Abstract
This paper describes the use of laminar flow of liquids in capillary systems to pattern the cell culture substrate, to perform patterned cell deposition, and to pattern the cell culture media. We demonstrate the patterning of the cell culture substrate with different proteins, the patterning of different types of cells adjacent to each other, the patterned delivery of chemicals to adhered cells, and performing enzymatic reactions over select cells or over a portion of a cell. This method offers a way to simultaneously control the characteristics of the surface to which cells are attached, the type of cells that are in their vicinity, and the kind of media that cells or part of a cell are exposed to. The method is experimentally simple, highly adaptable, and requires no special equipment except for an elastomeric relief that can be readily prepared by rapid prototyping.
Efforts to understand the interaction of cells in culture with their environment would benefit from general procedures for patterning both the position of the cells and the characteristics of the environment—that is, the molecular structure of the surface to which the cells are attached, the nature and position of other cells in their vicinity, and the composition of the fluid medium surrounding them. Here we describe procedures that use the laminar flow of multiple parallel liquid streams in rectangular capillaries to pattern the surface of the substrate with adhesion promoters and inhibitors, to deliver cells to the surface of the substrate in patterns, and to localize chemicals—fluorescent labels, nutrients, growth factors, toxins, enzymes, drugs—available to attached cells in the medium. We believe that these procedures will enable new types of studies in fundamental cell biology and cellular metabolism, and that they will also be useful in the fabrication of analytical systems that use cells as sensors.
These methods rely on the fact that the flow of liquids in capillaries often has a low Reynolds number (Re) and is laminar (1–4). When two or more streams with low Re are joined into a single stream, also with low Re, by using a junction of appropriate connectivity, the combined streams flow parallel to each other without turbulent mixing. This ability to generate and sustain parallel streams of different solutions in capillaries provides the capability required to pattern: (i) the substrate—by adsorption of adhesion promoters and inhibitors; (ii) the location of the cells—by exposure of patterned substrate to a suspension of cells or by selective deposition of cells onto an unpatterned substrate from laminar streams; or (iii) the medium—by patterned flow. These methods easily generate patterns of parallel stripes, but by using appropriately designed capillary systems (5–7) or multistep procedures, it is possible to generate more complex patterns.
MATERIALS AND METHODS
Device Fabrication.
A negative relief of poly(dimethylsiloxane) (PDMS) was formed by curing the prepolymer (Sylgard 184, Dow-Corning) on a silanized Si master having a positive relief (55 μm) of the capillary channels formed in photoresist (SU-8–50, MicroChem) on its surface (5). This PDMS membrane with a negative relief was placed on a Petri dish (bacteriological, VWR Scientific) resulting in formation of the capillary channels. The PDMS membrane seals against the Petri dish by conformal contact. We found no need to make the PDMS hydrophilic by plasma oxidation or other methods before use.
Cell Cultures.
Escherichia coli (strain designated RB 128) were grown for 18 hr at 37°C in M9 media (42 mM sodium phosphate dibasic/22 mM potassium phosphate monobasic/18.7 mM ammonium chloride/8.5 mM sodium chloride/1 mM magnesium sulfate heptahydrate/11.1 mM glucose) to an OD600 of 1.2. RB 128 is a clinical isolate from patients with acute pyelonephritis. The strains were obtained from Shaw Warren at Massachusetts General Hospital (Boston MA).
Chicken erythrocytes (12 day old, 5 ml cells in 165 ml Alsever’s solution) were obtained from SPAFAS (Preston, CT) and were used as received.
Bovine adrenal capillary endothelial (BCE) cells were cultured and harvested as previously reported (8). In brief, cells were cultured under 10% CO2 on Petri dishes (Falcon) coated with gelatin in DMEM (GIBCO) containing 10% calf serum, 2 mM glutamine, 100 μg/ml streptomycin, 100 μg/ml penicillin, 1 ng/ml basic fibroblast growth factor (bFGF). Cells were dissociated from culture plates with trypsin/EDTA and washed in DMEM containing 1% wt/vol BSA (BSA/DMEM). These cells were suspended in chemically defined medium (10 μg/ml high density lipoprotein/5 μg/ml transferrin/5 ng/ml bFGF in BSA/DMEM) (9), introduced into capillary networks (pretreated with 50 μg/ml fibronectin for 1 hr) from the reservoirs, and incubated in 10% CO2 at 37 °C for 4–6 hr before patterning.
Chemicals and Proteins.
BSA, BSA colabeled with α-d-mannopyranosyl phenylisothiocyante and FITC (man–FITC–BSA) and phosphate-buffered saline pouch (pH 7.4) was obtained from Sigma. Trypsin/EDTA (0.05% trypsin/0.53 mM EDTA) was obtained from GIBCO. Syto 9 fluorescent nucleic acid stain was obtained from Molecular Probes.
RESULTS AND DISCUSSION
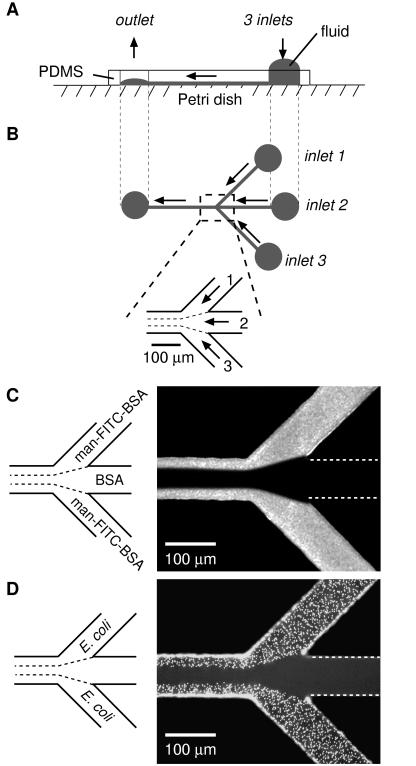
A representative procedure is outlined in Fig. 1. We fabricated a network of capillaries having three inlet channels that converge into a single main channel by bringing a PDMS membrane with the pattern of channels molded into its surface into contact with the flat surface of a polystyrene Petri dish (Fig. 1 A and B). (Other substrates—PDMS, glass, Si/SiO2, and evaporated gold films on glass—can also be used.) The capillary system was initially filled with H2O by filling the inlet reservoirs and applying vacuum at the outlet. Once liquid was flowing smoothly through the channels, a solution of a fluorescent neoglycoprotein (man–FITC–BSA, 0.5 mg/ml in PBS) was placed in inlets 1 and 3, and a solution of BSA (10 mg/ml in PBS) was placed in inlet 2; these solutions were allowed to flow into the main channel under the influence of gentle aspiration at the outlet. Proteins adsorbed nonspecifically to the regions of the surface over which the solutions containing them flowed. Fluorescence microscopy visualized the resulting pattern (Fig. 1C). The system of capillaries was then filled with a suspension of E. coli RB 128, a strain that has been shown to bind to mannose-presenting surfaces (M.N.L. and G.M.W., unpublished results). Cells that did not adhere strongly were washed away with PBS; the remaining adherent cells were visualized with a fluorescent nucleic acid stain (Fig. 1D). E. coli RB 128 adhered only to those portions of the channel that had been patterned by adsorption of man–FITC–BSA (compare Fig. 1 C and D).
Figure 1.
Schematic representation of a laminar flow patterning experiment. (A) Side view perpendicular to the central channel and in the plane of the Petri dish surface. A PDMS membrane containing micron-sized channels molded in its surface was placed on the flat surface of a Petri dish to form a network of capillaries. Fluids were pumped by gentle aspiration at the outlet, or by pressure created at the inlet reservoirs by gravity acting on the liquids in them. (B) Top view of the capillary network. Micrographs show the junction where the inlets converge. (C) Patterns of adsorbed protein created by laminar flow. Solutions of man–FITC–BSA (0.5 mg/ml in PBS) and BSA (10 mg/ml in PBS) were allowed to flow from the designated inlets into the main channel for 15 min under gentle aspiration, and the system was washed for 3 min with PBS. (D) The channels shown in C were filled with a suspension of E. coli RB 128 and allowed to stand for 10 min to allow adhesion; nonadherent cells were removed by washing with PBS. Cells were visualized with a fluorescent nucleic acid stain (Syto 9, 15 μM in PBS). Both micrographs were taken from the top of the capillary network looking through the PDMS. White dotted lines identify channels not visible with fluorescence microscopy.
The types of patterns that can be formed by the delivery of molecules and cells within capillary channels by using laminar flows are determined by the physics of these flows (1–4). By using channels with a height of ≈55 μm and widths of 75–300 μm at flow rates that are conveniently generated and controlled by gravity or gentle aspiration (0.01–10 cm/sec, calculated from volumetric flow rates based on an approximation that the fluid flow is plug-like), it is straightforward to generate flows with Re < 1 (the transition to turbulent flow is commonly assumed to occur around Re ≈ 2,000) (1–4). When two liquids flow in parallel at low values of Re, there is no turbulence at the interface between them; thus, blurring of the interface between the fluid streams occurs primarily by diffusion of particles across that interface (1–4, 10). With small particles that diffuse rapidly—for example, sucrose (molecular weight 342, diffusion coefficient D ≈ 5 × 10−6 cm2/s) (11)—diffusional broadening at the liquid/liquid interface between two aqueous streams flowing at 10 cm/sec will be ≈10 μm after 1 cm (11). Larger particles such as proteins and cells mix by diffusion more slowly: BSA, for example, has a D ≈ 6 × 10−7 cm2/s (11) and will diffuse ≈0.6 μm in an aqueous solution at a flow rate of 10 cm/sec; for a flow rate of 0.1 cm/sec, diffusion will be ≈6 μm after 1 cm. Cells—particles with diameters of ≈0.5 μm or more—will diffuse two orders of magnitude less rapidly than BSA (4, 11).
The width of the flowing streams can be readily controlled at values between 5 and 100 μm by adjusting the relative volumetric flow rates of the streams that combine. It is thus straightforward to generate patterns of three to five stripes (of adsorbed molecules and/or cells or of fluid streams containing molecules or cells) in a 75-μm-wide capillary, with stripes having widths of tens of microns and with the interfaces between neighboring flows localized to within a few microns.
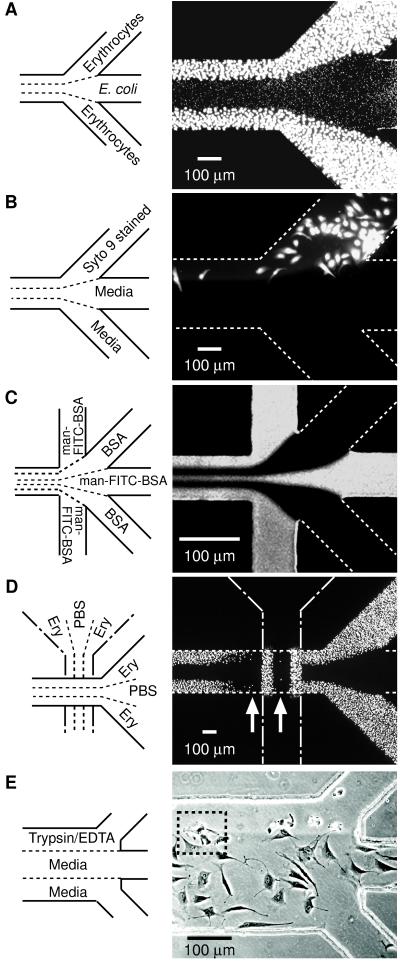
Fig. 2 shows representative patterns that can be formed by using multiple laminar flows. In Fig. 2A, two different cell types are deposited next to each other. In contrast to the pattern of cells in Fig. 1D, where the pattern of cells arises from the underlying pattern of proteins, the pattern of cells in Fig. 2A results from the selective deposition of cells from a patterned flow of suspended cells. Fig. 2B shows the selective labeling of BCE cells by using a fluorescent reagent. In this experiment, BCE cells covered the entire bottom face of the capillary; this fluorescence micrograph shows selective labeling only of those cells over which a solution of the reagent flowed. Fig. 2C shows a junction that combined five streams—three containing man–FITC–BSA and two containing BSA—in a 75-μm channel. Fig. 2D shows a pattern of erythrocytes obtained by performing two sequential laminar flow depositions rotated 90° from each other. The first array of erythrocytes (vertical) affects the second deposition of erythrocytes (horizontal); the difference between the two is most obvious in the observation of less adhesion of erythrocytes in regions immediately downstream of the first erythrocyte pattern than in other regions (see arrows in Fig. 2D). Fig. 2E demonstrates patterned release of attached BCE cells by using trypsin/EDTA. Digestion of fibronectin on the channel surface and sequestering of calcium by EDTA causes cells to detach and contract (12). When a solution of trypsin/EDTA flowed over only a portion of a cell, the treated part of the cell detached and contracted; the untreated part remained spread (for example, see inside dotted square in Fig. 2E). Because no physical barriers are required to separate the different liquid streams, different liquids can flow over different portions of a single cell.
Figure 2.
Examples of types of patterns that can be formed by laminar flow patterning. (A) Two different cell types patterned next to each other. A suspension of chicken erythrocytes was placed in inlets 1 and 3, and PBS in inlet 2 and allowed to flow by gravitational force for 5 min followed by a 3-min PBS wash; this flow formed the pattern of bigger cells (outer lanes). Next, a suspension of E. coli (RB 128) was placed in inlet 2 and PBS in inlets 1 and 3 and allowed to flow by gravitational forces for 10 min followed by a 3-min PBS wash; this flow created the pattern of smaller cells (middle lane). Both cell types adhered to the Petri dish by nonspecific adsorption. Cells were visualized with Syto 9 (15 μM in PBS). (B) Pattern of selectively stained BCE cells. BCE cells suspended in chemically defined medium (≈106 cells per ml) were introduced into the capillary network (pretreated with fibronectin) and incubated in 10% CO2 at 37 °C for 4 hr. After removing nonadherent cells by washing with media (1% wt/vol BSA/DMEM), Syto 9 (15 μM in BSA/DMEM) and media were allowed to flow from the designated inlets for 5 min under gentle aspiration, and the system was washed for 3 min with media. (C) Pattern obtained at a junction where five inlets converge into a 75-μm channel (see caption Fig. 1C for procedures). (D) Crisscross patterning of chicken erythrocytes. Erythrocytes were patterned initially in the vertical direction (procedure same as A). The PDMS membrane used in this initial patterning was demounted and a different PDMS membrane placed in a direction 90° rotated from the first pattern (that is, horizontal). The capillary system created by the second PDMS membrane was used to pattern erythrocytes in the horizontal (right-to-left) direction. The resulting pattern of erythrocytes in the second capillary system was visualized by Syto 9 (15 μM in PBS). (E) Patterned detachment of BCE cells by treatment with trypsin/EDTA. Cells were allowed to adhere and spread in a fibronectin-treated capillary network for 6 hr and nonadherent cells removed by washing. Trypsin/EDTA and media were allowed to flow from the designated inlets for 12 min by gravity. A–D are fluorescence micrographs taken from the top through PDMS. E is a phase-contrast image observed by an inverted microscope looking through the polystyrene Petri dish. White dotted lines identify channels not visible with fluorescence microscopy.
The ability to pattern adsorbed proteins (or other adhesion promoters or inhibitors), cells, and fluid streams containing labels, enzymes, or biologically active molecules by using simple experimental procedures offers a broadly useful new capability to biology. A number of methods have been demonstrated previously that pattern adhesion promoters on surfaces by using soft lithographic or photolithographic techniques (13–18). The procedures described in this paper are complementary: they produce less complex patterns when used for surface modification, but they have six characteristics that make them especially versatile: (i) They take advantage of the easily generated multiphase laminar flows to pattern fluids and to deliver components for patterning. The design and generation of patterns in capillaries is straightforward (5), and the method uses readily controlled interfacial processes—especially adsorption of proteins and cells—to generate the desired patterns. (ii) They are experimentally simple. Multiple-component patterns can be made in a short sequence of steps, without the need for multiple stages of pattern transfer with registration required by other patterning methods. (iii) They use PDMS. There are a number of methods to fabricate capillary networks conveniently in this material (5–7). The capillary networks are readily assembled and disassembled by using nonadhesive contact between molded PDMS and a flat surface, allowing direct access to the cells after an experiment or sequential patterning in multiple directions on a single substrate (as in Fig. 2D). Biological applications also benefit from the optical transparency and gas permeability of PDMS. (iv) They generate patterns inside the channels of microfluidic networks and are thus directly compatible with microfluidic systems designed for studying metabolism, or as sensors. (v) They can pattern over delicate structures such as mammalian cells. This type of patterning is not possible by other techniques. (vi) They can pattern the growth medium itself; no lithographic process has this capability. The principal limitation of the methodology is that it is most directly applicable to the generation of patterns of parallel stripes and is not generalizable to all patterns; certain more complex patterns can be produced but at the cost of additional steps.
We believe that these methods will enable the fabrication of patterned surfaces and fluids useful in studies of cell-surface adhesion, chemotaxis, haptotaxis, cell–cell communication, and cellular ecology; these methods will also be useful in fabricating microfluidic systems for studies of metabolism and toxicology, for screening by using arrays of cells, and in cell-based sensors.
Acknowledgments
Supported by the Defense Advanced Research Planning Agency (DARPA)/Space and Naval Warfare Systems Command, DARPA/Office of Naval Research, the National Science Foundation (NSF ECS-9729405), and the National Institutes of Health (NIH GM30367). We thank Prof. D. E. Ingber (Harvard Medical School) for use of his facilities and Dr. S. Warren (Massachusetts General Hospital) for the gift of E. coli (RB 128). S. T. is a Leukemia Society of America Fellow and thanks the society for a postdoctoral fellowship. J. C. M. thanks the National Science Foundation for a predoctoral fellowship. P. J. A. K. thanks the Netherlands Organization for Scientific Research (NWO) for a postdoctoral fellowship.
ABBREVIATIONS
- Re
Reynolds number
- BCE
bovine capillary endothelial
- PDMS
poly(dimethylsiloxane)
- man
mannopyranosyl phenylisothiocyante
References
- 1.Kovacs G T A. Micromachined Transducers Sourcebook. Boston: WCB/McGraw–Hill; 1998. [Google Scholar]
- 2.Brody J P, Yager P, Goldstein R E, Austin R H. Biophys J. 1996;71:3430–3441. doi: 10.1016/S0006-3495(96)79538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel S. Life in Moving Fluids. Princeton: Princeton University Press; 1994. [Google Scholar]
- 4.Weigl B H, Yager P. Science. 1999;283:346–347. [Google Scholar]
- 5.Duffy D C, McDonald J C, Schueller O J A, Whitesides G M. Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Whitesides G M. Angew Chem Int Ed Engl. 1998;37:551–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Qin D, Xia Y, Whitesides G M. Adv Mater. 1996;8:917–919. [Google Scholar]
- 8.Ingber D E, Folkman J. J Cell Biol. 1989;109:317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingber D E. Proc Natl Acad Sci USA. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight J B, Vishwanath A, Brody J P, Austin R H. Phys Rev Lett. 1998;80:3863–3866. [Google Scholar]
- 11.Atkins P W. Physical Chemistry. New York: Freeman; 1994. [Google Scholar]
- 12.Saunders S, Bernfield M. J Cell Biol. 1988;106:423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mrksich M, Whitesides G M. Annu Rev Biophys Biomol Struct. 1996;25:55–78. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 14.St. John P M, Davis R, Cady N, Czajka J, Batt C A, Craighead H G. Anal Chem. 1998;70:1108–1111. doi: 10.1021/ac9711302. [DOI] [PubMed] [Google Scholar]
- 15.Bernard A, Delamarche E, Schmid H, Michel B, Bosshard H R, Biebuyck H. Langmuir. 1998;14:2225–2229. [Google Scholar]
- 16.Chen C S, Mrksich M, Huang S, Whitesides G M, Ingber D E. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 17.Delamarche E, Bernard A, Schmid H, Michel B, Biebuyck H. Science. 1997;276:779–781. doi: 10.1126/science.276.5313.779. [DOI] [PubMed] [Google Scholar]
- 18.Singhvi R, Kumar A, Lopez G P, Stephanopoulos G N, Wang D I C, Whitesides G M, Ingber D E. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]