Abstract
ARA70 was first identified as a gene fused to the ret oncogene in thyroid carcinoma and subsequently as a co-activator for androgen receptor (AR). Two isoforms of ARA70 have been identified: a 70-kDa version called ARA70α and an internally spliced 35-kDa variant termed ARA70β. We have previously reported that ARA70α expression is reduced in prostate cancer, and its overexpression inhibits proliferation of LNCaP prostate cancer cells. However, the function of the ARA70β isoform in prostate cancer is not understood. In this report we examined the effects of ARA70β on AR transcriptional regulation as well as prostate cancer cellular proliferation and invasion. Although both ARA70α and ARA70β functioned as transcriptional co-activators of AR in cell-based reporter assays, ARA70β overexpression, in contrast to ARA70α, promoted prostate cancer cellular proliferation and invasion through Matrigel. Interestingly, genome-wide expression profiling of cells expressing ARA70β revealed an increase in the expression of genes involved in the control of cell division and adhesion, compatible with a role for ARA70β in proliferation and invasion. Consistent with its function in promoting cell growth and invasion, ARA70β expression was increased in prostate cancer. Our findings implicate ARA70β as a regulator of tumor cell growth and metastasis by affecting gene expression.
The androgen receptor (AR) is a transcription factor that regulates prostate cell growth and differentiation.1 AR mediates transcription through a series of events including ligand binding, DNA binding to cognate androgen response elements (AREs), and interaction with various co-activators. This results in activation of the general transcriptional machinery.2 Co-activators are components that are required for activator-dependent transcription but appear to be dispensable for basal transcription. AR co-activators are thought to facilitate AR-dependent transcription by linking the receptor to the basal transcription machinery or by modulating chromatin through methylation and acetylation.3,4 An increasing number of AR-interacting proteins have been identified, and several have been shown to function as AR co-activators in cell-based reporter assay.5,6,7 Interestingly, a number of AR co-activators display diverse patterns of expression in prostate cancer. For example, the AR cofactors ARA24, PIAS1,8 cyclinD1,9 SRC1,10 and FHL211 appear overexpressed, whereas ARA708 and ART-2712 are reduced in human prostate cancers compared to adjacent benign tissue. In addition, although most co-factors are expressed in prostatic epithelium, ARA55 is expressed exclusively in prostatic stroma, with reduced expression in prostate cancer. These findings suggest that AR co-activators perform distinct functions in prostate growth.
One of the earliest described AR co-activators is ARA70. It was initially identified as a gene fused to the ret oncogene13,14 and subsequently characterized as an AR co-activator.15 ARA70 interacts with the AR ligand-binding domain (LBD) via an FXXLF motif and with the N-terminal AF1 region of AR in an FXXLF-independent manner.16,17,18,19 ARA70 can enhance AR ligand-dependent transcriptional activation, possibly by interacting with PCAF and TFIIB.20
ARA70 exists as two isoforms that are generated by alternative splicing: full-length ARA70α (70 kDa) and an internally spliced variant ARA70β (35 kDa).20 We and others have shown that ARA70α expression is reduced in prostate cancer,8,20,21 and its overexpression inhibits LNCaP cell proliferation.8 In contrast to ARA70α, studies on the expression and function of ARA70β are limited. It has been shown that ARA70β is expressed in the androgen-independent PC3 prostate cancer cell line and in the estrogen-dependent MCF7 breast cancer cell line.22 Interestingly, in a study of four matched breast cancer cases, ARA70β expression appeared to be confined to malignant but not benign breast tissue.22 These results suggest that the expression of ARA70β is associated with prostate and breast cancers. To illuminate the function of ARA70β, we examined the regulatory effects of ARA70β on AR-mediated transcription activation, prostate cancer cell proliferation, and invasion. Whereas ARA70α inhibits LNCaP cell growth when overexpressed, the ARA70β isoform stimulates prostate cancer cellular proliferation and invasion. Thus, ARA70β has functions distinct from ARA70α in the regulation of prostate cancer cell growth and invasion.
Materials and Methods
Construction of an ARA70β Retroviral Vector
A FLAG (f)-tagged ARA70β retroviral construct was created by inserting f-ARA70β as an NdeI/BamHI fragment into the pBabe retroviral vector, resulting in pBabe-f-ARA70β. Expression of ARA70β was be detected by FLAG or ARA70β antibodies.
ARA70β-Specific Antibody Production and ARA70β-Specific mRNA Detection
An 18-amino acid peptide (QKQTLENSQQEVLLNSPL) spanning the region of the internal splice site was used to generate a rabbit ARA70β-specific polyclonal antibody.23 This antibody was affinity purified using a recombinant Escherichia coli-expressed and -purified ARA70β protein linked to Sepharose 4B beads. The eluted antibody fractions were examined for ARA70β-specific immune reactivity. Primers for the detection of the ARA70β-specific transcript by reverse transcriptase-polymerase chain reaction (RT-PCR) were OPL315: 5′-ACCTTGGAGAACAGTCAGCA-3′ and OPL316: 5′-TCACATCTGTAGAGGAGTTCGAT-3′. The OPL315 primer has a sequence (cagca) at its 3′ end that is unique to the ARA70β transcript.
Cell Culture, Transient Transfection, and Luciferase Assays
Prostate cancer cell lines LNCaP and PC3 were obtained from the American Type Culture Collection (Manassas, VA). PC3/AR is a PC3 cell line stably expressing the human AR and was generated by transfecting PC3 cells with pcDNA-human AR followed by selection in G418. The AR and ARA70β expression vectors for transfection assays were constructed by inserting their corresponding cDNA sequences into pcDNA3.1. The AR luciferase reporter contained multiple androgen response elements (4xAREs) in front of the E4 basal promoter and the luciferase gene. The PSA luciferase reporter contains a 4-kb region that includes the upstream regulatory region and promoter of the PSA gene (gift from Dr. L.G. Wang, New York University, New York, NY). LNCaP, PC3, and PC3/AR cells were maintained in RPMI 1640 medium plus 10% fetal bovine serum (standard media). Transfections were performed with Lipofectamine reagent (Invitrogen, Carlsbad, CA). Cells (105) were plated in each well of a 24-well plate 24 hours before transfection. After being washed with phosphate-buffered saline, cells in each well were transfected with 30 ng or 100 ng of an AR expression vector, 100 ng of the reporter plasmid, 2.5 ng of the pR-LUC internal control plasmid, and different amounts of the ARA70α and ARA70β expression vectors. The total amount of DNA was adjusted to 1 μg with pcDNA3.1. Transfections were conducted in phenol red-free RPMI 1640 medium. After 2 hours the medium was changed to phenol red-free RPMI 1640 plus charcoal-treated fetal bovine serum (10%), without or with 10 nmol/L R1881. Cells were cultured for another 48 hours and harvested for the dual luciferase assay (Promega, Madison, WI).
Retroviral Infection and Stable Cell Line Selection
The pBabe-f-ARA70β retroviral construct was transfected into phoenix A amphotropic packaging cells via Lipofectamine-mediated transfection to produce virus. After transfection, the retroviral packaging cells were maintained at 37°C in RPMI 1640 media with the addition of fetal bovine serum and penicillin/streptomycin for 24 hours and subsequently incubated for another 24 hours at 32°C to increase viral titer. The virus-containing supernatant was collected, centrifuged, and filtered before retroviral infection. Cells were transduced with viral supernatants containing pBabe or pBabe-f-ARA70β in the presence of 4 μg/ml of polybrene for 24 to 48 hours. Individual puromycin-resistant colonies as well as pools of cells expressing ARA70β were used to examine growth and invasion.
Cell Proliferation and Anchorage-Independent Assays
Cell proliferation was measured by counting cells cultured in complete (phenol red-containing media with serum), androgen-free (phenol red-free medium with charcoal-stripped serum), and androgen-containing (androgen-free media supplemented with 10 nmol/L synthetic androgen R1881) media. Cells (1 × 104) were plated into six-well plates and counted with a hemocytometer every other day. The assays were done in triplicate.
Anchorage-independent cell growth in soft agar was performed in triplicate on cells (104) suspended in 2 ml of medium with 0.35% agar (Difco; Becton Dickinson, Sparks, MD) and placed on top of 5 ml of 0.7% solidified agar. Cells were maintained for 2 to 3 weeks, and fresh media was added every 3 to 5 days. The volume of colonies, from the average radius, was calculated, and numbers of colonies were determined by counting. Controls included parental LNCaP, PC3, and PC3/AR cells transduced with the empty pBabe vector.
In Vitro Matrigel Cancer Cell Invasion Assay
Media (750 μl) containing 10% fetal bovine serum as the chemoattractant was added to the lower chamber of a BD Biocoat Matrigel invasion chamber (BD Bioscience, Bedford, MA). A cell suspension (5 × 104) in 0.5 ml of Dulbecco’s modified Eagle’s medium with 0.1% bovine serum albumin was placed on the insert of the 24-well chambers. After 24 hours, the noninvading cells on the upper surface of the membrane were removed using a cotton swab. Invasive cells on the lower surface of the membrane were stained with Diff Quik stain and counted under the light microscope. The average number of cells from three representative high-power fields (×400 magnifications) was recorded.
Affymetrix Microarray Analysis
Total RNA was extracted and purified from cell lines with and without ARA70β overexpressed using RNAqueous-4 PCR Kit (Ambion, Austin, TX). RNA was run on a denaturing agarose gel to examine its integrity. Gene expression analysis was performed using Affymetrix (Santa Clara, CA) U133A and U133B human gene arrays for individual gene and expressed sequence tag clusters using instruments and protocols recommended by the manufacturer. The U133A and B set consists of 200,000 distinct probe sets, representing 100,000 genes and expressed sequence tag transcripts. Two response measures, the average difference and absolute call, were extracted for each gene on every sample, as determined by the default settings of Affymetrix Microarray Suite 4.0. Average difference was used as the primary measure of expression level, and absolute call was retained as a secondary measure. Expression values on each array were scaled to have an average expression of 2500 across the central 96%. Specific genes corresponding to individual probe sets were identified using an Affymetrix database. The GenBank accession number of the clones used to produce the oligonucleotide probe set were annotated through various internet resources (Genecards, http://nciarray.nci.nih.gov/cards/; Locus Link, which has been superseded by Entrez Gene, http://www.ncbi.nlm. nih.gov/sites/entrez?db = gene; Online Mendelian Inheritance in Man, http://www.ncbi.nlm.nih.gov/sites/entrez?db = OMIM; Gene Ontology Browser, http://cgap.nci.nih. gov/Genes) and publications identified in PubMed (http://www.ncbi.nlm.nih.gov/PubMed/).
Immunoprecipitation and Western Blot Analysis
Whole cell extracts were prepared from LNCaP, PC3, and PC3/AR cells with either pBabe vector only or pBabe-f-ARA70β. For immunoprecipitation, 20 μg of proteins were incubated with 5 μl of rabbit anti-AR antibody and 40 μl of protein A agarose beads for 30 minutes. The protein A beads were washed with RIPA buffer. The products of each immunoprecipitation or whole cell lysates were subjected to electrophoresis on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane for Western blot analysis. The membrane was blocked in 5% nonfat dry milk in TBS-T (20 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, and 0.1% Tween 20) for 30 minutes. Blots were incubated with an affinity-purified ARA70β antibody overnight at 4°C, washed with TBS-T three times, and incubated for 1.5 hours with the horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:2000) (Amersham Biosciences, Piscataway, NJ). Antibodies were diluted into 5% nonfat milk in TBS-T. The protein was detected using an enhanced chemiluminescence detection kit (GE Biosciences, Piscataway, NJ).
Immunohistochemistry
Archival formalin-fixed, paraffin-embedded tissues of 50 prostate cancer cases were used in this study with New York University institutional review board approval. The ARA70β immunohistochemical staining was performed using single label immunohistochemistry by the NexES, automated immunostainer and detection system (Ventana Medical Systems, Tucson, AZ). Four-μm sections were deparaffinized in xylene, rehydrated through graded alcohols, and rinsed in distilled water. All incubations were performed at 37°C unless otherwise noted. After deparaffinization, heat-induced epitope retrieval was performed by microwaving sections with 0.01 mol/L citrate buffer, pH 6.0, for 20 minutes in a 1200 W microwave oven. Endogenous peroxidase was blocked by application of hydrogen peroxide for 4 minutes. ARA70β-specific antibody was detected by the application of a biotinylated goat anti-rabbit antibody for 8 minutes, followed by the application of streptavidin-horseradish peroxidase for 8 minutes. The chromogen, 3,3′-diaminobenzidine/hydrogen peroxide mix was applied for 8 minutes and then enhanced with copper sulfate for 4 minutes. Slides were then counterstained with hematoxylin, dehydrated, and mounted in permanent media. The levels of ARA70β expression were scored as 0 for negative staining, 1+ for weak staining, 2+ for moderate level of staining, and 3+ for strong staining. Because the levels of ARA70β staining are relatively constant among cells in a given case, the percentage of positive cells is not included as scoring criteria.
Yeast Two-Hybrid Assay
To construct vector pLexA:ARA70β, ARA70β was amplified by PCR using pcDNA-ARA70β as the template. The PCR product was digested with EcoRI and BamHI restriction endonucleases and inserted into EcoRI and BamHI sites of pEG202-NLS [HIS3, 2 μ, PADH1:LexA::NLS] (Origene Technologies, Rockville, MD). Vector pB42: NTD (N-terminal domain, amino acids 1 to 481 of human AR) was obtained from Dr. Susan Logan (New York University). All constructs were confirmed by sequencing, and their expression in yeast was confirmed by Western blot.
Yeast media were prepared as described.24 Yeast strain W303-1A (MATa leu2-3112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) was transformed25 with pSH18-34 reporter vector [URA3, 2 μ, 8xLexAop::lacZ] (Origene Technologies), pLexA, or pLexA::ARA70β, and one of the following vectors pB42, pB42::NTD, pB42::DBD, and pB42::LBD. Transformants were selected on synthetic dextrose agar medium lacking histidine, tryptophan, and uracil (SD-HTU). To perform the two-hybrid assay, selected transformants were grown overnight in synthetic liquid raffinose medium lacking histidine, tryptophan, and uracil (SR-HTU) and then diluted in three parts of SR-HTU and grown for a further 3 hours. Galactose was added to the final concentration of 2% w/v to induce AR expression. After 4 hours of galactose induction, optical density of the cultures was measured at 600 nm to determine cell density. The cell pellet was washed in water, centrifuged again, and resuspended in 150 μl of buffer Z (60 mmol/L Na2HPO4, 40 mmol/L NaH2PO4, 10 mmol/L KCl, 1 mmol/L MgSO4, 0.27% β-mercaptoethanol). Cells were lysed by vortexing with 50 μl of chloroform and 20 μl of 0.1% sodium dodecyl sulfate for 15 seconds. Next, 700 μl of 0.1% w/v solution of chlorophenol red-β-d-galactoside (CPRG; Fluka, Buchs, Switzerland) in buffer Z was added to the lysates and incubated at 30°C for 3 hours. Color development was stopped by the addition of 50 μl of 30 mmol/L ZnCl2. The mix was centrifuged, and the absorbance of the supernatant was measured at 578 nm. The β-galactosidase units were calculated as 1000 × A578/[A600 × reaction time (minute) × volume (ml)].
Results
Production of an ARA70β-Specific Antibody
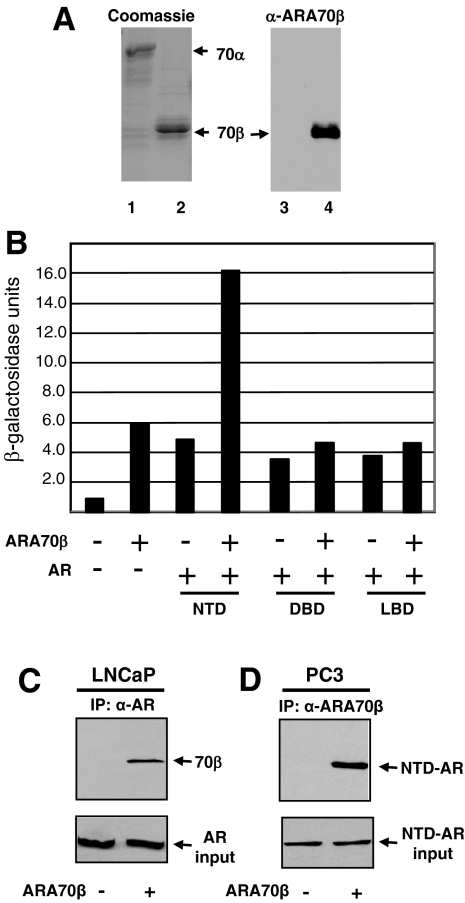
To determine the expression of ARA70β at the protein level, we generated an ARA70β isoform-specific antibody.23 The two amino acids (QQ) at the splice site are unique to ARA70β, forming the basis for the production of ARA70β-specific antibody. ARA70β-specific polyclonal antibody was generated using an 18-amino acid peptide (QKQTLENSQQEVLLNSPL) spanning the junction region of internal splicing as the immunogen. The ARA70β antibody was affinity purified and tested for specificity against recombinant ARA70α and ARA70β. Although there was an equivalent amount of ARA70α and ARA70β in each lane as revealed by Coomassie staining (Figure 1A, left), the ARA70β antibody did not recognize the ARA70α protein but showed substantial immunoreactivity toward ARA70β (Figure 1A, right). Thus, the antibody recognized ARA70β specifically.
Figure 1.
ARA70β isoform-specific antibody production and interaction between AR and ARA70β. A: Coomassie blue-stained SDS-PAGE gel showing recombinant purified ARA70α (lane 1) and ARA70β (lane 2) (right). ARA70β isoform-specific antibody recognizes ARA70β (lane 4) and not ARA70α (lane 3) (left). B: Interaction of ARA70β with the AR NTD, AR DBD, and the AR LBD was analyzed using the yeast two-hybrid assay. The strength of interaction was determined by a quantitative liquid β-galactosidase assay after 24 hours of incubation in galactose media at 30°C. Note the threefold increase in β-galactosidase activity with the AR NTD and ARA70β compared to other domains of AR. C: ARA70β interacts with endogenous AR in LNCaP cell extracts by co-immunoprecipitation. LNCaP cells were stably transfected with pBabe-ARA70β or the pBabe (vector control) and whole cell extracts were prepared as described in Materials and Methods and incubated with an antibody against AR. Immune complexes were collected on protein A-Sepharose beads, washed, eluted, resolved by SDS-PAGE, and transferred to a membrane. The filter was probed with antibody against AR and ARA70β. D: ARA70β interacts with N terminus of AR in PC3 cells overexpressing ARA70β and transiently transfected with N terminus of AR, as determined by co-immunoprecipitation.
Interaction between AR and ARA70β
We first examined the interaction between AR and ARA70β using the yeast two-hybrid system. Although ARA70α interacts primarily with the AR LBD, it can also interact with the AR NTD. Given that the interacting surface of ARA70α with the AR LBD is not present in the ARA70β splice variant, we reasoned that the interaction between ARA70β and AR most likely occurs via the AR NTD. To test this, we performed a yeast two-hybrid assay to detect the interaction between ARA70β and the functional domains of AR. ARA70β was fused to the DNA-binding domain of LexA and the individual domains of AR, including the NTD, DNA binding domain (DBD) and LBD were fused to the B42 transactivation domain. As shown in Figure 1B, ARA70β increased β-galactosidase activity up to threefold with the AR NTD, but not with the AR DBD or LBD. Because there are no AR or ARA70 homologues in yeast, the interaction between AR and ARA70β is likely direct.
To confirm the interaction between ARA70β and AR, we next tested the ability of ARA70β to interact with AR in LNCaP cells stably expressing ARA70β. Whole cell extracts from vector control- or ARA70β-transfected LNCaP cells were subjected to immunoprecipitation with an antibody against AR and analyzed by immunoblotting with an antibody to ARA70β. AR was co-immunoprecipitated in cells expressing ARA70β (Figure 1C, top). As expected, similar amounts of AR was immunoprecipitated by the AR antibody in both the control LNCaP-pBabe- and LNCaP-ARA70β-overexpressing cells (Figure 1C, bottom). We also performed co-immunoprecipitation experiments of ARA70β with the N terminus of AR (transiently transfected) in PC3 cells overexpressing ARA70β (PC3-ARA70β) or control PC3-pBabe using ARA70β-specific antibody. We were able to detect ARA70β co-immunoprecipitated with the N terminus of AR (Figure 1D, top). These results confirm the interaction between the N terminus of AR and ARA70β.
ARA70α and ARA70β Enhance AR-Mediated Transcriptional Activation
Multiple studies have demonstrated that ARA70α overexpression enhances AR-mediated transcriptional activation in a ligand-dependent manner. However, the extent to which transcription by AR is activated varies from 2- to 10-fold,15,18,20 which may reflect cell-type or other experimental differences among the studies. In addition, a direct comparison of the co-activator potential ARA70α versus ARA70β in cell based reporter assays has not been described.
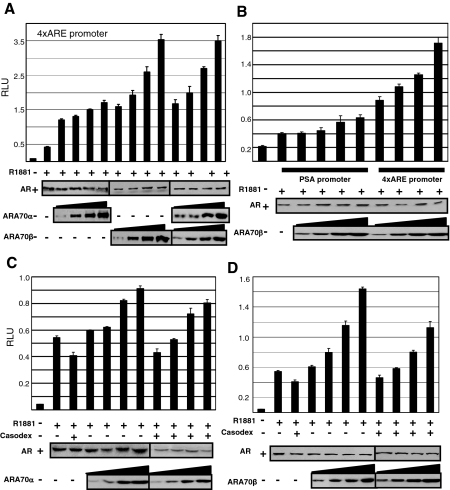
A transient transfection assay was used to examine the extent of ARA70α and ARA70β regulation of AR transcriptional activation. AR-deficient PC3 cells were transfected with a constant amount of AR and increasing concentrations of expression vectors encoding either ARA70α or ARA70β, along with an AR-responsive luciferase reporter gene (4xARE). As shown in Figure 2, the hormone-induced AR transcriptional activation was increased in a dose-dependent manner by overexpressed ARA70α and ARA70β. We were able to achieve a threefold ligand-dependent increase in AR-mediated transcriptional activation with ARA70α expression (Figure 2A) and a sixfold activation of AR transcriptional enhancement by ARA70β (Figure 2A). We also performed luciferase assays with a reporter gene containing the PSA promoter and regulatory region. Similarly, ARA70β was able to enhance AR-mediated transcriptional activation, but to a lesser extent as compared to the 4xARE reporter gene (Figure 2B). Although both ARA70α and ARA70β can act as positive regulators of AR transcriptional activity in cell-based reporter assays, ARA70β appears to be more potent than ARA70α at enhancing AR transcriptional activity, suggesting functional differences between the two isoforms.
Figure 2.
ARA70α and ARA70β function as AR co-activators in prostate cell lines. PC3 cells were transiently transfected with AR and 4XARE-Luc or PSA-Luc reporter genes along with increasing amounts of ARA70α or ARA70β or empty expression vector to equalize the total amount of DNA per condition. Cells were treated with 10 nmol/L R1881 (+) or ethanol vehicle (−) for 12 hours and receptor transcriptional activation was assayed as described in Materials and Methods, normalized for transfection efficiency, and expressed as relative luminescence units (RLUs). The average of three independent experiments and SE is shown. A: Transcriptional activation of ARA70α and ARA70β on 4XARE-Luc-reporter gene. B: Transcriptional activation of ARA70β on PSA-Luc reporter gene. C: Antagonist effects of Casodex on ARA70α transcriptional activation. D: Antagonist effects of Casodex on ARA70β transcriptional activation.
Because some anti-androgens may have androgenic activity under the influence of a co-activator, we determined the effects of the nonsteroidal anti-androgen bicalutamide (Casodex) on the transcriptional activation of AR as a function of ARA70α and ARA70β overexpression. Casodex did not exhibit androgenic activity in the presence of either ARA70 isoform (Figure 2, C and D). To determine whether ARA70β affects the transcriptional activation function of ARA70α, we performed luciferase assays in the presence of both ARA70α and ARA70β. We did not observe antagonistic or synergistic effects of ARA70α on ARA70β (Figure 2A).
ARA70β Enhances Prostate Cancer Cell Proliferation and Anchorage-Independent Growth
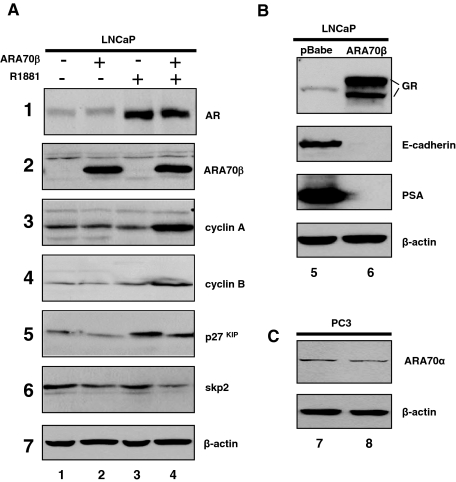
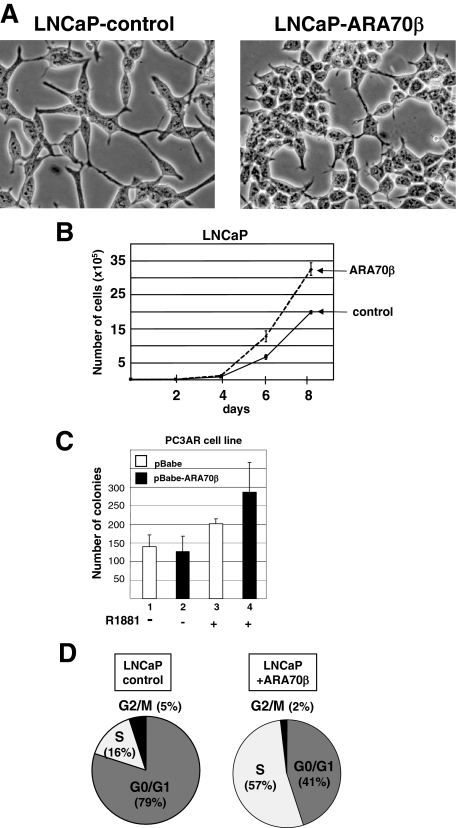
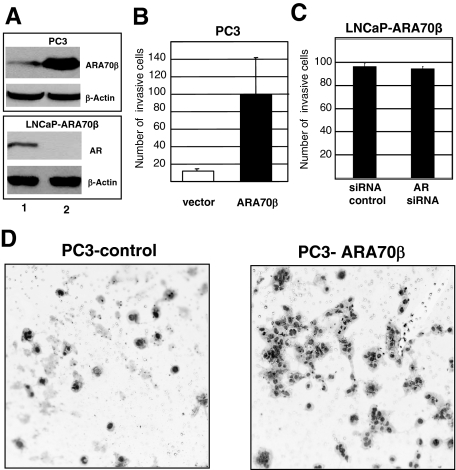
To determine the growth-regulatory effects of ARA70β on prostate cancer cells in vitro, we generated stable cell lines expressing ARA70β by retroviral transduction of LNCaP prostate cancer cells (Figure 3A2, lanes 2 and 4). Multiple clones were generated and ARA70β expression was verified by RT-PCR and immunoblotting with the ARA70β-specific antibody (data not shown). Morphologically, LNCaP cells expressing ARA70β became round with few processes, in contrast to cells without ARA70β that were flat with many processes (Figure 4A).
Figure 3.
Stable expression of ARA70β and expression of cell cycle regulatory proteins in ARA70β-containing LNCaP cells. A: Western blots were performed with whole cell lysates prepared from LNCaP cells expressing ARA70β or LNCaP with vector control cells were cultured in the absence (−) or presence (+) of 10 nmol/L R881 (cell were treated for 24 hours). On adjusting the total protein concentration in each sample, the proteins were resolved on 10 or 12.5% SDS-PAGE, transferred to Immobilon paper, and the blots were probed with antibodies against AR, ARA70β, cyclin A, cyclin B, p27, and skp2. Each blot is representative of at least three independent experiments. B: Western blot showing increased expression of glucocorticoid receptor (GR), decreased E-cadherin, and PSA intracellular concentration in LNCaP cells overexpressing ARA70β (lane 5, LNCaP-pBabe; lane 6, LNCaP-ARA70β). C: Western blot showing expression of ARA70α (top) and β-actin (bottom) in PC3 (lane 7) and PC3-ARA70β cells (lane 8).
Figure 4.
ARA70β promotes LNCaP cellular proliferation. Morphology of LNCaP-ARA70β cells. A: Photomicrographs of LNCaP cell line transduced with a control vector (pBabe) (left) or expressing ARA70β (right) are shown. Note the altered cellular morphology in the ARA70β-expressing cells. B: Cell growth kinetics of LNCaP stable lines. LNCaP-ARA70β (dotted line) or control LNCaP cells (solid line) were seeded on day 0 in androgen media and the total numbers of viable cells were determined on the indicated days using the trypan blue exclusion method. The graph represents one of at least three independent experiments. C: PC3/AR cells overexpressing ARA70β showed an increased number (lane 4) of colonies in soft agar compared with PC3/AR cells with control vector (lane 3) in androgen media. There is no significant difference in the number of colonies for PC3/AR cells with (lane 2) and without (lane 1) ARA70β overexpression in androgen-free media. D: Shift of LNCaP cells from G0/G1 (left) to S (right) phase of cell cycle with ARA70β overexpression. LNCaP cells were cultured in the presence of 10 nmol/L R881 for 48 hours. The DNA was stained with propidium iodide and the nuclear emitted fluorescence measured in a FACScan flow cytometer. Pie chart displays the proportion of cells in particular phases of the cell cycle, with percentage of S phase cells shown. The experiment was repeated three times with similar results. Original magnifications, ×400.
We next examined whether ARA70β expression affected LNCaP cellular proliferation. We measured proliferation of LNCaP cells expressing ARA70β and compared that to an LNCaP pBabe vector control line. Expression of ARA70β cells caused an increase in cell number compared to control cells in the presence of 10 nmol/L R1881 (Figure 4B). This effect was confirmed in three independent clones as well as a pooled line (data not shown).
We also examined cell proliferation and anchorage-independent growth of PC3/AR cells expressing ARA70β. Similar to LNCaP cells, PC3/AR cells overexpressing ARA70β showed increased cell growth compared to control cells (data not shown). Interestingly, anchorage-independent growth, as determined by the number of colonies capable of growing in soft agar, was greater for PC3AR-ARA70β in androgen-containing compared to androgen-free media compared to control cells (Figure 4C, lane 4 versus lane 3 and lane 2 versus lane 1). Of note, the anchorage-independent assays showed a slight increase in the number of colonies in PC/3AR cells on androgen treatment (Figure 4C, lanes 1 and 3). This is in contrast to previous reports that androgen treatment inhibits PC3/AR cell growth.26,27 Our result could be explained by several factors. For example, this current study measured PC3/AR growth in soft agar rather than on a plate. Different levels of AR expression among PC3/AR clones could also account for such differences. Indeed, PC3/AR lines with the highest AR expression display the least amount of androgen-dependent growth inhibition.27 ARA70β overexpression in PC3 cells had no significant impact on the morphology, cellular proliferation, or growth in soft agar (data not shown). Together, these data suggest that the effects of ARA70β on cell proliferation are AR-dependent.
Expression of Cell Cycle Regulatory Proteins in LNCaP Cells Expressing ARA70β
We asked whether the ARA70β-dependent increase in LNCaP cell number reflected a stage-specific effect on the cell cycle. Cell cycle distribution of LNCaP-ARA70β and LNCaP control cells was assessed by flow cytometry. LNCaP-ARA70β cells showed an increase in the population of cells in S phase to 57% compared to 16% observed in control LNCaP cells cultured in androgen-containing media (Figure 4D).
A number of mechanisms could account for the S phase growth stimulation in ARA70β-expressing LNCaP cells. ARA70β may increase the level or activity of growth-promoting factors involved in the G1 to S phase transition, such as the cyclins. Alternatively, the ARA70β may repress the production of growth-inhibiting factors, such as the p27Kip1. To determine which mechanism(s) may be operating to enhance cell proliferation, we examined the expression of cell cycle regulators including cyclin A, cyclin B, p27Kip1, and skp2. ARA70β-expressing and control LNCaP cells were cultured in the absence or presence of 10 nmol/L R1881. Whole cell extracts were prepared and subjected to immunoblot analysis using antibodies against the proteins described above. Androgen-treatment of ARA70β-expressing LNCaP cells resulted in an increase in the steady state level of cyclin A (Figure 3, panel 3) and to a lesser extent cyclin B1 (Figure 3, panel 4). We also found that the level of p27Kip1 (Figure 3, panel 5) was decreased in ARA70β-expressing cells treated with androgen. Interestingly, the level of p21 expression was not altered by the expression of ARA70β in either complete or androgen-containing media (data not shown), indicating p21 is not involved in the growth regulation by ARA70β. Together, these results suggest that the ARA70β-dependent growth stimulation in LNCaP cells is associated with AR’s ability to decrease the level of growth inhibitory proteins, including p27Kip1, and increase the concentration of growth promoting factors such as cyclin A.
Androgen-Independent Increase in Prostate Cancer Cell Invasion by ARA70β
We further examined the ability of LNCaP and PC3 cells expressing ARA70β to invade through a basement membrane in vitro. The invasion assays were performed using Matrigel invasion chambers with 10% fetal bovine serum as the chemoattractant. Parental LNCaP cells do not readily invade through Matrigel. ARA70β overexpression stimulated the invasion of LNCaP cells through Matrigel by threefold (Table 1). Importantly, there is a 10-fold increase in the number of LNCaP cells invading through the Matrigel28 when assayed using benign immortalized prostatic stromal cells as the chemoattractant (Table 1). The prostatic stromal cells were immortalized using telomerase and characterized as myofibroblasts by electron microscopy with normal karyotype (P.L., unpublished data). This finding suggests that a paracrine factor released by the stroma further augments invasion mediated by ARA70β in LNCaP cells.
PC3 cells overexpressing ARA70β (Figure 5A, top) were also examined for their ability to invade through Matrigel. An increase in the number of invading cells was observed in ARA70β-overexpressing PC3 cells compared to control cells (Figure 5, B and D). Because ARA70β increased cell invasion in both AR-expressing LNCaP and non/low-AR-expressing PC3 cells, these results suggest that the enhanced cell invasion by ARA70β is AR-independent. To determine whether the promotion of LNCaP cell invasion by ARA70β requires AR, we eliminated AR by RNA interference in LNCaP-ARA70β cells and examined cell invasion (Figure 5A, bottom). Our results demonstrate that cell invasion by ARA70β was unaffected by the loss of AR in LNCaP cells (Figure 5C), indicating that the increased cell invasion by ARA70β is AR-independent.
Figure 5.
Increased invasion ability of PC3 cells when transfected with ARA70β. A: ARA70β expression in PC3-ARA70β cells (top) and reduced AR expression (bottom) by AR siRNA in LNCaP cells. B: Increasing numbers of PC3 cells invade through Matrigel membrane when transfected with ARA70β as compared to pBabe vector alone control cells in regular media. C: Comparable numbers of LNCaP-ARA70β cells invade when AR is reduced by AR siRNA compared to control siRNA. D: Photomicrographs showing increased numbers of PC3 cells expressing ARA70β invading through Matrigel and forming clusters (right) relative to vector control cells (left). Original magnifications, ×400.
Expression Profiling Reveals Global Changes in Gene Expression on ARA70β Expression
To elucidate the genes and pathways regulated by ARA70β in LNCaP cells, we performed an expression profiling experiment using Affymetrix microarray technology.29 Gene expression levels were calculated from the CEL files using the multiarray averaging method, after quantile normalization. The expression of each transcript between ARA70β-overexpressing and control pBabe vector-expressing cells were compared using the two-sample t-test, and P values <0.05 were declared statistically significant.
We observed a substantial change in the pattern of gene expression when ARA70β is overexpressed in LNCaP cells. There were ∼2000 genes with expression changes of more than fivefold and 953 genes with expression changes of greater than 10-fold. Among the 953 genes, 404 are repressed and 549 are activated on ARA70β overexpression. The gene with the greatest increase in expression is HOXA9 (160-fold), whereas neutral endopeptidase (NEP) shows the largest decrease in expression (1700-fold) on ARA70β expression. Many of the changes in gene expression appear related to increased cellular metabolism, perhaps as a result of enhanced cell proliferation (Table 2). Examples for metabolic genes with higher expression include aldehyde dehydrogenase (34-fold) and aldo-keto reductase (15-fold). Because prostate cancer cells are rich in lipids, genes involved in fatty acid and lipid metabolism are also increased, including fatty acid desaturase (58-fold), fatty acid-binding protein (33-fold), and phospholipid transfer protein (14-fold). The levels of certain transcription factors are also elevated. For example, there is a 61-fold increase in a POU domain transcription factor and a 14-fold up-regulation of the GATA binding protein 4. Interestingly, the Homeobox transcription factors HOXD13 and HOXA9, key regulators of development, also show a sharp increase in expression on ARA70β overexpression.
Evidence substantiating that ARA70β increased cellular proliferation is reflected in the fact that certain oncogenes and growth regulatory factors were also up-regulated. We observed an increase in the expression of the proto-oncogene Fyn (54-fold), and growth regulators including the glucocorticoid receptor (98-fold) and fibroblast growth factor receptor 3 (53-fold). Indeed, we confirmed the increased glucocorticoid receptor protein expression in LNCaP cells overexpressing ARA70β (Figure 3B).
Because ARA70β functions as an AR co-activator, we were particularly interested in the gene expression changes of AR target genes. Both NEP and NKX3.1 are AR target genes and have tumor suppressor function.30,31,32,33 Interestingly, NEP and NKX3.1 show a dramatic decrease in expression as a function of ARA70β (1700-fold and 55-fold, respectively). ARA70β overexpression also decreased the mRNA expression of PSA (1138-fold), which was confirmed by real-time RT-PCR (data not shown) and Western blot analysis (Figure 3B). This may seem counterintuitive, given that ARA70β functions as an AR transcriptional co-activator. However, immunohistochemistry of AR and PSA on 20 prostate cancer cases found decreased levels of PSA in the prostate tissue despite ample AR expression (data not shown). This is consistent with a previous report showing decreased PSA expression in prostate cancer tissue,34 despite high serum levels of PSA. This implies PSA expression in response to cellular and environmental changes that occur during transformation is complex.
We have shown that ARA70β stimulated LNCaP and PC3 prostate cancer cell invasion. Interestingly, genes involved in cell adhesion are decreased on ARA70β overexpression, including E-cadherin and claudins. We confirmed decreased E-cadherin expression on ARA70β overexpression by Western blot analysis (Figure 3B). Reduced expression of E-cadherin has also been associated with less differentiated prostate cancers.35,36 Claudin 3 and claudin 7 are calcium-dependent adhesion proteins in the tight junction of epithelial cells. Decreased expression would be expected to facilitate cancer invasion and metastasis. Taken together, these gene expression changes are consistent with ARA70β promoting prostate cancer cell proliferation and invasion.
Increased Expression of ARA70β in Human Prostate Cancer
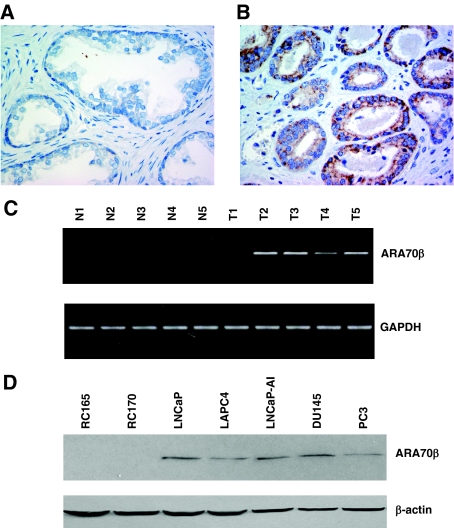
To determine the expression levels of ARA70β in prostate cancer, we performed immunohistochemistry on 50 prostatectomy specimens from prostate cancer patients using an ARA70β-specific antibody. Although ARA70β is not expressed in benign prostatic epithelium (Figure 6A), we did observe ARA70β expression in 15 of the 50 (30%) cancer cases examined (Figure 6B). Of the 15 cases with increased ARA70β expression, 9 showed moderate to strong expression of ARA70β expression in cancer (not shown). Because of the small sample size in high-grade tumor in this series, we were unable to determine whether ARA70β expression correlates with tumor grade and stage. The positive signal was blocked by antigenic peptide, and preimmune serum showed no immunoreactivity above background. These data correlate well with previous reports demonstrating that whereas ARA70α expression is decreased,8,21 total ARA70 (α and β) is increased in prostate cancer,17 suggesting ARA70β expression is increased in cancer.
Figure 6.
Increased ARA70β expression in prostate cancer. A: ARA70β is not expressed in benign prostate tissue by immunohistochemistry using an ARA70β isoform-specific antibody. B: ARA70β is expressed primarily in the cytoplasm of a subset of prostate cancer cells. Preimmune serum and peptide antigen were used as negative controls. C: ARA70β expression in five matched benign (N1 to N5) and malignant (T1 to T5) prostate tissues by RT-PCR. N, Normal prostate, T, tumor. D: ARA70β expression in prostate cancer cell lines (LNCaP, LAPC4, PC3, and DU145) compared to benign prostate cells (RC165 and RC170) by Western blot analysis. Original magnifications, ×400.
We also examined ARA70β expression by RT-PCR and Western blot from benign and malignant prostate tissue and cell lines. ARA70β mRNA was more highly expressed in four of five malignant prostate cases examined relative to the matched benign tissue by RT-PCR (Figure 6C). Likewise, ARA70β protein expression is higher in the prostate cancer cell lines LNCaP, LAPC4, PC3, and DU145 relative to the benign telomeraseimmortalized prostate epithelial lines RC165 and RC170 (Figure 6D).37 Thus, ARA70β expression is elevated in prostate cancer cells compared to their normal counterparts.
Discussion
Although activation of AR can induce cell proliferation in the prostate, receptor activation also inhibits the proliferation of prostate cells under certain circumstances. For example, ectopic expression of AR appears to restrain cell growth in response to androgens in PC3 cells cultured on plastic.26,27 Furthermore, an immortalized nontumorigenic rat prostatic cell line engineered to stably express AR also showed reduced growth and differentiated on androgen treatment compared to control cells lacking AR.38 Here we demonstrate that the ARA70β splice variant has profound effects on prostate cancer cell growth, invasion, and gene regulation. Like ARA70α, we show that ARA70β can function as an AR co-activator in cell-based reporter gene assays. However, despite ARA70β and ARA70α exhibiting AR co-activator function, their effect on prostate cancer growth is distinct: ARA70α inhibits whereas ARA70β promotes androgen-dependent cellular proliferation. This differential response of ARA70β and ARA70α may in part underlie the switch between AR-dependent proliferation versus growth arrest and differentiation.
In prostate cancer it has been shown that ARA70α is expressed in LNCaP, PC3, and DU145, with its levels of expression decreased in primary benign prostatic epithelial cells.21 We have reported that ARA70α mRNA is decreased in prostate cancer specimens by in situ hybridization.8 Consistent with low ARA70α expression in cancer, overexpression of ARA70α reduced LNCaP cell proliferation.8
It has previously been shown that ARA70β is expressed most highly in PC3, relative to DU145 and LNCaP cells.22 Using a novel ARA70β-specific antibody, we now show that ARA70β is also expressed in LNCaP cells at the protein level (Figure 6D). In breast, Kollara and colleagues22 showed that ARA70β is highly expressed in breast cancer relative to matched normal tissue. Our findings also show that expression of ARA70β is elevated in prostate cancer compared to benign cell lines and tissues. These results indicate that ARA70α and ARA70β might have different expression profiles and functions in cancer including prostate. One appealing hypothesis is that ARA70α may interact with the AR in normal prostate epithelial cells to suppress cell proliferation, whereas ARA70β would do the opposite and endow prostate cells with the ability to grow and invade. We propose that distinct functions of ARA70α and ARA70β are likely because of differential expression of genes controlled by the ARA70 isoforms.
Unlike ARA70α, which is nuclear, ARA70β is expressed primarily in the cytoplasm in human prostate cancer. A previous report also shows cytoplasmic localization of ARA70β, although the antibody used in that experiment recognizes both α and β forms.17 The localization of ARA70β in the cytoplasm may suggest an indirect or class II AR co-activator function, whereas ARA70α likely functions as classic nuclear or class I co-activator.17
Together with its increased expression in prostate cancer, ARA70β stimulates prostate cancer growth in an androgen-dependent manner. Indeed, enhanced cellular proliferation and anchorage-independent growth by ARA70β was only observed in AR-expressing prostate cells (LNCaP and PC3/AR). These results indicate that the ARA70β-mediated growth is androgen-dependent, possibly through activation of a novel subset of AR target genes involved in proliferation such as cyclin B (Y.L. and P.L., unpublished data). Repression of growth inhibitory molecules, including p27, also appeared enhanced by ARA70β overexpression, which would further stimulate cell proliferation. It will also be interesting to determine the involvement of growth factor pathways, including IGF, TGF, FGF, and JAK/Stat, in the regulation of prostate cancer growth by ARA70β because these genes are sharply up-regulated in LNCaP cells overexpressing ARA70β, as determined by gene expression profiling.
ARA70β also dramatically increased the ability of PC3 and LNCaP cells to invade through Matrigel. In contrast to cell proliferation, this effect appears AR-independent because PC3, which lack endogenous AR, showed enhanced invasiveness on ARA70β overexpression. Likewise, LNCaP in which AR levels were reduced by siRNA remained invasive. This likely reflects differences in the target genes induced by ARA70β in the absence and presence of AR. Our results may also be clinically important for prostate cancers that overexpress ARA70β because androgen ablation may reduce growth but not invasion of the cancer cells, which could hasten recurrence and/or metastasis. Our future studies will be directed toward determining whether the candidate genes identified by expression profiling, such as E-cadherin, are directly involved in the regulation of the invasive processes by ARA70β and serve as potential targets to block cell invasion.
In summary, we have found that ARA70β stimulates prostate cancer growth through androgen- and AR-dependent pathways and also enhances prostate cancer invasion via androgen- and AR-independent mechanisms. Our results define a new and prominent role for ARA70β in prostate cancer growth and invasion.
Acknowledgments
We thank Dr. Susan Logan and Dr. Angel Pellicer for helpful discussion, Dr. John Hirst for flow cytometric analysis, Hongfeng Guo for assistance with the Matrigel invasion assays, and Susan Ha for her excellent technical assistance and editorial skills.
Footnotes
Address reprint requests to Peng Lee M.D., Ph.D., Department of Pathology, New York University School of Medicine, New York Harbor Healthcare System, 423 E. 23rd St., Room 6140N, New York, NY 10010. E-mail: peng.lee@med.nyu.edu.
Supported by the Department of Veterans Affairs (Seed funds), the Department of Defense (Prostate Cancer Research Program award PC041210 to P.L.), and the National Institutes of Health (grant DK058024 to M.J.G.).
Y.P. and C.X.L. contributed equally to this study.
References
- Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- Brinkmann AO, Blok LJ, de Ruiter PE, Doesburg P, Steketee K, Berrevoets CA, Trapman J. Mechanisms of androgen receptor activation and function. J Steroid Biochem Mol Biol. 1999;69:307–313. doi: 10.1016/s0960-0760(99)00049-7. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Xu J, Nawaz Z, Tsai SY, Tsai MJ, O’Malley BW. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J Steroid Biochem Mol Biol. 1999;69:3–12. doi: 10.1016/s0960-0760(98)00144-7. [DOI] [PubMed] [Google Scholar]
- Kumar R, Wang RA, Barnes CJ. Coregulators and chromatin remodeling in transcriptional control. Mol Carcinog. 2004;41:221–230. doi: 10.1002/mc.20056. [DOI] [PubMed] [Google Scholar]
- Janne OA, Moilanen AM, Poukka H, Rouleau N, Karvonen U, Kotaja N, Hakli M, Palvimo JJ. Androgen-receptor-interacting nuclear proteins. Biochem Soc Trans. 2000;28:401–405. [PubMed] [Google Scholar]
- Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- Li P, Yu X, Ge K, Melamed J, Roeder RG, Wang Z. Heterogeneous expression and functions of androgen receptor co-factors in primary prostate cancer. Am J Pathol. 2002;161:1467–1474. doi: 10.1016/S0002-9440(10)64422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnjak M, Osman I, Scher HI, Fazzari M, Cordon-Cardo C. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res. 2000;6:1891–1895. [PubMed] [Google Scholar]
- Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277:7076–7085. doi: 10.1074/jbc.M108255200. [DOI] [PubMed] [Google Scholar]
- Müller JM, Metzger E, Greschik H, Bosserhoff AK, Mercep L, Buettner R, Schule R. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J. 2002;21:736–748. doi: 10.1093/emboj/21.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja SS, Ha S, Swenson NK, Torra IP, Rome S, Walden PD, Huang HY, Shapiro E, Garabedian MJ, Logan SK. ART-27, an androgen receptor coactivator regulated in prostate development and cancer. J Biol Chem. 2004;279:13944–13952. doi: 10.1074/jbc.M306576200. [DOI] [PubMed] [Google Scholar]
- Santoro M, Dathan NA, Berlingieri MT, Bongarzone I, Paulin C, Grieco M, Pierotti MA, Vecchio G, Fusco A. Molecular characterization of RET/PTC3; a novel rearranged version of the RETproto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994;9:509–516. [PubMed] [Google Scholar]
- Bongarzone I, Butti MG, Coronelli S, Borrello MG, Santoro M, Mondellini P, Pilotti S, Fusco A, Della Porta G, Pierotti MA. Frequent activation of ret protooncogene by fusion with a new activating gene in papillary thyroid carcinomas. Cancer Res. 1994;54:2979–2985. [PubMed] [Google Scholar]
- Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Minges JT, Lee LW, Wilson EM. The FXXLF motif mediates androgen receptor-specific interactions with coregulators. J Biol Chem. 2002;277:10226–10235. doi: 10.1074/jbc.M111975200. [DOI] [PubMed] [Google Scholar]
- Hu YC, Yeh S, Yeh SD, Sampson ER, Huang J, Li P, Hsu CL, Ting HJ, Lin HK, Wang L, Kim E, Ni J, Chang C. Functional domain and motif analyses of androgen receptor coregulator ARA70 and its differential expression in prostate cancer. J Biol Chem. 2004;279:33438–33446. doi: 10.1074/jbc.M401781200. [DOI] [PubMed] [Google Scholar]
- Gao T, Brantley K, Bolu E, McPhaul MJ. RFG (ARA70, ELE1) interacts with the human androgen receptor in a ligand-dependent fashion, but functions only weakly as a coactivator in cotransfection assays. Mol Endocrinol. 1999;13:1645–1656. doi: 10.1210/mend.13.10.0352. [DOI] [PubMed] [Google Scholar]
- Zhou ZX, He B, Hall SH, Wilson EM, French FS. Domain interactions between coregulator ARA70 and the androgen receptor (AR). Mol Endocrinol. 2002;16:287–300. doi: 10.1210/mend.16.2.0765. [DOI] [PubMed] [Google Scholar]
- Alen P, Claessens F, Schoenmakers E, Swinnen JV, Verhoeven G, Rombauts W, Peeters B. Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1alpha with multiple steroid receptors and identification of an internally deleted ELE1beta isoform. Mol Endocrinol. 1999;13:117–128. doi: 10.1210/mend.13.1.0214. [DOI] [PubMed] [Google Scholar]
- Tekur S, Lau KM, Long J, Burnstein K, Ho SM. Expression of RFG/ELE1alpha/ARA70 in normal and malignant prostatic epithelial cell cultures and lines: regulation by methylation and sex steroids. Mol Carcinog. 2001;30:1–13. doi: 10.1002/1098-2744(200101)30:1<1::aid-mc1008>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kollara A, Kahn HJ, Marks A, Brown TJ. Loss of androgen receptor associated protein 70 (ARA70) expression in a subset of HER2-positive breast cancers. Breast Cancer Res Treat. 2001;67:245–253. doi: 10.1023/a:1017938608460. [DOI] [PubMed] [Google Scholar]
- Peng Y, Chiriboga L, Pei Z, Wang Z, Lee P: Androgen receptor coactivator ARA70α and ARA70β isoform-specific antibodies: new tools for studies of expression and immunohistochemical localization. Appl Immunohistochem Mol Morphol, [Epub ahead of press] [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–1311. [PubMed] [Google Scholar]
- Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura M, Miura H, Nonomura N, Takada S, Takahara S, Matsumoto K, Nakamura T, Matsumiya K. Prostate stromal cell-derived hepatocyte growth factor induces invasion of prostate cancer cell line DU145 through tumor-stromal interaction. Prostate. 1999;41:145–153. doi: 10.1002/(sici)1097-0045(19991101)41:3<145::aid-pros1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Osman I, Sorhaindo L, Navarro D, Taneja S, Lee P, Christos P, Shen R, Nanus D. Loss of neutral endopeptidase and Akt activation is associated with prostate cancer progression. Cancer. 2006;107:2628–2636. doi: 10.1002/cncr.22312. [DOI] [PubMed] [Google Scholar]
- Abdulkadir SA. Mechanisms of prostate tumorigenesis: roles for transcription factors Nkx3.1 and Egr1. Ann NY Acad Sci. 2005;1059:33–40. doi: 10.1196/annals.1339.018. [DOI] [PubMed] [Google Scholar]
- Asatiani E, Huang WX, Wang A, Rodriguez Ortner E, Cavalli LR, Haddad BR, Gelmann EP. Deletion, methylation, and expression of the NKX3.1 suppressor gene in primary human prostate cancer. Cancer Res. 2005;65:1164–1173. doi: 10.1158/0008-5472.CAN-04-2688. [DOI] [PubMed] [Google Scholar]
- Dai J, Shen R, Sumitomo M, Goldberg JS, Geng Y, Navarro D, Xu S, Koutcher JA, Garzotto M, Powell CT, Nanus DM. Tumor-suppressive effects of neutral endopeptidase in androgen-independent prostate cancer cells. Clin Cancer Res. 2001;7:1370–1377. [PubMed] [Google Scholar]
- Gallee MP, Visser-de Jong E, van der Korput JA, van der Kwast TH, ten Kate FJ, Schroeder FH, Trapman J. Variation of prostate-specific antigen expression in different tumour growth patterns present in prostatectomy specimens. Urol Res. 1990;18:181–187. doi: 10.1007/BF00295844. [DOI] [PubMed] [Google Scholar]
- Jaggi M, Johansson SL, Baker JJ, Smith LM, Galich A, Balaji KC. Aberrant expression of E-cadherin and beta-catenin in human prostate cancer. Urol Oncol. 2005;23:402–406. doi: 10.1016/j.urolonc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Paul R, Ewing CM, Jarrard DF, Isaacs WB. The cadherin cell-cell adhesion pathway in prostate cancer progression. Br J Urol. 1997;79(Suppl 1):37–43. doi: 10.1111/j.1464-410x.1997.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Gu Y, Li H, Miki J, Kim KH, Furusato B, Sesterhenn IA, Chu WS, McLeod DG, Srivastava S, Ewing CM, Isaacs WB, Rhim JS. Phenotypic characterization of telomerase-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines. Exp Cell Res. 2006;312:831–843. doi: 10.1016/j.yexcr.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Whitacre DC, Chauhan S, Davis T, Gordon D, Cress AE, Miesfeld RL. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ. 2002;13:1–11. [PubMed] [Google Scholar]