trigger the rise of the critical period but a mature suprathreshold level of inhibition opposes excitation to close the critical period. The two different classes of genes that show complementary patterns of bidirectional regulation due to age and dark rearing could similarly act both as cooperative partners and opposing factors at different time points of the critical period.
Munc13s and neuronal plasticity
Munc13 isoforms, mammalian homologues of the C. elegans uncoordinated gene (unc-13), play an important role in synaptic plasticity. All three Munc13 genes are phorbol ester receptors that bind syntaxin, are specifically targeted to presynaptic active zones, and play an essential role in synaptic vesicle release (Augustin et al., 1999; Betz et al., 1997, 1998; Brose et al., 1995). Munc13-1 deletion mouse mutants die near birth, but primary hippocampal cultures from these Munc13 mutant mice indicate an inability of docked vesicles to fuse in response to physiological stimuli and this effect is evident in glutamatergic and GABAergic neurons (Augustin et al., 1999b; Varoqueaux et al., 2002). Study of Munc13-1, Munc13-2 and combined mutants in the hippocampus indicate that differential expression of Munc13 isoforms occurs within individual synapses and may be a mechanism that controls short-term plasticity by producing a mix of facilitating and depressing synapses (Rosenmund et al., 2002). This result has several potential implications. First, different types of synapses formed by the same axon may allow a given nerve cell to transmit different information to different target cells. Second, transforming depressing synapses into augmenting ones or vice versa by exchanging the Munc13 isoforms may represent an mechanism to induce lasting changes in synaptic efficacy that result from development and environmental input. Such mechanisms should be relevant to the ocular dominance shifts that define the visual cortical critical period.
Munc13-3 mutants have been studied in cerebellum. These mutants thrive and show grossly normal brain morphology, but there are impairments of synaptic transmission at cerebellar synapses and of motor learning (Augustin et al., 2001). Munc13-3 mutants show enhanced paired pulse facilitation at parallel fiber-Purkinje cell synapses which is indicative of decreased neurotransmitter release probability (Zucker, 1989), suggesting that normally Munc13-3 functions to increase release probability and decrease paired pulse facilitation. Conversely, mutation of BDNF, a molecule which promotes neuronal plasticity (Berardi and Maffei, 1999), results in decreased paired pulse facilitation at parallel fiber-Purkinjie cell synapses (Carter et al., 2002). Thus, the data in cerebellum indicate that Munc13-3 acts to reduce synaptic plasticity, consistent with a potential repressor role in the visual cortical critical period. Firm conclusions must await determination of the function of Munc13-3 in visual cortex. The present results provide the basis for functional analysis of visual cortical critical period plasticity in Munc13-3 mutant mice. Overall, growing evidence indicates a role of Munc13 genes in presynaptic transmission and plasticity and Munc13-3 protein expression is a promising mechanism for regulating activity dependent critical period plasticity of visual cortex.
Acknowledgments
This work was supported by NIH R01 EY016724 and NSF 0090777.
Abbreviations
- BDNF
brain derived neurotrophic factor
- ECL
enhanced chemiluminescence
- D
dark reared
- CSPG
chondroitin sulphate proteoglycan
- GABA
γ-amino butyric acid
- GAD65
glutamic acid decarboxylase 65
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- kDa
kiloDalton
- N
normally reared
- SDS
sodium dodecyl sulphate
- TBST
tris buffered saline with 0.1% Tween 20
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Augustin I, Betz A, Herrmann C, Jo T, Brose N. Differential expression of two novel Munc13 proteins in rat brain. Biochem J. 1999a;337:363–371. [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999b;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Augustin I, Korte S, Rickmann M, Kretzschmar HA, Sudhof TC, Herms JW, Brose N. The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice. J Neurosci. 2001;21:10–17. doi: 10.1523/JNEUROSCI.21-01-00010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q-H, Daw NW. Layer differences in the effect of monocular vision in light and dark reared kittens. Vis Neurosci. 2001;18:811–820. doi: 10.1017/s0952523801185147. [DOI] [PubMed] [Google Scholar]
- Berardi N, Maffei L. From visual experience to visual function: roles of neurotrophins. J Neurobiol. 1999;41:119–126. [PubMed] [Google Scholar]
- Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J Biol Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- Brose N, Hofmann K, Hata Y, Sudhof TC. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270:25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- Carter AR, Chen C, Schwartz PM, Segal RA. Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J Neurosci. 2002;22:1316–1327. doi: 10.1523/JNEUROSCI.22-04-01316.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang C, Mower GD. Developmental changes in the expression of GABA(A) receptor subunits (alpha(1), alpha(2), alpha(3)) in the cat visual cortex and the effects of dark rearing. Mol Brain Res. 2001;88:135–143. doi: 10.1016/s0169-328x(01)00042-0. [DOI] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark reared cats. J Neurophysiol. 1980;43:1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Daly C, Ziff EB. Post-transcriptional regulation of synaptic vesicle protein expression and the developmental control of synaptic vesicle formation. J Neurosci. 1997;17:2365–2375. doi: 10.1523/JNEUROSCI.17-07-02365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NW. Mechanisms of plasticity in the visual cortex. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994;35:4168–4179. [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci USA. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guire ES, Lickey ME, Gordon B. Critical period for the monocular deprivation effect in rats: assessment with sweep visually evoked potentials. J Neurophysiol. 1999;81:121–128. doi: 10.1152/jn.1999.81.1.121. [DOI] [PubMed] [Google Scholar]
- Guo Y, Kaplan IV, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of the cat visual cortex. Dev Brain Res. 1997;103:127–141. doi: 10.1016/s0165-3806(97)81789-0. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–24. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaysu Y. Development of cortical inhibition in kitten striate cortex investigated by a slice preparation. Dev Brain Res. 1983;8:136–139. [Google Scholar]
- Lee WC, Nedivi E. Extended plasticity of visual cortex in dark-reared animals may result from prolonged expression of cpg15-like genes. J Neurosci. 2002;22:1807–1815. doi: 10.1523/JNEUROSCI.22-05-01807.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. Comparison of the effects of dark rearing and binocular suture on development and plasticity of cat visual cortex. Brain Res. 1981;220:255–267. doi: 10.1016/0006-8993(81)91216-6. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Rosen KM, McCormack MA, Villa-Komaroff L, Mower GD. Brief visual experience induces immediate early gene expression in the cat visual cortex. Proc Nat Acad Sci USA. 1992;89:5437–5411. doi: 10.1073/pnas.89.12.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Sigler A, Augustin I, Reim K, Brose N, Rhee JS. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron. 2002;33:411–424. doi: 10.1016/s0896-6273(02)00568-8. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel M, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in the adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Shaw C, Wilkinson M, Cynader M, Needler MC, Aoki C, Hall SE. The laminar distributions and postnatal development of neurotransmitter and neuromodulator receptors in cat visual cortex. Brain Res Bull. 1986;16:661–71. doi: 10.1016/0361-9230(86)90137-1. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982;62:738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Silver B, Ellis SR, Mower GD. Bidirectional regulation of mitochondrial gene expression during developmental neuroplasticity of visual cortex. Biochem Biophys Res Commun. 2001;287:1070–1074. doi: 10.1006/bbrc.2001.5706. [DOI] [PubMed] [Google Scholar]
- Yang CB, Zheng YT, Li GY, Mower GD. Identification of Munc13-3 as a candidate gene for critical period neuroplasticity in visual cortex. J Neurosci. 2002;22:8614–8618. doi: 10.1523/JNEUROSCI.22-19-08614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CB, Zheng YT, Kiser PJ, Mower GD. Identification of disabled-1 as a critical period neuroplasticity gene in cat and mouse visual cortex. Eur J Neurosci. 2006;23:2804–2808. doi: 10.1111/j.1460-9568.2006.04799.x. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]