Abstract
Stratum corneum comprises corneocytes, derived from outer stratum granulosum during terminal differentiation, embedded in a lipid-enriched extracellular matrix, secreted from epidermal lamellar bodies. Permeability barrier insults stimulate rapid secretion of preformed lamellar bodies from the outer stratum granulosum, regulated through modulations in ionic gradients and serine protease (SP)/protease-activated receptor type 2 (PAR2) signaling. Because corneocytes are also required for barrier function, we hypothesized that corneocyte formation could also be regulated by barrier function. Barrier abrogation by two unrelated methods initiated a wave of cornification, assessed as TdT-mediated dUTP nick end-labeling-positive cells in stratum granulosum and newly cornified cells by electron microscopy. Because cornification was blocked by occlusion, corneocytes formed specifically in response to barrier, rather than injury or cell replacement, requirements. SP inhibitors and hyperacidification (which decreases SP activity) blocked cornification after barrier disruption. Similarly, cornification was delayed in PAR2−/− mice. Although classical markers of apoptosis [poly(ADP-ribose)polymerase and caspase (Casp)-3] remained unchanged, barrier disruption activated Casp-14. Moreover, the pan-Casp inhibitor Z-VAD-FMK delayed cornification, and corneocytes were structurally aberrant in Casp14−/− mice. Thus, permeability barrier requirements coordinately drive both the generation of the stratum corneum lipid-enriched extracellular matrix and the transformation of granular cells into corneocytes, in an SP- and Casp-14-dependent manner, signaled by PAR2.
Formation of the stratum corneum (SC) permeability barrier in terrestrial mammals requires two parallel processes, corneocyte formation and deposition of a highly hydrophobic extracellular matrix. During the final stages of epidermal differentiation, outer stratum granulosum (granular) cells transform into anucleate corneocytes, with highly resilient cornified envelopes.1,2 Immediately before cornification, large quantities of polar lipids are secreted from epidermal lamellar bodies into the extracellular spaces of the SC.3,4,5 These lipids are then catabolized into a nonpolar mixture of ceramides, cholesterol, and free fatty acids,6,7,8 which then organize into a structurally unique system of parallel, lamellar membrane arrays, which pack the SC interstices.6 Many studies have demonstrated the importance of this membrane system for permeability barrier homeostasis, and much is known about the regulation of epidermal lipid synthesis and secretion in response to alterations in permeability barrier status.9 The initial response to acute barrier disruption, independent of method of abrogation, is the rapid (by 30 minutes) secretion of the contents of up to 70% of preformed lamellar bodies, from the outermost layer of granular cells,4,5 after which a variety of synthetic responses up-regulate in the underlying epidermis, which return toward basal levels in parallel with restoration of permeability barrier homeostasis.9
Two signaling mechanisms are known to regulate the lamellar body secretory response in the epidermis underlying disrupted skin sites: an abrupt decline in epidermal calcium levels stimulates secretion of preformed lamellar bodies from the outer granular layer;10,11,12,13,14 and acute barrier disruption also raises the ambient pH of normal SC transiently from its usual acidic levels (∼5.0) toward neutrality, which, in turn, activates serine proteases (SPs) in the outer epidermis, a sequence that retards barrier recovery kinetics.15,16,17 Conversely, either immediate reacidification of SC,18 or applications of SP inhibitors accelerates barrier recovery.15,19 The increase in SP activity that results from either barrier disruption or a discrete increase in the pH of SC, in turn, activates the protease-activated receptor type 2 (PAR2), which is expressed on the plasma membranes of cells of the stratum granulosum.16,20 Accordingly, PAR2 agonist peptides down-regulate lamellar body secretion, thus delaying permeability barrier recovery; and conversely, absence of PAR2 in transgenic PAR2−/− mice accelerates lamellar body secretion and permeability barrier recovery kinetics.16 Thus, SP-PAR2 down-regulation of lamellar body secretion represents a second potentially important regulatory mechanism of permeability barrier homeostasis.
Terminal differentiation results in both the abrupt cessation of epidermal synthetic activity and the blockade of further lamellar body secretion. However, how this terminal differentiation, also termed “physiological apoptosis” or “planned cell death,” is regulated is not known. The association of cornification with expression of caspase 14 (Casp-14), an epidermis-predominant, cysteine protease,21 suggests that this protease could be important for terminal differentiation, but its specific role remains undefined. Although cells transitioning from the outer stratum granulosum into corneocytes are only rarely encountered under basal conditions, we hypothesized that acute abrogation of the permeability barrier, which stimulates immediate secretion of the contents of preformed lamellar bodies,4,5 could coordinately stimulate a wave of new cornification, specifically in response to modulations in permeability status. Moreover, because the SP-mediated activation of PAR2 down-regulates lamellar body secretion, SP-PAR2 signaling could represent a mechanism that regulates the final transformation of outer stratum granulosum cells into corneocytes, allowing coordinate formation of corneocytes and the extracellular matrix of the SC.
Materials and Methods
Materials
Adult male and female hairless mice (Skh1), 8 to 10 weeks of age, were purchased from Charles River Laboratories (Wilmington, MA). Casp14−/− and wild-type mice were generated in the laboratory of Dr. Wim De Clercq (Ghent, Belgium), as described.22 PAR2−/− and wild-type mice were from Dr. Shaun R. Coughlin (University of California–San Francisco, San Francisco, CA). All animals had free access to food and water ad libitum. An affinity-purified, rabbit primary antibody, specific for the active form of Casp-3, was purchased from Sigma-Aldrich (St. Louis, MO). Secondary, fluorescein isothiocyanate-conjugated, goat anti-rabbit IgG was from Invitrogen-Molecular Probes (Carlsbad, CA). The Fluorescein-FragEL apoptosis detection kit and the general Casp inhibitor Z-VAD-FMK were purchased from EMD Biosciences (San Diego, CA). Lactobionic acid (LBA) and the SP inhibitors aprotinin, trans-4-(aminomethyl)cyclohexane carboxylic acid (t-AMCHA), and phenylmethyl sulfonyl fluoride (PMSF) were from Sigma-Aldrich. The Casp-3 antibody was purchased from Biosource (Camarillo, CA), the Casp-14 antibody was from Tebu-Bio (Boechout, Belgium), and the poly(ADP-ribose)polymerase (PARP) antibody was from Abcam (Cambridge, UK).
Animal Model and Tissue Preparation
Epidermal barrier disruption was achieved by two separate methods using either acetone or repeated applications of cellophane tape on the flanks until transepidermal water loss reached 1 ± 0.25 mg/cm2/hour, 2 to 4 mg/cm2/hour, or ≥6 mg/cm2/hour, as determined with an electrolytic water analyzer (Meeco, Warrington, PA). Immediately after barrier disruption, animals (n = 4 to 5 in each group) were treated with 30 μl of t-AMCHA (5%), PMSF (10 mmol/L), aprotinin (2 mg/ml), or LBA (10%) dissolved in propylene glycol:ethanol (7:3 ratio) or vehicle alone. The Casp inhibitor (Z-VAD-FMK, 2 mmol/L) was applied 1 hour before, as well as after, acute barrier disruption. Baseline control groups consisted of nontreated animals (n = 4 to 5). Harvesting of tissue samples was performed at 30 minutes, 1, 3, 6, 8, 18, and 24 hours after the treatment.
Microscopy and Imaging
Skin samples were fixed in 4% formalin, and 6-μm-thick paraffin-stained sections were examined under an Axioplan 2 light microscope (Zeiss, Jena, Germany) at ×20 magnification. Quantitation of TdT-mediated dUTP nick end-labeling-positive (TUNEL+) cells was performed on three to five pictures of each coded sample, taken at ×20 magnification. The total length of epidermis was measured on each picture using the planimeter PlanWheel PC (Scalex Corp., Carlsbad, CA). The ratio of TUNEL+ cells over the total length was then obtained and expressed as TUNEL+ cells per unit length. Using Photoshop software (Adobe Systems, Inc., San Jose, CA), nucleated cell layer thickness was obtained by measuring the distance between the basal membrane and the stratum granulosum/SC junction at 5 to 10 randomized points, on three to five fields of each coded sample. For each experiment, n represents the number of animals in each group. Experiments were repeated at least three times to confirm results.
Immunofluorescence
For Casp-3 immunostaining, frozen sections were fixed for 10 minutes at −20°C in 100% acetone. After incubation in blocking solution [4% bovine serum albumin, 0.5% cold water fish gelatin in phosphate-buffered saline (PBS)], sections were incubated with active Casp-3 primary antibody. After application of secondary fluorescein isothiocyanate-conjugated antibody, counterstaining of nuclei with propidium iodide was performed.
Casp-14 immunofluorescence was achieved on paraffin-embedded tissue. After deparaffinization 5-μm tissue sections were incubated first for 30 minutes in blocking solution, then overnight at 4°C with a 1:3000 dilution of Casp-14 primary antibody. Sections were then incubated with the Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody counterstained with propidium iodide. Visualization of Casp-3- and Casp-14-stained sections was performed at 480- and 588-nm wavelengths using a Leica TCS-SP confocal microscope (Leica, Wetzlar, Germany).
Detection of Apoptosis by TUNEL Assay
The Fluorescein-FragEL apoptosis detection kit and protocol were used for the detection of apoptotic cells in paraffin-embedded skin samples. After deparaffinization, tissue was incubated for 10 minutes with 2 mg/ml of proteinase K in 10 mmol/L Tris, pH 8 solution. After equilibration the buffer was applied to the samples for 30 minutes, labeling with TdT enzyme followed for 90 minutes at 37°C. Labeled nuclei were visualized using a standard fluorescein filter (465 to 495 nm).
Isolation of Cytoplasmic Fraction from Epidermal Keratinocytes
After skin excision and removal of subcutaneous fat with scalpel blades, samples were floated on 10 mmol/L dithiothreitol solution in PBS for 1.5 hours at 37°C. Subcellular cytoplasmic fractions were isolated using ReadyPrep protein extraction kits (Cytoplasm; Bio-Rad, Nazareth Eke, Belgium) according to the manufacturers’ protocol.
Western Immunoblotting
After protein isolation (see above), the protein content of whole, basal, and suprabasal epidermal extracts was determined using the Bio-Rad protein assay. Equal amounts of protein from each experimental group were loaded onto 12% sodium dodecyl sulfate-polyacrylamide gels (Novex gels; Invitrogen, Mereleke, Belgium). After electrophoresis in slab gels, proteins were transferred onto nitrocellulose membranes and immunoblotted to detect Casp-14 (1/1000), using the WesternBreeze chemiluminescence kit (Invitrogen).
Electron Microscopy
Samples were processed for electron microscopy using modified Karnovsky’s fixative and postfixed with either 0.5% ruthenium tetroxide or 2% reduced osmium tetroxide, as described previously.23 All samples were dehydrated in a graded ethanol series followed by propylene oxide and embedded in an Epon-epoxy mixture. As cytochemical indicator of the extent of lamellar body secretion, we performed ultrastructural cytochemistry for acid lipase, a lipid hydrolase concentrated in lamellar bodies.24 As described previously,25 aldehyde-prefixed samples were microwave-incubated with substrate containing 5% Tween 85 (+/− inhibitor: tetrahydrolipstatin, 200 μmol/L) followed by standard lead-capture cytochemical method that depicts the localization of acid lipase. Samples for ultrastructural cytochemistry were postfixed in reduced osmium and processed as above. Ultrathin sections were viewed in a Zeiss 10 C electron microscope, operated at 60 kV, after further contrasting with lead citrate and uranyl acetate. Images were captured using the DigitalMicrograph 3.10.0 software from Gatan, Inc. (Pleasanton, CA).
Statistics
All data are given as mean ± SEM. Statistical analyses were determined using the Student’s t-test in GraphPad Prism 3 software for Macintosh (version 3.0a, Sept. 2000; GraphPad Software, Inc., San Diego, CA).
Results
Tape Stripping Rapidly Stimulates a Wave of Cornification
To address the hypothesis that permeability barrier function regulates not only production of mortar lipids, but also the transformation of outer granular cells into corneocyte bricks, we first abrogated the barrier by tape stripping hairless mouse skin [transepidermal water loss (TEWL) levels ≥6 μg/cm2/hour] and then assessed the appearance of TUNEL+ cells in the outermost granular layer, identifying by this method cells that are potentially metamorphosing into corneocytes. Whereas few TUNEL+ cells were identified before tape stripping (Figure 1A1), between 60 minutes and 6 hours after tape stripping, an increased number of TUNEL+ cells appeared in the outer stratum granulosum (Figure 1A2), decreasing to pretreatment levels by 18 hours (Figure 1A3). Quantitative data showed a highly significant, four- to fivefold increase in TUNEL+ outer stratum granulosum cells 1, 3, and 6 hours after tape stripping (Figure 1B). To ascertain whether TUNEL+ cells appear even sooner after acute barrier disruption, we next examined epidermis 30 minutes after tape stripping. Even at this early time point, the density of TUNEL+ cells increased by threefold (Figure 1B, P < 0.05).
Figure 1.
Tape stripping-induced barrier disruption increases TUNEL+ cells in the outer stratum granulosum. Although TUNEL+ cells (white arrows) are barely present at baseline (A1), the number of TUNEL+ cells markedly increases after barrier disruption by cellophane tape at 1 hour (A2), 3 hours and 6 hours (not shown), and return to normal by 18 hours (A3). B: Quantification of TUNEL+ cells is shown as percent from baseline at various time points after barrier disruption (n = 5; baseline = 0.27 ± 0.12 cells per unit length). B: TUNEL+ cells appear as early as 30 minutes after tape stripping. C: Dose-response relationship between the degree of barrier disruption, measured as changes in TEWL (mg/cm2/hour), and the number of TUNEL+ cells (n = 4), 3 hours after tape stripping (baseline = 0.18 ± 0.05 cells per unit length; 1 unit length = 5 μm). Scale bars = 10 μm.
We next determined both the threshold for tape stripping induction of cornification and whether there is a dose-response relationship between the extent of barrier disruption and increased cornification. As seen in Figure 1C, 3 hours after tape stripping, the number of TUNEL+ cells appeared to increase linearly in relation to the extent of barrier disruption, assessed as changes in TEWL rates. Although low levels of disruption (TEWL increased to ∼1 mg/cm2/hour, normal <0.2 mg/cm2/hour) appeared to increase TUNEL positivity, only at TEWL levels of 2 to 4 mg/cm2/hour does the increased density of TUNEL+ cells became statistically significant. Yet, at this level of disruption, the density of TUNEL+ cells was still significantly lower than the number of TUNEL+ cells in the outer granular layer when TEWL levels reach or exceed 6 mg/cm2/hour. Together, these results show that barrier disruption by repeated tape stripping stimulates the appearance of a wave of TUNEL+ cells in the outer stratum granulosum, in both a time- and a dose-dependent manner.
To ascertain whether the TUNEL+ cells reflect cells that have transitioned from outer stratum granulosum cells into corneocytes, we next assessed changes in the thickness of the epidermal nucleated layers immediately before, 1 hour, and 3 hours after tape stripping in hematoxylin and eosin-stained sections. Although the thickness of the stratum granulosum appeared unchanged 1 hour after barrier disruption, thinning of the nucleated layers of the epidermis (∼40% below the pretreatment thickness), with partial restoration of SC, became apparent by 3 hours (Figure 2). These results show that loss of outer stratum granulosum cells correlates with a wave of new cornification and that transient epidermal thinning is accompanied by the rapid reappearance of full-thickness SC.
Figure 2.
Acute barrier disruption transiently reduces thickness of epidermal nucleated layers. H&E-stained paraffin sections show a decrease in the thickness of the nucleated layers of the epidermis, ie, distance between the basal membrane and the stratum granulosum/SC junction, with a concurrent increase in SC thickness that is readily apparent by 3 hours after barrier disruption in comparison to baseline (A3 versus A1, brackets), whereas these changes are not yet observed at 1 hour (A2). Black arrows (A1 and A2) indicate stratum granulosum cells that cannot be seen at 3 hours after TS as they transform into corneocytes (arrowheads). Percentage changes from baseline show a statistically significant decrease (40%) in the thickness of the nucleated layers at 3 hours (B, P < 0.02; baseline = 122.67 ± 9.37 mm on ×20 micrographs) (n = 3). Thickness was measured on 10 different points of three randomized micrographs of each coded section from three animals in each group. Scale bars = 10 μm.
We next examined electron micrographs of skin biopsies obtained before, 30 minutes, 1 hour, and 3 hours after tape stripping (TEWL levels ≥6 mg/cm2/hour). Although tape stripping under these conditions removed most of the SC, cytotoxicity was not evident (Figures 2A and 3B). Although pretreated epidermis rarely revealed outer stratum granulosum cells metamorphosing into corneocytes (ie, cells with cornified envelopes that encapsulate cytosol with nuclear and/or organelle remnants ≤1% of stratum granulosum cells at baseline), such transitional cells became evident by 30 minutes after tape stripping and were even more commonly encountered 3 hours after tape stripping, accounting for more than 50% of cells in the outer stratum granulosum (Figure 3, B and C, versus A: pretreatment normal with no transitional cells). Moreover, the proportion of granular cells metamorphosing into corneocytes appeared to correlate with the density of TUNEL+ cells in parallel samples from the same animals (Figure 1A2). Together, these results show that tape stripping rapidly induces a wave of cornification, characterized by the transformation of numerous outer stratum granulosum cells into nascent corneocytes.
Figure 3.
Barrier disruption stimulates transformation of outer stratum granulosum cells into corneocytes. A: Electron micrographs of osmium tetroxide-postfixed samples show no transitioning cells under basal conditions (untreated skin) (black arrows show lamellar bodies, white arrows indicate normal lamellar body secretion). However, transitional cells (ie, cells in the process of transitioning into corneocytes) are present in large amounts 3 hours after TS. These cells display cornified envelopes (B and C, white arrows) and encapsulate cytosol replete with nuclear and other organelle remnants (B and C, black arrows). Scale bars = 500 nm.
TUNEL+ Cells Cornify, but Not by Classical Apoptosis
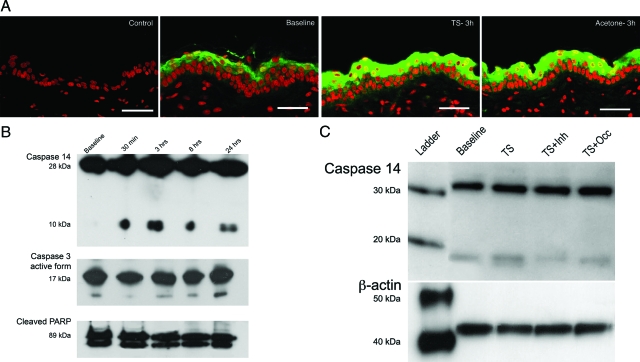
The appearance of a wave of TUNEL+ cells suggests that outer stratum granulosum cells undergo apoptosis in response to tape stripping. Therefore, we next evaluated whether this sequence involves classical apoptosis by assessing changes in both epidermal Casp-3 and PARP expression levels. Whereas oxidative stress, such as UV-B, increases Casp-3 expression,26 neither the extent nor the localization of epidermal immunostaining of the active form of Casp-3 protein appeared to change after tape stripping (supplemental Figure 1, see http://ajp. amjpathol.org). In addition, despite strong TUNEL positivity in the outer stratum granulosum at this time point (Figure 1B), neither the active form of Casp-3 nor cleaved PARP content appeared to change by Western immunoblotting 3 hours after tape stripping (TEWL ≥6 mg/cm2/hour) (Figure 4B). In contrast, epidermal protein expression of Casp-14, an epidermis-predominant Casp that is linked to cornification,27,28,29,30 increased 3 hours after tape stripping, as assessed by immunofluorescence (Figure 4A). Likewise, epidermal protein levels of the active form (ie, the 10-kDa subunit) of Casp-14 increased by 30 minutes after tape stripping and remained elevated for at least 24 hours (Figure 4B). These findings again contrast sharply with effects of oxidative stressors, such as UV-B, which do not induce Casp-14 activation.26 Together, these results show that the wave of cornification that occurs after tape stripping does not occur through induction of classical apoptosis, but rather that it represents a specific form of physiological apoptosis associated with Casp-14 activation.
Figure 4.
TUNEL positivity in outer stratum granulosum stimulates Casp-14 activation, but classic markers of apoptosis remain unchanged. A: Casp-14 immunofluorescence shows increased levels of the protein 3 hours after tape stripping and acetone treatment as compared to baseline (green staining, Casp-14; red staining, nuclei). B: Western immunoblotting shows no changes in the active form of Casp-3 (17 kDA) or cleaved PARP (89 kDa) levels after barrier disruption by tape stripping (TS). Although Casp-14 proenzyme (28 kDa) levels also remain unchanged after barrier disruption, levels of the small subunit (10 kDa) of the active enzyme increase as soon as 30 minutes after TS, and remain elevated at 3, 8, and 24 hours when compared to baseline. C: This increase in the active form of Casp-14 after tape stripping (TS) is blocked by topical application of the pan Casp inhibitor Z-VAD-FMK(TS + inh) or occlusion (TS + Occ) immediately after tape stripping as shown by Western immunoblotting. Scale bars = 20 μm.
Corneocyte Formation Is Regulated by Permeability Barrier Function
Although the above results show that a wave of cornification occurs after tape stripping, they leave unanswered its physiological role. For example, does this response represent either cell replacement or an injury response? Or alternatively, does it represent a specific response aimed at normalizing permeability barrier homeostasis? To address this issue, we first assessed whether acute permeability barrier disruption by an unrelated method (solvent treatment), which removes few, if any, corneocytes, also stimulates cornification. Acetone treatment (TEWL ≥6 mg/cm2/hour) again stimulated a large increase in the density of TUNEL+ cells in the outer granular layer (Figure 5, B1 and B2) that was at least comparable to the increase that follows tape stripping (Figure 1, A and B). As reported in numerous prior publications,4 cytotoxicity was not evident in acetone-treated animals. As with tape stripping, the maximum increase in TUNEL+ cells appeared by 1 hour, remaining significantly elevated at 3 hours (Figure 5D). Finally, acetone treatment stimulated the appearance of transitional cells, identified by cornified envelopes surrounding residual cytosolic organelles on electron microscopy, as early as 30 minutes, with a further increase in transitional cells between 1 and 3 hours (Figure 6A). Thus, two unrelated methods of acute permeability barrier disruption, tape stripping and solvent treatment, produce a comparable increase in the density of TUNEL+ cells in the outer stratum granulosum.
Figure 5.
Occlusion blocks both TS and acetone-induced stimulation of TUNEL+ cells. Increase in the number of TUNEL+ cells (white arrows) is shown after two unrelated methods of barrier disruption, ie, TS (A1 and A2) and acetone treatment (B1 and B2). Immediately after barrier disruption by either method, occlusion with a vapor-impermeable Latex wrap blocks the expected increase in TUNEL+ cells [occlusion after TS: A3 and C (n = 5, baseline = 0.27 ± 0.12 cells per unit length); occlusion after acetone: B3 and D (n = 3, baseline = 0.30 ± 0.13 cells per unit length)] (1 unit length = 5 μm). Scale bars = 10 mm.
Figure 6.
Barrier disruption rapidly entombs unsecreted lamellar bodies in nascent corneocytes. Ultrastructural studies of epidermis 30 minutes after acetone-induced barrier abrogation shows a wave of outer stratum granulosum cells transitioning into corneocytes with complete cornified envelopes (A, arrows) and entombed organelles (see Figure 3). Lipase cytochemical staining, a marker of lamellar body contents and secretion, shows entombed lamellar bodies in nascent corneocytes 3 hours after acetone treatment (C, arrowheads), whereas at baseline, all lamellar body contents have been secreted, with lipase staining only seen in the extracellular spaces (B,arrowheads). Scale bars = 500 nm.
To ascertain further the potential link between changes in permeability barrier status and cornification, we next assessed whether artificial permeability barrier restoration, immediately after acute barrier disruption, blocks the expected wave of new cornification. Acute disruption by tape stripping (≥6 mg/cm2/hour) again produced a marked increase in the number of TUNEL+ cells in the outer stratum granulosum at 3 hours (Figure 5A; quantitative data are in Figure 5C). Yet, placement of a vapor-impermeable, Latex wrap on newly stripped sites blocked the expected increase in TUNEL+ cells, restoring their density to near-normal levels (Figure 5). Likewise, occlusion primarily blocked the acetone-induced increase in TUNEL+ cells in the outer granular layer at 1 hour, while completely reversing the expected acetone-induced increase at 3 hours (Figure 5, B and D), as well as preventing the formation of transitional cornified cells (supplemental Figure 2, see http://ajp.amjpathol.org). Finally, occlusion also blocked Casp-14 activation (Fig. 4C). Together, these results are fully consistent with the hypothesis that modulations in permeability barrier function regulate the kinetics of terminal differentiation.
Accelerated Cornification Blocks Further Secretion of Lamellar Bodies
Formation of the permeability barrier under basal conditions requires not only the gradual transformation of outer granular cells into corneocytes but also continuous, low rates of lamellar body secretion from the outer stratum granulosum. Acute permeability barrier disruption amplifies lamellar body secretion so that 50 to 70% of the preformed pool of these organelles is secreted from the outer granular cells between 0 to 30 minutes.4,5 We next asked whether the barrier disruption-induced wave of cornification, which begins by 30 minutes after acute barrier disruption, impedes further secretion of lamellar body contents from the outer stratum granulosum. In standard electron microscopy images, remnants of organelles, including keratohyalin granules and lamellar bodies, surrounded by nascent cornified envelopes, again identified cells that were transitioning into corneocytes (Figure 3, B and C; Figure 6A). Using acid lipase as an ultrastructural marker for lamellar body contents and secretion,24,31 abundant enzyme activity became entombed within the cytosol of transitional cells as early as 30 minutes after either tape stripping or acetone treatment (3-hour results shown in Figure 6C; untreated controls show no lipase activity trapped within corneocytes27,28). Thus, the 30-minute delay in cornification allows an initial burst of lamellar body secretion, which is terminated by the subsequent wave of cornification.
Cornification Requires Casp-14
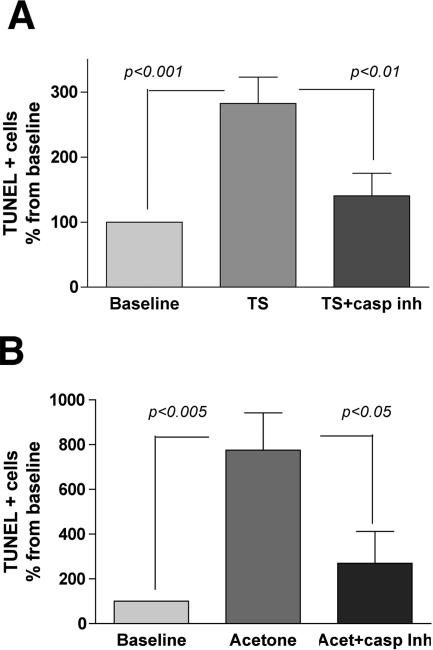
Casp-14 is linked to epidermal terminal differentiation,27,32 and although its putative role still remains unclear, the corneocyte filament-aggregating protein profilaggrin recently has been shown to be a physiological substrate.26 Because Casp-14 was activated in parallel with accelerated cornification after tape stripping (Figure 4), we next asked whether Casp-14 activity is required for this response. To address this issue, we first assessed changes in TUNEL+ cell number after application of the global Casp inhibitor Z-VAD-FMK to tape stripped skin. In parallel, we assessed cornification in Casp14−/− versus wild-type mice. Although tape stripping and acetone treatment again stimulated a two- to sixfold increase in the density of TUNEL+ cells, the expected increase in TUNEL positivity was entirely abrogated by topical applications of the pan-Casp inhibitor Z-VAD-FMK immediately after acute barrier disruption (Figure 7, A and B). Moreover, levels of the active form of Casp-14, as assessed by Western immunoblotting, declined after a single topical application of Z-VAD-FMK, applied immediately after tape stripping (Figure 4C).
Figure 7.
Topical pan-Casp inhibitor blocks appearance of TUNEL+ cells after barrier disruption. The pan-Casp inhibitor, Z-VAD-FMK, applied immediately before and after acute barrier disruption, inhibits the increase in TUNEL+ cells seen 3 hours after TS (A, baseline = 0.22 ± 0.16 cells per unit length) (n = 8). Similar results were observed using an alternate method of barrier disruption by acetone treatment (B, baseline = 0.22 ± 0.03 cells per unit length) (n = 4) (1 unit length = 5 μm). Variations in epidermal thickness were observed among different groups of animals.
To address further whether Casp-14 is required for terminal differentiation, we next assessed cornification in Casp14−/− versus wild-type murine epidermis under basal conditions and 3 hours after tape stripping. After tape stripping, the epidermis of wild-type mice revealed transitional cells with normal cornified envelopes, as described for normal hairless mice above (Figure 3, B and C; and Figure 8A, black arrowheads). In contrast, cornified envelopes either failed to form or appeared highly attenuated in transitional cells in Casp14−/− epidermis (Figure 8B, white arrowheads). Moreover, the defect in cornified envelope formation extended several SC layers above the stratum granulosum-SC junction. The inhibitor studies, in conjunction with the results in Casp14−/− mice, demonstrate that Casp-14 is not only a marker but also an effector of epidermal terminal differentiation, where it appears to be required for cornified envelope formation.
Figure 8.
Aberrant cornification in Casp-14-deficient epidermis. After acute barrier disruption (3 hours), transitional cells appear with a complete cornified envelope in wild-type epidermis (A, black arrowheads), whereas Casp14−/− corneocytes display primarily an absence of cornified envelopes (B, white arrowheads), with scattered images of attenuated envelope (B, arrows). Abnormal cornified envelopes persist high into the SC of Casp14−/− mice (not shown). Scale bars = 500 nm.
pH-Dependent Activation of Serine Proteases (SP) Accelerates Cornification by Activating PAR2
Acute barrier disruption leads to an increase in pH, which in turn, increases SP activity in the SC.15,16 This increase in SP activity activates PAR2 on the membranes of stratum granulosum cells, leading to blockade of lamellar body secretion, whereas in contrast, SP inhibitors accelerate lamellar body secretion by inhibiting activation of PAR2.16 To assess whether SP-PAR2 signaling also regulates cornification, we first applied three different types of SP inhibitors (aprotinin, PMSF, or the highly-specific plasminogen activator inhibitor t-AMCHA) immediately after acute barrier disruption by tape stripping. Although the tape stripping-induced increase in the density of TUNEL+ cells was abrogated entirely by applications of t-AMCHA (Figure 9, A and B; P < 0.01), aprotinin only partially blocked this increase (results do not achieve statistical significance), and PMSF, although previously shown to accelerate lamellar body secretion and permeability barrier recovery,16,19 also did not inhibit the development of TUNEL positivity after tape stripping (Figure 9C; data for PMSF not shown). These results suggest that the tape-stripping-induced wave of cornification is signaled, at least in part, by the plasminogen activator subfamily of SP.
Figure 9.
Serine protease inhibition with t- AMCHA, SC acidification, or absence of PAR2 prevent increase in TUNEL+ cells after barrier disruption. Topical application of the plasminogen activator inhibitor, t-AMCHA immediately after tape stripping inhibited the increase in the number of TUNEL+ cells (white arrows) (A3) that is seen after TS (A2), when compared to baseline (bb) (1 unit length = 5 μm). Quantitative data are shown in B (results are expressed as the ratio of TUNEL+ cells per epidermal length compared to baseline, baseline = 0.21 ± 0.06 cells per unit length) (n = 5). In parallel, aprotinin showed a partial, although not statistically significant, blockade of TUNEL increase whereas PMSF (not shown) did not significantly inhibit the increase in TUNEL+ cell number observed 3 hours after TS (C, baseline = 0.14 ± 0.05 cells per unit length) (n = 4). Topical application of the polyhydroxylacid LBA, immediately after TS, blocked the expected increase in the density of TUNEL+ cells 3 hours after barrier disruption (D, baseline = 0.16 ± 0.12 cells per unit length) (n = 8). Moreover, although tape stripping induced the expected increase in TUNEL+ cells in the outer stratum granulosum of wt mice, no increase in TUNEL+ cells occurred in PAR2−/− mice 3 hours after tape stripping (E) (n = 5). Scale bars = 10 μm.
Because acute barrier disruption increases the pH of SC, which in turn, activates SP,15 we next assessed whether reversal of the increased pH that both follows barrier disruption and is responsible for the increase in SP activity15 would also block the expected increase in TUNEL positivity. For these studies, we applied the nontoxic, polyhydroxylacid LBA immediately after tape stripping, to rapidly restore an acidic pH to the SC. Tape stripping increased both surface pH and epidermal SP activity, as described previously,15,16 and it again increased the density of TUNEL+ cells at 3 hours (Figure 9D). However, the increase in TUNEL+ was primarily blocked when SC pH was rapidly reacidified to normal (pretreatment) levels with topical LBA (Figure 9D). These results show that pH-induced changes in SP activity signal the wave of cornification that occurs after barrier disruption.
Because SPs signal downstream metabolic responses via the PAR2 receptor, we next assessed whether cornification proceeds normally in PAR2−/− versus wild-type mice after tape stripping. In wild-type mice, barrier disruption induced the expected increase in TUNEL+ cells in the stratum granulosum, but a significant increase did not occur in PAR2−/− epidermis after tape stripping (Figure 9E). Together, these results demonstrate the role of SPs, via activation of PAR2, in signaling new cornification.
Discussion
In this study, we assessed whether permeability barrier function regulates not only epidermal lipid synthesis/secretion but also the terminal differentiation of outer granular cells into corneocytes. Previous studies have focused solely on events in the extracellular matrix. At the earliest phase (0 to 30 minutes) after acute permeability barrier perturbation, these studies showed that cells of the outer stratum granulosum secrete the majority, but not all, of their preformed lamellar bodies into the extracellular spaces at the stratum granulosum-SC interface.4,5 This initial burst is followed by a marked increase in lipid synthesis and ongoing amplified secretion of nascent lamellar bodies (1 to 6 hours), resulting in rapid restoration of permeability barrier homeostasis as lamellar membranes are redeposited throughout the SC extracellular spaces.9 Accordingly, inhibition of either lamellar body secretion33 or of key enzymes of cholesterol or fatty acid synthesis delays permeability barrier recovery.34,35 Moreover, genetic and pharmacologically induced deficiencies in lipid hydrolases that metabolize extracellular, secreted lipids into their nonpolar products36,37,38 result in impaired barrier in parallel with delayed maturation of extracellular lamellar bilayers8,39,40,41 and are up-regulated after barrier perturbations.31,42
Yet, in addition to the extracellular matrix, corneocytes also are required for permeability barrier homeostasis both through their spacer function that forces water molecules to transverse a torturous, elongated pathway43 and by providing a scaffold necessary for the supramolecular organization of the lipid matrix into lamellar membrane arrays.44,45 This critical role for corneocytes is demonstrated most convincingly in genetic disorders, such as Vohwinkel’s disease (loricrin keratoderma) and transglutaminase 1-deficient lamellar ichthyosis, in which a defective cornified envelope leads to abnormal permeability barrier function attributable to alterations in lamellar membrane organization.44,45 Therefore, we asked here whether permeability barrier function not only dictates the generation of the extracellular lipids but also the formation of corneocytes. Finally, it should be noted that tight junction proteins also are important for the formation of a competent permeability barrier,46 but how they impact permeability barrier function remains unclear.
In the present study, we used two independent techniques to identify cells that are metamorphosing from outer stratum granulosum cells into corneocytes; ie, by electron microscopy, which identifies new (transitional) corneocytes by the presence of a cornified envelope encasing remnant organelles, and the TUNEL assay, a standard method for the detection of specific nuclear alterations in apoptotic cells. After acute permeability barrier disruption, a rapid (by 30 minutes) increase in the number of TUNEL+ cells occurs in the outer granular cells, persisting for 6 hours after barrier disruption. Appearance of TUNEL+ cells is paralleled by the appearance of numerous transitional cells in the outer stratum granulosum with nascent cornified envelopes and organelle remnants. Supporting our hypothesis that these new corneocytes, now clearly identified by both TUNEL assay and electron microscopy, form specifically in response to barrier alterations, and not only as a cell replacement or injury response to tape stripping, we assessed an equally effective, alternate method that alters permeability barrier function while leaving the SC layers intact, ie, acetone treatment, which induced a fully-comparable wave of cornification. After either method of barrier disruption, there was no evidence of cell death accompanied by evidence of cellular toxicity or autophagy; specifically, keratinocytes did not display evidence of cytotoxicity, nor the presence of intracellular vacuoles containing cell debris, a classical feature of autophagy.47,48 The link to barrier function is shown further by the linear, dose-response relationship between the extent of the increase in TUNEL+ cells and the degree of barrier disruption, and most specifically, the link to barrier function is shown by the absence of new cornification after artificial barrier restoration with occlusion. The occlusion results also further exclude nonspecific mechanisms of cell death, such as cell repair in response to cytotoxicity or autophagy. The presence of lipase activity, a lamellar body content marker,31 in these transitional cells shows further that those lamellar bodies that have not been secreted before the 30-minute lag period, become entombed during the subsequent wave of cornification. Thus, permeability barrier requirements regulate both responses required for its restoration: the replenishment of the extracellular, lamellar membrane-enriched matrix, and the formation of new corneocytes. It remains possible, however, that injury to the epidermis could stimulate new corneocyte formation, independent of abnormalities in permeability barrier function.
The present study also assessed mechanisms whereby changes in permeability barrier status could signal the underlying granular layer to transform into corneocytes. Previous studies by our and other laboratories have demonstrated that disruption of the permeability barrier results in a decrease in calcium and other ions in the outer nucleated layers of the epidermis,10,49 modifying the distinctive calcium gradient of the epidermis50,51,52,53,54 and stimulating lamellar body secretion. Yet, although decreases in calcium stimulate lamellar body secretion, they simultaneously inhibit epidermal differentiation52; hence, we suspect that a decrease in calcium in the outer epidermis would not stimulate terminal differentiation. But the potential role of calcium in signaling of terminal differentiation has not yet been investigated.
Very recently, we identified another homeostatic signaling system between the SC and the underlying epidermis. After acute permeability barrier disruption, the pH of SC increases from its highly acidic values (≈5.0) toward a more neutral pH, stimulating a pH-dependent increase in SP activity15,55 that, in turn, activates PAR2, which localizes to the membranes of outer stratum granulosum cells.16 SP binding to PAR2 impairs barrier recovery through down-regulation of lamellar body secretion, which can be reversed either by inhibiting the increase in SC pH or by applying topical SP inhibitors, or in PAR2−/− mice.16,19 In parallel, we show here that 1) reversal of the increase in pH by application of a polyhydroxyl acid, 2) application of certain protease inhibitors, or 3) lack of PAR2 in PAR2−/− mice blocks the expected wave of cornification. The results of the present series of experiments provide an explanation for the seemingly paradoxical observations that pH-induced increase in SP activity simultaneously inhibits lamellar body secretion while stimulating cornification. The barrier-initiated SP PAR2 signaled acceleration of cornification of outer stratum granulosum cells is delayed by 30 minutes, a time frame that allows secretion (escape) of much of the preformed pool of lamellar bodies before cornification. Thereafter, residual lamellar bodies become entombed within newly formed corneocytes. This synchronized response allows an initial burst of lipid secretion, with only a transient delay in new cornification, both of which are required for normal permeability barrier homeostasis.
Our studies also shed light on the intracellular mechanisms that are required for the terminal differentiation of outer granular cells into corneocytes. Classic apoptotic mechanisms do not appear to be involved, because Casp-3 and PARP levels were not altered by acute barrier disruption. In contrast, Casp-14, a Casp unique to keratinizing epithelia,21 appears to be involved and required for cornification. Casp-14 activation increased in parallel with the wave of new cornification, and pharmacological blockade of Casp-14 prevented the expected increase in TUNEL+ cells in the outer stratum granulosum after acute disruption. Because Casp14−/− mice display delayed cornification, and barrier disruption fails to stimulate cornification, this enzyme is clearly required for terminal differentiation. Moreover, these results are consistent with the recent demonstration that Casp14−/− mice display abnormal barrier function.26 Although Casp-14 is a known member of the Casp family of cysteine proteases that is activated within the SC,56 and one of its endogenous substrates has recently been discovered,26 its precise role in the regulation of keratinocyte terminal differentiation remains unclear. In contrast to other caspases, its activation does not require typical apoptotic stressors or stimuli.57 The observation here that corneocytes in Casp14−/− mice display strikingly attenuated cornified envelopes is consistent with immunoelectron microscopy studies that have shown localization of this enzyme in the vicinity of cornified envelopes,58 suggesting together that Casp-14 regulates cornified envelope formation. Yet, although Casp-14 activation is both important for normal cornification and required for the transition of outer stratum granulosum cells into corneocytes after acute barrier perturbations, these studies did not define the physiological substrate(s) for Casp-14 nor did they exclude a role for other mechanisms, eg, stimulation of TNF and TRAIL activation,59,60,61 in the regulation of cornification.
In summary, the present study demonstrates that changes in permeability requirements specifically regulate the transformation of outer stratum granulosum into corneocytes. At least two signaling pathways emanate from the SC that signal the viable epidermis. Decreases in calcium in the outer stratum granulosum stimulate lamellar body secretion, while transiently inhibiting terminal differentiation, whereas the SP/PAR2, pH-dependent pathway signals terminal differentiation and inhibits lamellar body secretion (Figure 10). We propose that the interplay of these two signaling pathways, which display opposing effects, allows for the sequential orchestration of two parallel processes that provide for barrier function and allows more precise responses to injury or perturbations than could a single signaling mechanism. Moreover, these studies show that an increase in Casp-14 activity is essential both for accelerated cornification in response to barrier disruption and for the formation of normal corneocytes (Figure 10).
Figure 10.
Involvement of PAR2 and Casp-14 in corneocyte formation. Decreases in calcium in the outer stratum granulosum after barrier disruption stimulate lamellar body secretion, while transiently inhibiting keratinocyte differentiation. The SP/PAR2, pH-dependent pathway signals terminal differentiation and inhibits lamellar body secretion. Moreover, an increase in Casp-14 activity is essential for accelerated cornification in response to barrier disruption and for the formation of normal corneocytes.
Supplementary Material
Acknowledgments
We thank Joan Wakefield for her assistance in preparing and editing the manuscript.
Footnotes
Address reprint requests to Peter M. Elias, M.D., Dermatology Service (190), VA Medical Center, 4510 Clement St., San Francisco, CA 94121. E-mail: eliasp@derm.ucsf.edu.
Supported by the National Institutes of Health (grants AR 19098, AI 059311, AR 050629, AR 050629, and AR 39448 to P.P.) and the Centre de Recherches et D’Investigation Epidemiques et Sensorielles Foundation, Paris (research grant).
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Hohl D. Cornified cell envelope. Dermatologica. 1990;180:201–211. doi: 10.1159/000248031. [DOI] [PubMed] [Google Scholar]
- Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- Bickenbach JR, Greer JM, Bundman DS, Rothnagel JA, Roop DR. Loricrin expression is coordinated with other epidermal proteins and the appearance of lipid lamellar granules in development. J Invest Dermatol. 1995;104:405–410. doi: 10.1111/1523-1747.ep12665896. [DOI] [PubMed] [Google Scholar]
- Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992;98:279–289. doi: 10.1111/1523-1747.ep12497866. [DOI] [PubMed] [Google Scholar]
- Elias PM, Cullander C, Mauro T, Rassner U, Komuves L, Brown BE, Menon GK. The secretory granular cell: the outermost granular cell as a specialized secretory cell. J Invest Dermatol Symp Proc. 1998;3:87–100. doi: 10.1038/jidsymp.1998.20. [DOI] [PubMed] [Google Scholar]
- Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Takagi Y, Menon GK, Legler G, Feingold KR, Elias PM. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J Clin Invest. 1993;91:1656–1664. doi: 10.1172/JCI116374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran WM, Ginns EI, Menon GK, Grundmann JU, Fartasch M, McKinney CE, Elias PM, Sidransky E. Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J Clin Invest. 1994;93:1756–1764. doi: 10.1172/JCI117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold KR. The regulation and role of epidermal lipid synthesis. Adv Lipid Res. 1991;24:57–82. doi: 10.1016/b978-0-12-024924-4.50007-9. [DOI] [PubMed] [Google Scholar]
- Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992;89:530–538. doi: 10.1172/JCI115617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Elias PM, Feingold KR, Mauro T. A role for ions in barrier recovery after acute perturbation. J Invest Dermatol. 1994;102:976–979. doi: 10.1111/1523-1747.ep12384225. [DOI] [PubMed] [Google Scholar]
- Denda M, Fuziwara S, Inoue K. Influx of calcium and chloride ions into epidermal keratinocytes regulates exocytosis of epidermal lamellar bodies and skin permeability barrier homeostasis. J Invest Dermatol. 2003;121:362–367. doi: 10.1046/j.1523-1747.2003.12367.x. [DOI] [PubMed] [Google Scholar]
- Menon GK, Price LF, Bommannan B, Elias PM, Feingold KR. Selective obliteration of the epidermal calcium gradient leads to enhanced lamellar body secretion. J Invest Dermatol. 1994;102:789–795. doi: 10.1111/1523-1747.ep12377921. [DOI] [PubMed] [Google Scholar]
- Denda M, Kumazawa N. Negative electric potential induces alteration of ion gradient and lamellar body secretion in the epidermis, and accelerates skin barrier recovery after barrier disruption. J Invest Dermatol. 2002;118:65–72. doi: 10.1046/j.0022-202x.2001.01500.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, Choi EH, Uchida Y, Brown BE, Feingold KR, Elias PM. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126:2074–2086. doi: 10.1038/sj.jid.5700351. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Mao-Qiang M, Brown BE, Hachem JP, Moskowitz DG, Demerjian M, Haftek M, Serre G, Crumrine D, Mauro TM, Elias PM, Feingold KR. Functional consequences of a neutral pH in neonatal rat stratum corneum. J Invest Dermatol. 2004;123:140–151. doi: 10.1111/j.0022-202X.2004.22726.x. [DOI] [PubMed] [Google Scholar]
- Mauro T, Holleran WM, Grayson S, Gao WN, Man MQ, Kriehuber E, Behne M, Feingold KR, Elias PM. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290:215–222. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- Denda M, Kitamura K, Elias PM, Feingold KR. trans-4-(Aminomethyl) cyclohexane carboxylic acid (T-AMCHA), an anti-fibrinolytic agent, accelerates barrier recovery and prevents the epidermal hyperplasia induced by epidermal injury in hairless mice and humans. J Invest Dermatol. 1997;109:84–90. doi: 10.1111/1523-1747.ep12276640. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, Ansel JC, Bunnett NW. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Lippens S, VandenBroecke C, Van Damme E, Tschachler E, Vandenabeele P, Declercq W. Caspase-14 is expressed in the epidermis, the choroid plexus, the retinal pigment epithelium and thymic Hassall’s bodies. Cell Death Differ. 2003;10:257–259. doi: 10.1038/sj.cdd.4401141. [DOI] [PubMed] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, Hochepied T, Lippens S, Ovaere P, Van den Broucke C, Van Damme P, D’Herde K, Hachem JP, Borgonie G, Schoonjans L, Libert C, Vandekerckhove H, Gevaert K, Vandenabeele P, Declercq W. Caspase-14 protects against epidermal UVB photo-damage and water loss. Nat Cell Biol. 2007;9:666-674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- Hou SY, Mitra AK, White SH, Menon GK, Ghadially R, Elias PM. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991;96:215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Menon GK, Grayson S, Elias PM. Cytochemical and biochemical localization of lipase and sphingomyelinase activity in mammalian epidermis. J Invest Dermatol. 1986;86:591–597. doi: 10.1111/1523-1747.ep12355263. [DOI] [PubMed] [Google Scholar]
- Rassner UA, Crumrine DA, Nau P, Elias PM. Microwave incubation improves lipolytic enzyme preservation for ultrastructural cytochemistry. Histochem J. 1997;29:387–392. doi: 10.1023/a:1026438917856. [DOI] [PubMed] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, Van den Broecke C, Van Damme P, D’Herde K, Hachem JP, Borgonie G, Presland RB, Schoonjans L, Libert C, Vandekerckhove J, Gevaert K, Vandenabeele P, Declercq W. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Declercq W, Ban J, Rendl M, Lengauer B, Mayer C, Lippens S, Vandenabeele P, Tschachler E. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol. 2000;115:1148–1151. doi: 10.1046/j.1523-1747.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- Fischer H, Stichenwirth M, Dockal M, Ghannadan M, Buchberger M, Bach J, Kapetanopoulos A, Declercq W, Tschachler E, Eckhart L. Stratum corneum-derived caspase-14 is catalytically active. FEBS Lett. 2004;577:446–450. doi: 10.1016/j.febslet.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Fischer H, Rossiter H, Ghannadan M, Jaeger K, Barresi C, Declercq W, Tschachler E, Eckhart L. Caspase-14 but not caspase-3 is processed during the development of fetal mouse epidermis. Differentiation. 2005;73:406–413. doi: 10.1111/j.1432-0436.2005.00046.x. [DOI] [PubMed] [Google Scholar]
- Kuechle MK, Predd HM, Fleckman P, Dale BA, Presland RB. Caspase-14, a keratinocyte specific caspase: mRNA splice variants and expression pattern in embryonic and adult mouse. Cell Death Differ. 2001;8:868–870. doi: 10.1038/sj.cdd.4400897. [DOI] [PubMed] [Google Scholar]
- Menon GK, Ghadially R, Williams ML, Elias PM. Lamellar bodies as delivery systems of hydrolytic enzymes: implications for normal and abnormal desquamation. Br J Dermatol. 1992;126:337–345. doi: 10.1111/j.1365-2133.1992.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Presland RB, Kuechle MK. Processing of native caspase-14 occurs at an atypical cleavage site in normal epidermal differentiation. Biochem Biophys Res Commun. 2002;296:911–917. doi: 10.1016/s0006-291x(02)02015-6. [DOI] [PubMed] [Google Scholar]
- Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131:809–816. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Man MQ, Menon GK, Cho SS, Brown BE, Elias PM. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86:1738–1745. doi: 10.1172/JCI114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao-Qiang M, Feingold KR, Elias PM. Inhibition of cholesterol and sphingolipid synthesis causes paradoxical effects on permeability barrier homeostasis. J Invest Dermatol. 1993;101:185–190. doi: 10.1111/1523-1747.ep12363729. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, Suzuki A, Elias PM, Holleran WM, Hamanaka S. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000;41:2071–2082. [PubMed] [Google Scholar]
- Mao-Qiang M, Feingold KR, Jain M, Elias PM. Extracellular processing of phospholipids is required for permeability barrier homeostasis. J Lipid Res. 1995;36:1925–1935. [PubMed] [Google Scholar]
- Elias PM, Williams ML, Maloney ME, Bonifas JA, Brown BE, Grayson S, Epstein EHJ. Stratum corneum lipids in disorders of cornification. Steroid sulfatase and cholesterol sulfate in normal desquamation and the pathogenesis of recessive X-linked ichthyosis. J Clin Invest. 1984;74:1414–1421. doi: 10.1172/JCI111552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JM, Schutze S, Forl M, Kronke M, Proksch E. Roles for tumor necrosis factor receptor p55 and sphingomyelinase in repairing the cutaneous permeability barrier. J Clin Invest. 1999;104:1761–1770. doi: 10.1172/JCI5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Man MQ, Weber F, Gao W, Feingold KR, Fritsch P, Elias PM, Holleran WM. Permeability barrier disorder in Niemann-Pick disease: sphingomyelin-ceramide processing required for normal barrier homeostasis. J Invest Dermatol. 2000;115:459–466. doi: 10.1046/j.1523-1747.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Crumrine D, Rassner U, Hachem JP, Menon GK, Man W, Choy MH, Leypoldt L, Feingold KR, Williams ML. Basis for abnormal desquamation and permeability barrier dysfunction in RXLI. J Invest Dermatol. 2004;122:314–319. doi: 10.1046/j.1523-1747.2003.22258.x. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Takagi Y, Menon GK, Jackson SM, Lee JM, Feingold KR, Elias PM. Permeability barrier requirements regulate epidermal beta-glucocerebrosidase. J Lipid Res. 1994;35:905–912. [PubMed] [Google Scholar]
- Potts RO, Francoeur ML. Lipid biophysics of water loss through the skin. Proc Natl Acad Sci USA. 1990;87:3871–3873. doi: 10.1073/pnas.87.10.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Fluhr JW, Crumrine DC, Uchida Y, Hachem JP, Behne M, Moskowitz DG, Christiano AM, Feingold KR, Elias PM. Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis). J Invest Dermatol. 2004;122:909–922. doi: 10.1111/j.0022-202X.2004.22431.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Schmuth M, Uchida Y, Rice RH, Behne M, Crumrine D, Feingold KR, Holleran WM, Pharm D. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exp Dermatol. 2002;11:248–256. doi: 10.1034/j.1600-0625.2001.110308.x. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12(Suppl 2):1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro T, Bench G, Sidderas-Haddad E, Feingold K, Elias P, Cullander C. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. J Invest Dermatol. 1998;111:1198–1201. doi: 10.1046/j.1523-1747.1998.00421.x. [DOI] [PubMed] [Google Scholar]
- Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- Denda M, Hosoi J, Asida Y. Visual imaging of ion distribution in human epidermis. Biochem Biophys Res Commun. 2000;272:134–137. doi: 10.1006/bbrc.2000.2739. [DOI] [PubMed] [Google Scholar]
- Elias P, Ahn S, Brown B, Crumrine D, Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002;119:1269–1274. doi: 10.1046/j.1523-1747.2002.19622.x. [DOI] [PubMed] [Google Scholar]
- Menon GK, Bommannan DB, Elias PM. High-frequency sonophoresis: permeation pathways and structural basis for enhanced permeability. Skin Pharmacol. 1994;7:130–139. doi: 10.1159/000211287. [DOI] [PubMed] [Google Scholar]
- Lee SH, Choi EH, Feingold KR, Jiang S, Ahn SK. Iontophoresis itself on hairless mouse skin induces the loss of the epidermal calcium gradient without skin barrier impairment. J Invest Dermatol. 1998;111:39–43. doi: 10.1046/j.1523-1747.1998.00226.x. [DOI] [PubMed] [Google Scholar]
- Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005;124:198–203. doi: 10.1111/j.0022-202X.2004.23547.x. [DOI] [PubMed] [Google Scholar]
- Park K, Kuechle MK, Choe Y, Craik CS, Lawrence OT, Presland RB. Expression and characterization of constitutively active human caspase-14. Biochem Biophys Res Commun. 2006;347:941–948. doi: 10.1016/j.bbrc.2006.06.156. [DOI] [PubMed] [Google Scholar]
- Lippens S, Kockx M, Knaapen M, Mortier L, Polakowska R, Verheyen A, Garmyn M, Zwijsen A, Formstecher P, Huylebroeck D, Vandenabeele P, Declercq W. Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 2000;7:1218–1224. doi: 10.1038/sj.cdd.4400785. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Dockal M, Reinisch C, Tschachler E, Eckhart L. Ultrastructural localization of caspase-14 in human epidermis. J Histochem Cytochem. 2004;52:1561–1574. doi: 10.1369/jhc.4A6300.2004. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Sitailo LA, Bodner B, Denning MF, Nickoloff BJ. Defining the caspase-containing apoptotic machinery contributing to cornification in human epidermal equivalents. Exp Dermatol. 2006;15:14–22. doi: 10.1111/j.0906-6705.2005.00383.x. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Bacon P, Chaturvedi V, Nickoloff BJ. Role of NF-kappaB activity in apoptotic response of keratinocytes mediated by interferon-gamma, tumor necrosis factor-alpha, and tumor-necrosis-factor-related apoptosis-inducing ligand. J Invest Dermatol. 2001;117:898–907. doi: 10.1046/j.0022-202x.2001.01477.x. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Bacon P, Panella J, Sitailo LA, Denning MF, Nickoloff BJ. Low-dose UV-radiation sensitizes keratinocytes to TRAIL-induced apoptosis. J Cell Physiol. 2004;200:155–166. doi: 10.1002/jcp.20017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.