Abstract
Nonconjugative plasmids were transferred by protoplast fusion among Streptococcus faecalis strains and from Streptococcus sanguis to S. faecalis. S. faecalis protoplasts were also transformed with several different plasmids, including the Tn917 delivery vehicle pTV1. Transformation was reproducible, but low in frequency (10(-6) transformants per viable protoplast). A new shuttle vector (pAM610), able to replicate in Escherichia coli and S. faecalis, was constructed and transformed into S. faecalis protoplasts. pAM610 was mobilized by the conjugative plasmid pAM beta 1 in matings among S. faecalis strains and from S. sanguis to S. faecalis. Chimeric derivatives of pAM610 were also transformed into S. faecalis.
Full text
PDF
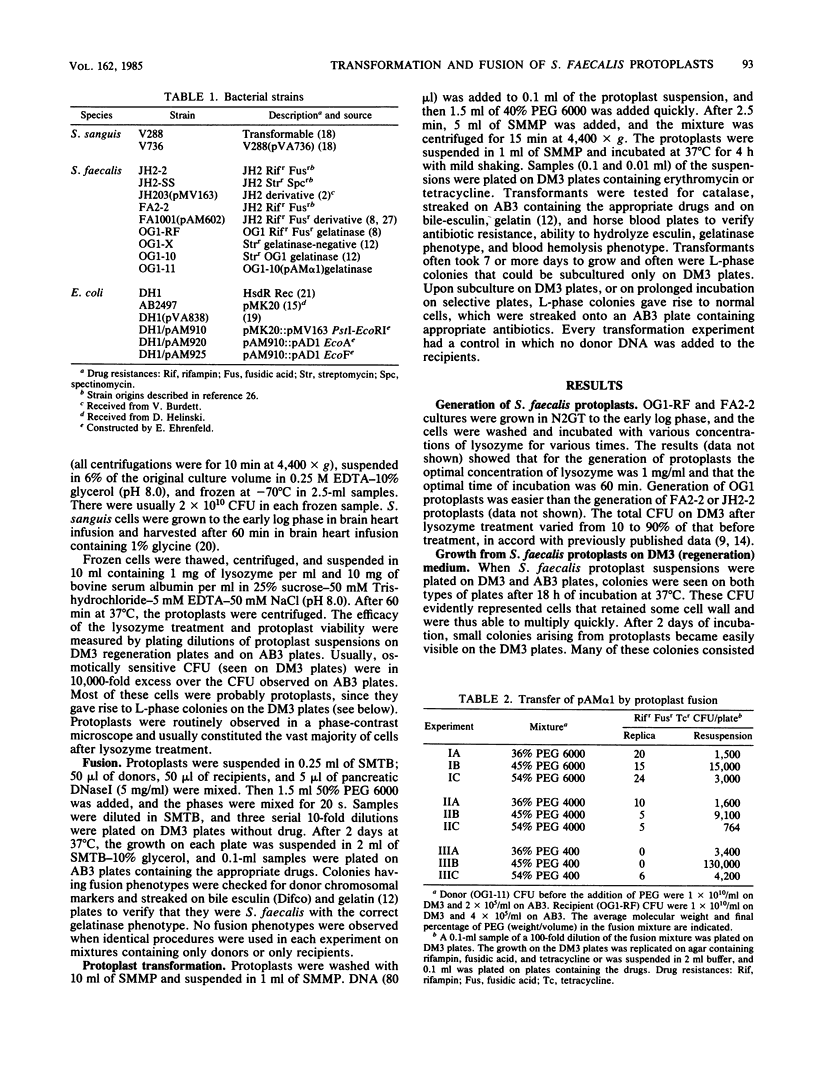
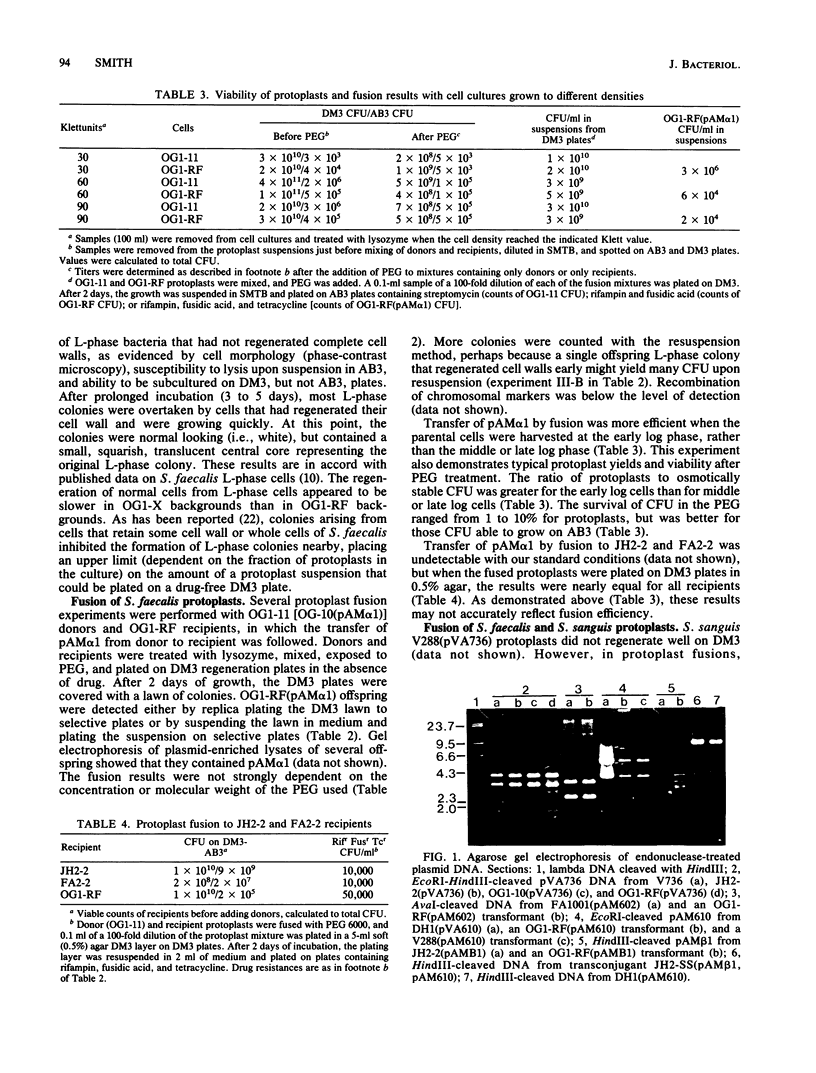
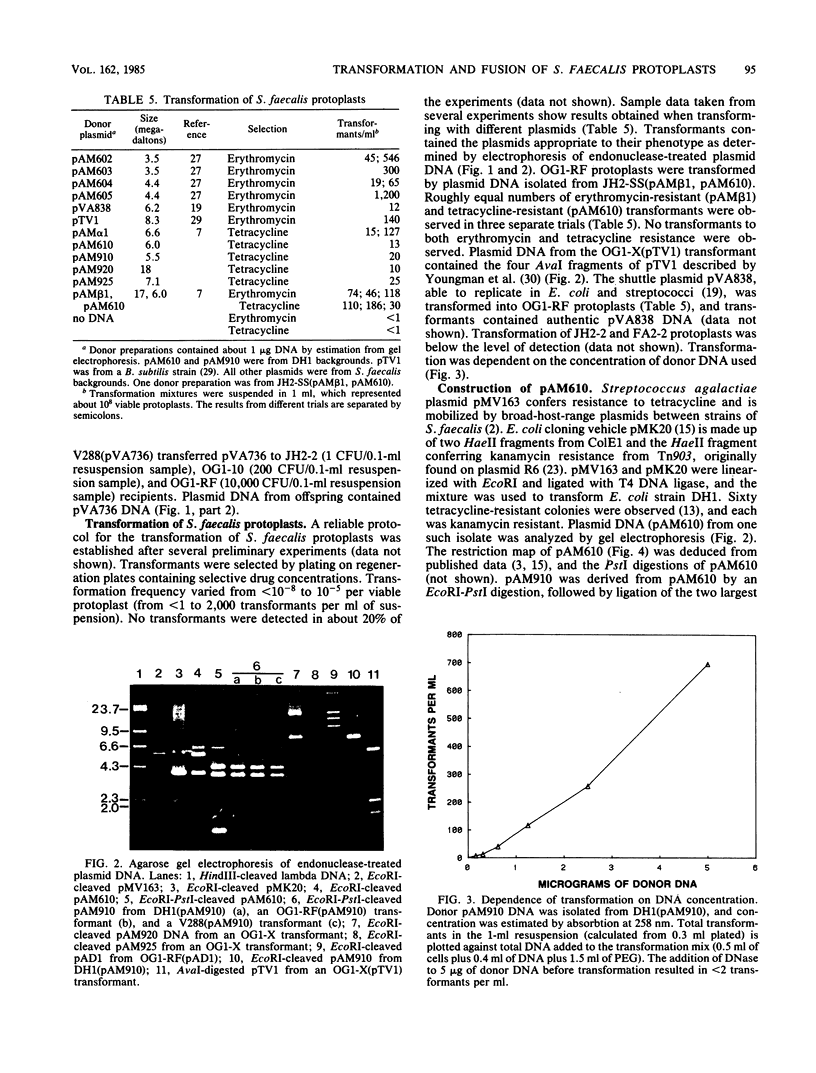
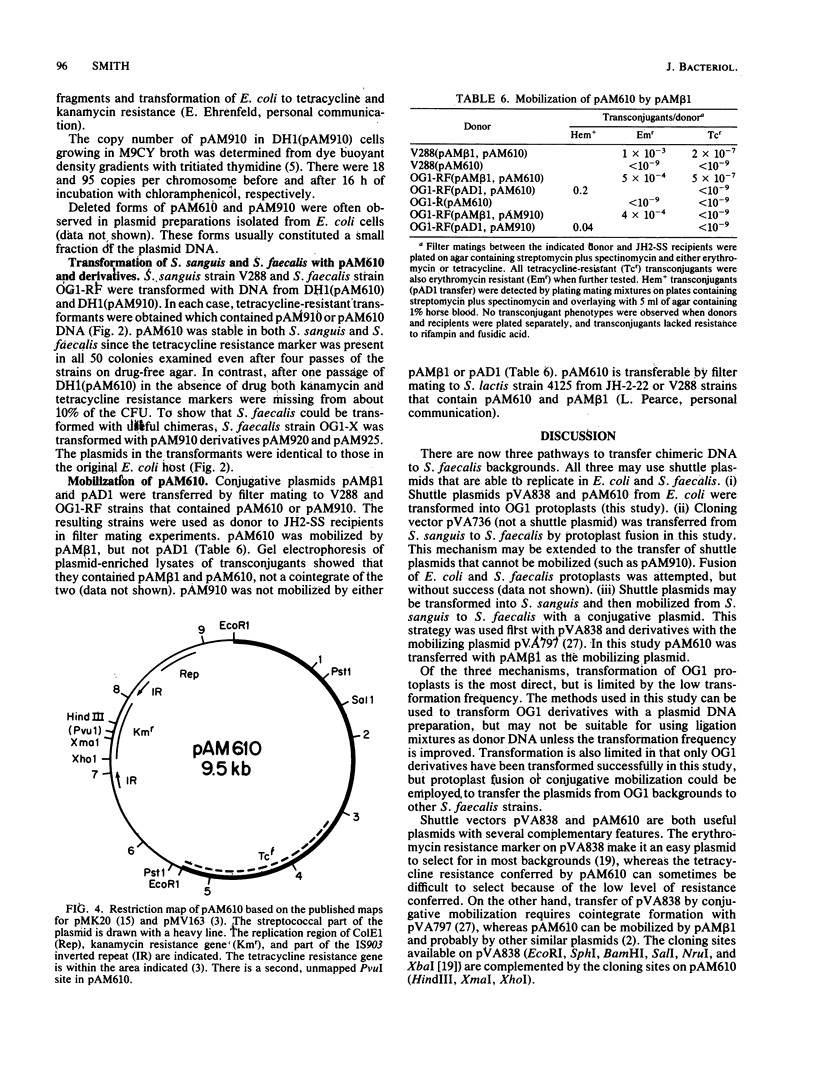

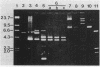
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1980 Nov;18(5):753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Ike Y., Craig R. A., White B. A., Yagi Y., Clewell D. B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. R., Gooder H. Reversion to the streptococcal state of enterococcal protoplasts, spheroplasts, and L-forms. J Bacteriol. 1970 Sep;103(3):692–696. doi: 10.1128/jb.103.3.692-696.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Kondo J. K., McKay L. L. Transformation of Streptococcus lactis Protoplasts by Plasmid DNA. Appl Environ Microbiol. 1982 May;43(5):1213–1215. doi: 10.1128/aem.43.5.1213-1215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J. W., Gooder H. Growth and development of competence in the group H streptococci. J Bacteriol. 1970 Jun;102(3):820–825. doi: 10.1128/jb.102.3.820-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Wood P. H. Chimeric streptococcal plasmids and their use as molecular cloning vehicles in Streptococcus sanguis (Challis). J Bacteriol. 1980 Sep;143(3):1425–1435. doi: 10.1128/jb.143.3.1425-1435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Simple method for demonstrating small plasmid deoxyribonucleic acid molecules in oral streptococci. Appl Environ Microbiol. 1980 May;39(5):1070–1073. doi: 10.1128/aem.39.5.1070-1073.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraro R. V., Pfister R. M., Rheins M. S. Growth inhibition of bacterial variants. Can J Microbiol. 1970 Jul;16(7):640–642. doi: 10.1139/m70-109. [DOI] [PubMed] [Google Scholar]
- Nomura N., Yamagishi H., Oka A. Isolation and characterization of transducing coliphage fd carrying a kanamycin resistance gene. Gene. 1978 Feb;3(1):39–51. doi: 10.1016/0378-1119(78)90006-9. [DOI] [PubMed] [Google Scholar]
- PERRY D., SLADE H. D. Transformation of streptococci to streptomycin resistance. J Bacteriol. 1962 Mar;83:443–449. doi: 10.1128/jb.83.3.443-449.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Cami B., Hotchkiss R. D. Fusion of bacterial protoplasts. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2151–2155. doi: 10.1073/pnas.73.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Clewell D. B. Return of Streptococcus faecalis DNA cloned in Escherichia coli to its original host via transformation of Streptococcus sanguis followed by conjugative mobilization. J Bacteriol. 1984 Dec;160(3):1109–1114. doi: 10.1128/jb.160.3.1109-1114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Guild W. R. Improved method for conjugative transfer by filter mating of Streptococcus pneumoniae. J Bacteriol. 1980 Oct;144(1):457–459. doi: 10.1128/jb.144.1.457-459.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M. L., Pattee P. A. Computer-assisted chromosome mapping by protoplast fusion in Staphylococcus aureus. J Bacteriol. 1983 Apr;154(1):395–405. doi: 10.1128/jb.154.1.395-405.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P. J., Perkins J. B., Losick R. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2305–2309. doi: 10.1073/pnas.80.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]