Abstract
We have previously shown that glycosaminoglycan (GAG) storage in animal models of the mucopolysaccharidoses (MPS) leads to inflammation and apoptosis within cartilage. We have now extended these findings to synovial tissue and further explored the mechanism underlying GAG-mediated disease. Analysis of MPS rats, cats, and/or dogs revealed that MPS synovial fibroblasts and fluid displayed elevated expression of numerous inflammatory molecules, including several proteins important for lipopolysaccharide signaling (eg, Toll-like receptor 4 and lipoprotein-binding protein). The expression of tumor necrosis factor, in particular, was elevated up to 50-fold, leading to up-regulation of the osteoclast survival factor, receptor activator of nuclear factor-κB ligand, and the appearance of multinucleated osteoclast-like cells in the MPS bone marrow. Treatment of normal synovial fibroblasts with GAGs also led to production of the prosurvival lipid sphingosine-1-phosphate, resulting in enhanced cell proliferation, consistent with the hyperplastic synovial tissue observed in MPS patients. In contrast, GAG treatment of normal chondrocytes led to production of the proapoptotic lipid ceramide, confirming the enhanced cell death we had previously observed in MPS cartilage. These findings have important implications for the pathogenesis and treatment of MPS and have further defined the mechanism of GAG-stimulated disease.
The mucopolysaccharidoses (MPS) are a group of 11 distinct lysosomal storage disorders attributable to defective catabolism of glycosaminoglycans (GAGs), leading to severe joint and bone disease.1 Our previous studies in MPS animal models showed that inflammation is a critical aspect of these disorders, secondary to GAG accumulation.2,3 As part of this inflammatory cascade, tumor necrosis factor (TNF-α) and other inflammatory cytokines [eg, interleukin (IL-1β)] are released from chondrocytes, resulting in apoptosis. In addition, matrix metalloproteinases (MMPs) are released, contributing to the joint and bone destruction.
Lipopolysaccharide (LPS), a molecule that is structurally similar to GAGs, stimulates a signaling pathway that is pivotal to the pathogenesis of many chronic inflammatory diseases, including rheumatoid arthritis (RA).4 LPS signaling occurs through Toll-like receptor 4 (TLR4) and can result in the release of TNF-α and other proinflammatory cytokines. Based on these observations, we put forth a hypothesis suggesting that GAG accumulation in MPS connective tissues induces TNF-α release and inflammation through stimulation of the LPS signaling pathway.3 Notably, although TNF-α promotes apoptosis in cartilage,5 this cytokine has a proliferative effect in synovial cells.6 This would be consistent with the hyperplasia observed in MPS synovial tissue.7,8
Abnormal GAG metabolism has been observed in several common autoimmune diseases, including RA, scleroderma, systemic lupus erythematosus, and others.9,10 For example, patients with RA have elevated concentrations of GAGs in blood and synovial fluid, and the destruction of joints in these patients correlates positively with high GAG levels in synovial fluid. Injection of GAGs into normal mice also induces arthritis, tendosynovitis, dermatitis, and other pathological conditions, and it has been suggested that GAGs stimulate expansion of inflammatory cells, provoking autoimmune dysfunction.11
Thus, the aim of the current study was to characterize further the mechanism of GAG-mediated lesions in the MPS disorders, with the long-term goal of using these findings to develop novel treatment strategies for these and other GAG-mediated diseases. Gene and protein expression analysis was performed on synovial fibroblasts obtained from rats with one MPS type (MPS VI, Maroteaux-Lamy disease), revealing a markedly abnormal, proinflammatory expression pattern. Of note, several molecules important for LPS signaling were elevated in the MPS cells [eg, LPS-binding protein (LBP), TLR4, CD14, MyD88]. We also investigated how GAG storage influenced the levels of two important signaling lipids, ceramide and sphingosine-1-phosphate (S1P), known mediators of LPS activation. GAG treatment of chondrocytes led to elevation of the proapoptotic lipid, ceramide, consistent with enhanced apoptosis in these cells.2 In contrast, a decrease in ceramide and elevated production of the prosurvival lipid, S1P, was observed in MPS synovial cells, leading to an enhanced proliferation rate and contributing to the hyperplastic MPS synovial membranes.
We further studied the effects of elevated TNF-α expression on MPS joint and bone pathogenesis. TNF-α and receptor activator of nuclear factor (NF-κB) ligand (RANKL) stimulate osteoclast differentiation in RA.12 RANKL is essential for osteoclast differentiation, expressed on T cells and fibroblasts within inflamed synovial tissue, and regulated by proinflammatory cytokines. We found that RANKL expression and activity were markedly elevated in MPS synovial tissues and bone marrow. In addition, MPS bone marrow cultures had tartrate-resistant acid phosphatase (TRAP)-positive multinucleated osteoclast-like cells, consistent with the osteopenia previously observed in these animals.13
Lastly, in MPS animals we previously showed that MMPs play a crucial role in the degradation of the articular cartilage.3 This is also likely attributable to elevated TNF-α, because this cytokine has been shown to promote cartilage degradation by stimulating the synthesis and release of MMPs.14 In the present study we demonstrate that MMP-13 (collagenase 3) and MMP-1 are elevated in MPS synovial membranes, contributing to the pathological remodeling of the extracellular matrix in these diseases.
Thus, these studies have provided new insights into the molecular mechanisms leading to MPS joint and bone disease, suggesting new therapeutic strategies that may be used to treat these and other GAG-mediated disorders. In addition, some of the molecules identified in this study may be used as biomarkers to monitor disease progression and the response to therapy, not only for the MPS disorders, but perhaps other GAG-mediated diseases as well.
Materials and Methods
Animals
The MPS VI cats and rats and MPS VII dogs have been previously described.15,16,17,18 Affected and control animals were raised under National Institutes of Health and United States Department of Agriculture guidelines for the care and use of animals in research. Rats were maintained at the Mount Sinai School of Medicine, whereas the larger animal models were maintained at the University of Pennsylvania School of Veterinary Medicine. The animals were housed with ad libitum food and water, 12-hour light cycles at 21°C, with 12 to 15 air changes per hour. Euthanasia was performed on cats and dogs using 80 mg/kg of sodium pentobarbital (Veterinary Laboratories, Lenexa, KS) in accordance with the American Veterinary Medical Association guidelines. Euthanasia of rats was performed using carbon dioxide inhalation. Breeding colonies were maintained under Institutional Animal Care and Use Committee-approved protocols, and the diagnosis of affected animals was performed by enzymatic assays and/or by polymerase chain reaction (PCR)-based genotyping methods.19,20
Tissue Isolation and Cell Culture
Proximal humeri, distal femurs, and proximal tibias were collected from age-matched normal and MPS animals, and either placed in phosphate-buffered saline (PBS) for the isolation of fibroblast-like synoviocytes (FLS) (see below) or fixed in neutral buffered 10% formalin (Sigma Chemical, St. Louis, MO) for immunohistochemical assessment (see below). For immunohistochemistry, the formalin-fixed bones were further decalcified in 8% formic acid (Sigma Chemical) for 5 days, embedded in paraffin, and sectioned (5 μm). The tissues were obtained from four animals in each age group, ranging from 12 to 20 months in cats and dogs and 9 to 12 months in rats.
To establish primary FLS cultures, the synovium was sliced from the underlying cartilage and washed in minimal essential medium (Invitrogen, Carlsbad, CA). The tissue was then minced and digested with 1 mg/ml of collagenase type IV (Sigma Chemical) and DNase (Invitrogen) suspended in RPMI 1640 (Life Technologies, Inc., Gaithersburg, MD) for 2 hours at 37°C. Cells were isolated by centrifugation (450 × g), and resuspended in RPMI 1640 complete medium supplemented with 10% fetal bovine serum, 2 mmol/L l-glutamine, penicillin (100 U/ml), and streptomycin (10 μg/ml), cultured in 100-mm2 plates until confluent, and passed at a density of 1 × 104/cm2. After 24 hours the media was changed to serum-free media, and the cells were either grown at 37°C or stored at −20°C. FLS cultures were generally used for experiments between passages 2 to 6. For RNA isolation the cell pellets were stored at −70°C (see below).
To establish primary chondrocyte cultures, the articular cartilage was sliced from the underlying bone and washed in minimal essential medium (Invitrogen). The cartilage was minced and digested with 1% hyaluronidase for 1 hour at 37°C, washed three times with PBS containing 1 mmol/L ethylenediaminetetraacetic acid, and sequentially incubated with 2.5% trypsin (Invitrogen) and 0.01 mol/L ethylenediaminetetraacetic acid for 1 hour at 37°C, followed by further digestion at 37°C for 8 hours with 0.2% collagenase (Sigma) prepared in minimal essential medium containing 10% fetal calf serum. Cells were isolated by centrifugation (450 × g), washed twice, and plated in 96-well plates at a concentration of 7 × 104 cells/well in minimal essential medium containing 10% fetal bovine serum, 1% glutamine, penicillin (150 U/ml), and streptomycin (50 μg/ml). After 24 hours the media were changed to serum-free media, and the cells were either grown at 37°C or stored at −20°C.
Isolation of Synovial Fluid
Samples of synovial fluid were obtained from the joints of normal dogs and cats, MPS VI cats, and MPS VII dogs. Animals were euthanized, and the joints were examined to confirm the lack or presence of gross pathological changes. Samples of synovial fluid were collected from the following joints: stifle (knee), elbow, shoulder, and coxofemoral. After isolation, synovial samples were divided into aliquots and stored frozen at −70°C until analyzed. Synovial membranes from MPS VII and normal dogs also were collected from the stifle after euthanasia, embedded in OCT media (Sakura Finetek, Torrance, CA), and stored at −70°C until analyzed.
Ceramide and Sphingosine-1-Phosphate Quantification
Chondrocytes and FLSs from normal and MPS VI rats were cultured for 10 minutes to 2 days in the presence or absence of 100 μg/ml of dermatan sulfate (Sigma). After the incubation period was complete, the cells were collected, centrifuged, and washed with PBS, and total cell lysates were prepared by three cycles of freeze/thaw. Lipids were extracted by mixing 150 μl of the cell lysate with chloroform:methanol (1:2, v/v), and sonicated for 5 minutes. After sonication, 100 μl of 1 mol/L NaCl and 10 μl of concentrated HCl were added, vortexed, and centrifuged at 13,000 × g for 2 minutes. The lower organic phase was transferred to a new tube, dried with a SpeedVac concentrator, and resuspended in 10 μl of ethanol.
For S1P analysis, the 10-μl lipid extract in ethanol was added to 20 μl of NDA derivatization reaction mixture (25 mmol/L borate buffer, pH 9.0, containing 1.25 mmol/L each of NDA and NaCN). The reaction mixture was diluted 1:3 with ethanol, centrifuged at 13,000 × g for 5 minutes, and incubated at 50°C for 10 minutes. Thirty μl of the supernatant was transferred to a sampling glass vial and 5 μl was applied onto a high performance liquid chromatography system (Waters, Milford, MA) for analysis. The fluorescent derivatives were monitored using a 474 scanning fluorescence detector (Waters) at an excitation wavelength of 252 nm and an emission wavelength of 483 nm. Quantification of the S1P peak was calculated from a S1P standard calibration curve using the Waters Millennium software. A detailed protocol for this method is currently under preparation (X.H. and E.H.S., manuscript is preparation). Ceramide was quantified from the lipid extracts using the diacylglycerol kinase method.21 The combined data from triplicate experiments were subjected to a t-test analysis, and the results were considered significant at P < 0.01.
Nitrite Quantification
Chondrocyte and FLS cultures were maintained under the conditions described above (with or without dermatan sulfate) for 48 hours, and the media were collected for nitrite assays using the Griess reagent.22 The combined data from triplicate experiments were subjected to a t-test analysis, and the results were considered significant at P < 0.005.
Gene Expression Microarray Analyses
Total RNA from normal and MPS VI rat FLS cultures was extracted using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions and then treated with a DNA-free DNase treatment and removal kit (Ambion, Austin, TX). Three μg of total RNA were reverse-transcribed using T7-polydT primer and converted into double-stranded cDNA using a one-cycle cDNA synthesis kit (Affymetrix Inc., Santa Clara, CA). The resulting templates were then used for in vitro transcription at 37°C for 16 hours to yield biotin-labeled antisense RNA (GeneChip HT IVT labeling kit, Affymetrix Inc.). The labeled, antisense RNA was chemically fragmented and made into a hybridization cocktail according to the Affymetrix GeneChip protocol, which was then hybridized to the Rat Genome 230 Plus 2 array (Affymetrix Inc.). The array image was generated by a high-resolution GeneChip 7G scanner (Affymetrix Inc.) and then converted into digitized data matrix by the Affymetrix MAS 5.0 algorithm. Complete microarray data have been deposited to Array Express (Accession E-TABM-306; www.ebi.ac.uk/arrayexpress).
TLR4, MIP-1α, and MMP-13 Immunohistochemistry
Bone sections were deparaffinized, rehydrated in a series of descending ethanol concentrations, and digested for 1 hour with testicular hyaluronidase (2 mg/ml, Sigma) in PBS. Immunohistochemistry for TLR4, MIP-1α, and MMP-13 was performed using polyclonal anti-goat TLR4 (Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal anti-rat MIP-1α antibodies (Peprotech Inc., Rocky Hill, NJ) and polyclonal anti-goat MMP-13, respectively. Dilutions of the antisera were applied in PBS containing 1% bovine serum albumin, and the sections were incubated overnight at 4°C. After several rinses with PBS, visualization of TLR4 was accomplished using a fluorescent secondary antibody, Cy-3, whereas MMP-13 was incubated with Alexa FluorProbe 488 anti-mouse or Alexa FluorProbe 594 anti-rabbit IgG. Nuclei were stained with a bis-benzimide Hoechst dye (1 μg/μl) for 10 minutes, rinsed, and sections were mounted with an anti-bleaching mounting media. On the other hand, MIP-1α was visualized nonfluorescently using the streptavidin-biotin complex method (Histostain-Plus kit; Zymed, San Francisco, CA). The slides were stained with diaminobenzidine, and counterstained with hematoxylin. All slides (TLR4, MIP-1α and MMP-13) were visualized and photographed with a confocal laser-scanning microscope (Carl Zeiss, Thornwood, NY).
Reverse Transcriptase (RT)-PCR
Total RNA was extracted from cultured FLSs using the RNeasy mini kit (Qiagen, Valencia, CA), and processed according to the manufacturer’s instructions. The RNA was first reverse-transcribed into first-strand complementary DNA (cDNA) using the SuperScript II RT kit (Invitrogen) and random hexanucleotide primers. For cDNA amplification, 1 μg of cDNA, 10× PCR buffer (100 mmol/L Tris-HCl, 500 mmol/L KCl, 25 mmol/L MgCl2), 2.5 mmol/L dNTPs, 20 pmol of sense and anti-sense primers, and recombinant Taq polymerase (Invitrogen) was mixed in a total reaction volume of 25 μl. PCR amplification was performed using primers corresponding to cDNA sequences for GAPDH,23 MMP-1,24 MMP-13,23 and TNF-α.25 Changes in gene expression were calculated as the ratio of molecules of the target gene/number of molecules of GAPDH.
Immunoassays
IL-1β and TNF-α were quantified in normal and MPS FLS cells and media, and synovial fluid, by immunoassays using rat and human Quantikine immunoassay kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocols. RANKL and MMP-13 activities also were measured using commercial assay kits (Chemicon International, Temecula, CA, and AnaSpec Inc., San Jose, CA, respectively) according to the manufacturers’ protocols. All immuno- and enzymatic assay experiments were performed in triplicate.
Western Blot Analysis
FLSs and articular chondrocytes from 1-year-old normal and MPS VI rats were grown to subconfluence, washed with cold PBS, and then counted using a hemocytometer. To compare directly the protein levels in MPS versus normal animals, an equal number of cells (7.2 × 105 FLSs and 1.6 × 106 chondrocytes) were used to prepare the cell extracts. Cells were lysed by freeze/thaw in buffer containing 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 2 mmol/L ethylenediaminetetraacetic acid, 1% NP, 1 mmol/L vanadate, 5 mmol/L Naf, and 10 mg/ml aprotinine, pH 7.4. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% or 12% precast Nupage Bis/Tris gels under reducing conditions and MES running buffer (Invitrogen), and transferred onto a nitrocellulose membrane (Amersham Biosciences, Little Chalfont, UK) using a semidry transfer apparatus (Bio-Rad, Hercules, CA) and Nupage-MOPS transfer buffer. For immunoblot analysis, the membranes were blocked with TBS/Tween containing 5% dry milk, and then incubated with polyclonal anti-goat LBP, anti-rabbit TLR4, anti-rabbit MyD88, and anti-rabbit protein kinase C-α (PKC-α) as a loading control (Santa Cruz Biotechnology). The bound antibodies were recognized by secondary antibodies conjugated to horseradish peroxidase. Detection of the antibody complex was accomplished using an enhanced chemiluminescence detection reagent (Amersham Biosciences). Approximate molecular masses were determined by comparison with the migration of prestained protein standards (Bio-Rad). To calculate the density of the bands produced the blots were scanned using Image J 1.36b software (National Institutes of Health, Bethesda, MD), and absolute numbers were displayed as ratios.
TRAP Staining
Bone marrow was isolated from normal and MPS VI age-matched rats (four each) as previously described.25 To identify osteoclasts, bone marrow cells were fixed and stained using a TRAP kit according to the manufacturer’s protocol (Kamiya Biomedical Comp, Seattle, WA).
Cell Proliferation Assays
Normal and MPS VI FLSs were isolated as previously described from 9-month-old rats. Cells were plated at a density of 5 × 103 and followed for 72 hours. Proliferation was assayed using the CELLTiter96Aqueous One solution and performed according to the manufacturer’s protocol (Promega, Madison, WI). The combined data from triplicate experiments were subjected to a t-test analysis, and results were considered significant at P < 0.001.
Data Presentation and Statistical Analyses
All experiments were independently replicated at least three times. The combined data from the triplicate experiments were subjected to a two-tailed t-test analysis. Graphs represent the mean ± SEM of combined data from the triplicate experiments.
Results
Gene and Protein Expression Findings in MPS Synovial Cells and Tissue
RNA was prepared from FLSs of 9-month-old normal and MPS VI rats. Gene expression microarray analysis was performed using the Affymetrix 230 rat gene expression array and analyzed using the Affymetrix MAS 5.0 software. Ninety genes were found to be overexpressed at least fivefold in the MPS synovial cells, and 35 genes were underexpressed. As illustrated in Table 1, the majority of the genes overexpressed in the MPS VI cells were those involved in inflammation and/or immunity. Among the proteins encoded by these genes included numerous chemokines, cytokines, inflammatory cell receptors, inflammatory proteases.
Table 1.
Inflammatory Genes Elevated in MPS Fibroblast-Like Synovial Cells
| Fold change | Accession no. | Gene | Description |
|---|---|---|---|
| 13.6 | gb:AW434057 | C1qb | Complement component 1, q subcomponent, b |
| 12.6 | gb:AF268593.1 | β2 integrin | Integrin β2 a subunit |
| 11.8 | gb:AA945737 | CXCr4 | CXC chemokine receptor |
| 10.6 | gb:AB000818.1 | Aif1 | Allograft inflammatory factor 1 |
| 10.4 | gb:AF156540.1 | Mpeg1 | Macrophage-expressed gene 1 |
| 10.2 | gb:BI285793 | KFMS | Macrophage CSF receptor precursor |
| 9.8 | gb:M10072.1 | L-CA | Leukocyte common antigen |
| 9.6 | gb:U54791.1 | LCR1 | Chemokine receptor LCR1 |
| 9.6 | gb:BE111722 | FCγrIII | High-affinity Ig-ε receptor γ precursor |
| 9.2 | gb:AB003042.1 | C5r1 | Complement component 5, receptor 1 |
| 8.2 | gb:NM_013069.1 | CD74 | Invariant polypeptide-associated MHC II antigen |
| 8.2 | gb:U22414.1 | MIP-1α | Macrophage inflammatory protein-1α |
| 8.0 | gb:AB003042.1 | Bcl2a1 | BCL2-related protein A1 |
| 8.0 | gb:AF209406.1 | NMRK | Rat NK cell receptor 2B4 |
| 8.0 | gb:AB046592.1 | Laptm5 | Lysosomal-associated protein transmembrane 5 |
| 7.6 | gb:AB015308.1 | Gna15 | GTP binding protein α 15 |
| 7.4 | gb:J05155.1 | Plcg2 | Phospholipase C, γ 2 |
| 7.2 | gb:AF169636.1 | PIR-B | Paired Ig-like receptor-B |
| 7.0 | gb:AF298656.3 | Cybb | Endothelial type gp91-phox |
| 6.8 | gb:AF087943.1 | CD14 | CD14 antigen |
| 6.8 | gb:AJ222813.1 | IL-18 | Precursor interleukin 18 |
| 6.8 | gb:NM_017260.1 | Alox5ap | Arachidonate 5-lipoxygenase activating protein |
| 6.8 | gb:NM_017124.1 | CD37 | Leukocyte-specific protein |
| 6.2 | gb:BF282632 | CD53 | Leukocyte antigen |
| 6.0 | gb:BF550890 | CD43 | Leukosianin |
| 5.8 | gb:AF039033.1 | Ucp2 | Uncoupling protein 2 |
| 5.8 | gb:M64370.1 | FCγrIII | Fc receptor, IgG, low affinity III |
| 5.8 | gb:BF289368 | LBP | Lipopolysaccharide-binding protein |
| 5.8 | gb:U06434.1 | MIP-1β | Macrophage inflammatory protein-1 beta |
| 5.4 | gb:AI406496 | TEC | Protein tyrosine kinase |
| 5.2 | gb:NM_053963.1 | MMP-12 | Matrix metalloproteinase 12 |
| 5.0 | gb:AF208230.1 | LST1 | Leukocyte specific transcript 1 |
| 4.8 | gb:AF072411.1 | CD36 | Fatty acid translocaseCD36 |
| 4.4 | gb:M64711.1 | EDN1 | Endothelin 1 |
| 4.2 | gb:AB053449.1 | KIAA1775 | MT-protocadherin |
| 4.0 | gb:60616.1 | MMP-13 | Collagenase 3 |
| 4.0 | gb:AA893169 | Timp3 | Tissue inhibitor of metalloproteinase 3 |
| 3.8 | gb:M98820.1 | IL1-β | Interleukin 1-beta |
Bold indicates genes also elevated in rheumatoid arthritis. For complete microarray data see Array Express (Accession E-TABM-306; www.ebi.ac.uk/arrayexpress).
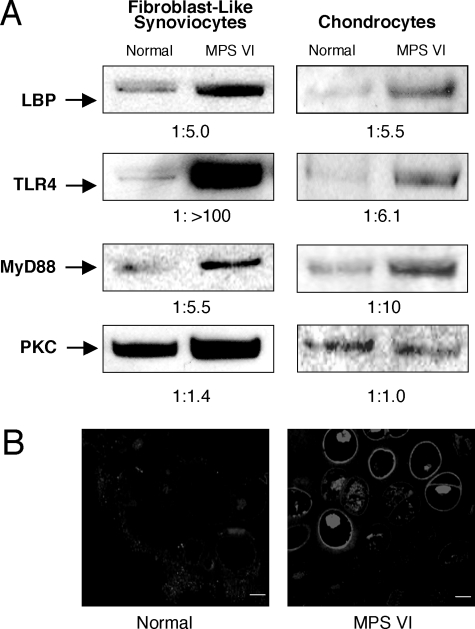
RA is one of the best-characterized inflammatory joint diseases in man. Interestingly, among the genes with elevated expression in the MPS VI synovial cells, many are also elevated in RA (Table 1, bold). The majority of these genes common to RA and MPS VI are involved in inflammation and/or immunity, and some are important for bone development and/or are involved in connective tissue cell differentiation. Interestingly, the gene encoding LPS-binding protein (LBP) was highly overexpressed in the MPS VI cells (elevated 5.8-fold; Table 1). This was consistent with our prior hypothesis that GAGs induce pathological changes in MPS connective tissues via activation of the LPS-mediated signaling pathway.3 Several other genes known to be involved in LPS signaling (eg, CXCR4, CD14) also were elevated in the MPS cells. To confirm these microarray findings, immunoblotting was performed to examine the expression of LBP in MPS FLSs and chondrocytes. As shown in Figure 1A, LBP expression was markedly up-regulated in the MPS cells, confirming the microarray results. Interestingly, immunoblotting also revealed that the expression of TLR4, a known mediator of LPS signaling, was similarly elevated in the MPS cells (Figure 1A). The fact that TLR4 gene expression was not elevated (as judged by microarray analysis) in the MPS VI cells, suggested a post-transcriptional regulation event. To confirm this Western blotting result, TLR4 immunohistochemistry analysis was performed on normal and MPS VI rat cartilage (Figure 1B). The results showed that TLR4 expression was markedly elevated in the MPS cells. The elevation of TLR4 and LBP protein expression in the MPS cells supports our hypothesis that GAGs are stimulating an inflammatory response in the MPS disorders through activation of the LPS signaling pathway. To further explore this hypothesis, we also determined the expression of MyD88, an adapter molecule that acts downstream of TLR4. As shown in Figure 1A, expression of this protein was also markedly elevated in MPS FLSs and chondrocytes.
Figure 1.
A: Detection of the TLR4, 192-kDa heterodimer; lipopolysaccharide-binding protein (LBP), 57-kDa monomer; adaptor molecule MyD88, 30-kDa monomer; and protein kinase C-α (PKC-α), 82-kDa monomer (as a loading control) by Western blot analysis in normal and MPS VI rat FLSs and chondrocytes. An equal number of cells (7.2 × 105 FLSs and 1.6 × 106 chondrocytes) were used to prepare the extracts, and an equal volume (20 μl) was loaded into each lane. B: TLR4 immunostaining in 12-month-old normal and MPS VI rat articular cartilage. Scale bars = 10 μm.
Ceramide and S1P Levels in MPS Cells
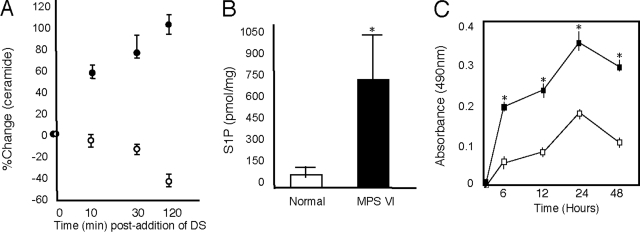
Stimulation of TLR4 by LPS is associated with activation of the lipid hydrolase, acid sphingomyelinase, elevation of ceramide, and reorganization of membrane rafts.26,27 To examine the effects of GAGs on ceramide, we performed an experiment in which normal rat chondrocytes and FLSs were grown in the presence of dermatan sulfate, and ceramide levels were determined. As shown in Figure 2A, when normal chondrocytes were treated with dermatan sulfate, ceramide levels increased by 10 minutes and proceeded through 120 minutes. This is consistent with the enhanced apoptosis we previously observed in these cells.2,3 In MPS VI chondrocytes the baseline levels of ceramide were higher than in normal cells, and supplementation of the culture media with additional dermatan sulfate did not significantly increase these levels (data not shown). This is also consistent with the high, baseline levels of apoptosis observed in these cells.2
Figure 2.
Effects of dermatan sulfate (DS) on ceramide and S1P levels in rat chondrocytes and FLSs. A: Normal chondrocytes (filled circles) and FLSs (open circles) were grown in standard culture media containing 100 μg/ml of DS for 120 minutes and ceramide levels were determined in cell lysates. B: Normal and MPS VI rat FLSs were grown in standard culture media for 48 hours and S1P levels were determined in cell lysates. Mean (n = 4) ± SEM. *P < 0.001. C: Proliferation studies in FLSs from 1-year-old normal (open boxes) and MPS VI rats (filled boxes). Cells were grown for 48 hours and the proliferation rates were determined. Data represent median values obtained from four independent experiments. MPS VI rat FLSs showed a greater than twofold increase. *P < 0.001.
In contrast to our findings with chondrocytes, when normal FLSs were treated with dermatan sulfate, ceramide levels declined throughout time (Figure 2A). We then measured the levels of the prosurvival lipid, S1P, in these cells. S1P levels were sixfold higher in MPS VI FLSs when compared to the age-matched normal FLSs (Figure 2B). Consistent with these findings was the observation that MPS VI FLSs proliferated approximately twofold faster than age-matched, normal FLSs (Figure 2C). In addition, no apoptosis was observed in these cells (in contrast to MPS chondrocytes), as revealed by TUNEL staining and low nitrite release (data not shown).
Immunohistochemistry and RIA Findings
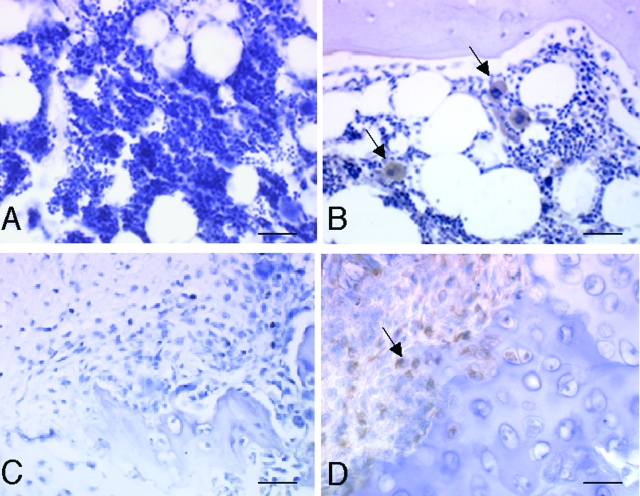
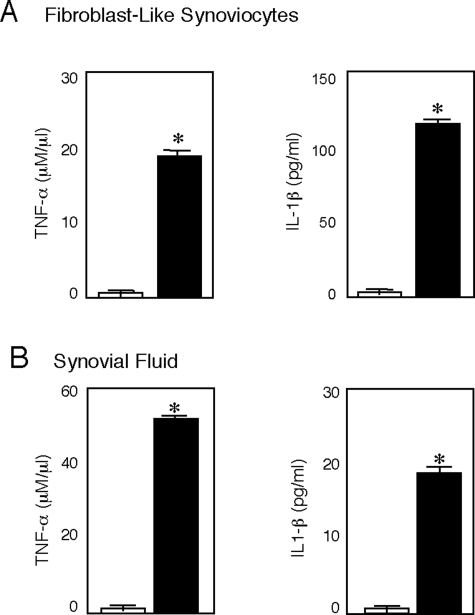
Immunohistochemistry and/or RIA assays were performed on articular cartilage, synovial tissue, and synovial fluid from several MPS animal models. Among the proteins studied were TNF-α, IL-1β, macrophage inflammatory protein (MIP-1α, TLR4), and several inflammatory proteases (MMP-1 and -13). These proteins were chosen based on the microarray gene expression data and/or their involvement in LPS signaling and RA. As shown in Figure 3, MIP-1α, an important inflammatory chemokine, was elevated in MPS VI synovial tissue and bone marrow. Also, as we had observed previously in MPS VI chondrocytes and articular cartilage,3 the cytokines TNF-α and IL-1β were substantially elevated in MPS VI FLSs (Figure 4A). In contrast to their effects on chondrocytes, however, TNF-α and IL-1β have an antiapoptotic function in FLSs,28,29 consistent with the hyperplasia in MPS synovial tissue.
Figure 3.
MIP-1α immunostaining in 12-month-old normal and MPS VII dog bone marrow and synovial membrane. A and C: Normal bone marrow and synovial membrane. B and D: MPS VII bone marrow and synovial membrane showing MIP-1α-positive (arrows) macrophages. Scale bars = 50 μm.
Figure 4.
TNF-α and IL-1β levels in synovial fluid from normal and MPS VII dogs, and MPS VI rat FLSs. A: TNF-α and IL-1β concentrations were determined in serum-free FLS-conditioned culture media from MPS VI rats collected after 48 hours. Normal controls are shown by open boxes, and filled boxes represent MPS VI animals. B: Synovial fluid from normal (open boxes) and MPS VII dogs (filled boxes). Mean (n = 4) ± SEM. *P < 0.001.
Synovial fluid can be readily obtained from MPS patients and could serve as an important biomarker source that reflects the pathogenic state of joints and bones. Therefore, we investigated whether these cytokines were elevated in synovial fluid from the MPS animals. As shown in Figure 4B, TNF-α and IL-1β were markedly elevated in synovial fluid from MPS VII dogs, confirming the usefulness of this fluid as a biomarker source.
MMP-13 Production
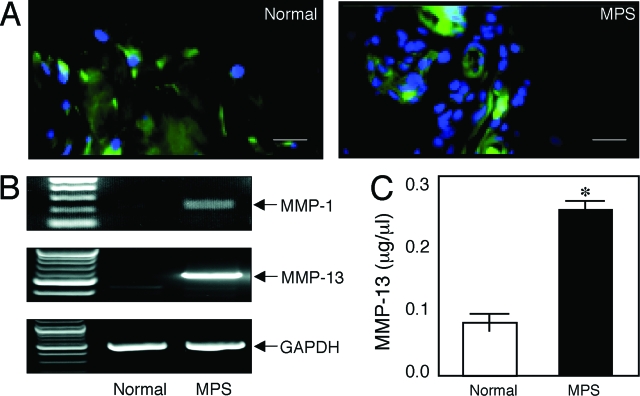
Another important component of inflammatory disease in RA and MPS is the activation of matrix-degrading proteases. Our microarray analysis of MPS VI FLSs had suggested that expression of MMP-13 (collagenase) was elevated in these diseases. To explore this observation further, we performed immunohistochemistry to determine MMP-13 activity in synovial tissue and RT-PCR to confirm the microarray results. As shown in Figure 5, expression of active MMP-13 was markedly elevated in MPS tissues and, importantly, could be detected in MPS synovial fluid as well. Similarly, the activity of several other inflammatory proteases, including MMP-1 (Figure 5), -2, and -9 (data not shown) also were elevated in the MPS tissues.
Figure 5.
MMP expression in MPS VI rats. A: MMP-13 immunostaining in age-matched (12-month) normal and MPS VI rat synovial membranes. B: MMP-13 and MMP-1 RNA expression in rat FLSs. PCR analysis for MMP-1 and -13 was performed using mRNA obtained from FLS cultures. C: MMP-13 released into FLS-conditioned, serum-free culture supernatants collected after 48 hours. Mean (n = 4) ± SEM. *P < 0.001. Scale bars = 50 μm.
RANKL Expression and Osteoclastogenesis
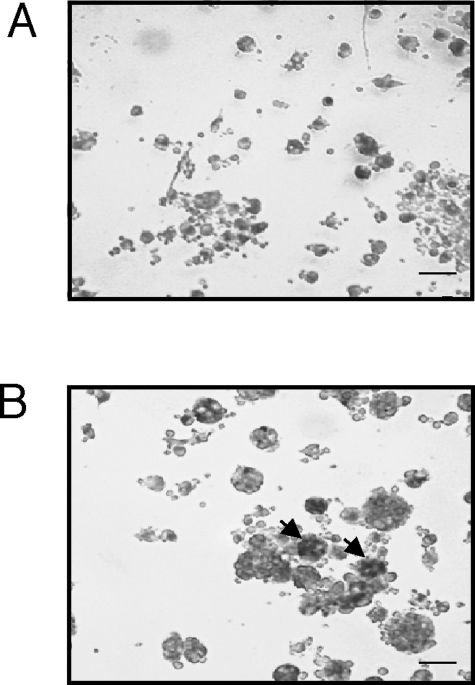
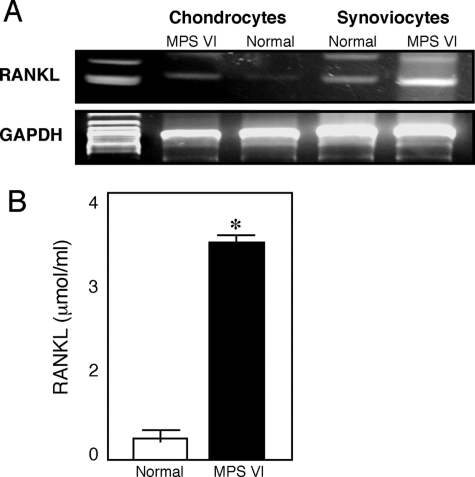
The link between inflammation and skeletal breakdown lies in the potential of the inflamed synovial tissue to activate osteoclastogenesis.29 In RA and the MPS disorders, the synovial membrane is infiltrated with proinflammatory cytokine-producing osteoclast precursors and other cells of the macrophage lineage that release proinflammatory cytokines and support osteoclastogenesis, resulting in chronic inflammation and bone loss. This inflammatory bone loss leads to the osteopenia that is characteristic of the MPS disorders.13 As shown in Figure 6, numerous TRAP-positive multinucleated osteoclast-like cells were observed in the bone marrow of MPS VI rats. In addition, an important molecule that triggers osteoclastogenesis, RANKL, was elevated in MPS connective tissues and cells (Figure 7). RANKL engages RANK on the surface of monocytes and induces the differentiation of monocytes to osteoclasts. RT-PCR analysis revealed that RANKL gene expression was more than threefold greater in MPS VI FLSs compared to normal.
Figure 6.
TRAP-positive multinuclear cells (multinucleated osteoclast-like cells) in bone marrow cultures from MPS VI rats. A: Bone marrow cultures from normal 12-month-old rats. B: Age-matched MPS VI bone marrow cultures showing TRAP-positive multinucleated osteoclast-like cells (arrows). TRAP was used as a marker for osteoclasts. Scale bars = 50 μm.
Figure 7.
RANKL expression in 12-month-old rat MPS VI chondrocytes and FLSs. A: PCR analysis was performed using mRNA obtained from chondrocyte and FLS cultures. B: RANKL concentrations were determined in serum-free FLS culture supernatants after 48 hours. Mean (n = 4) ± SEM. *P < 0.001.
Discussion
GAG accumulation is the primary pathological defect in the MPS diseases and has also been shown in many other connective tissue diseases, including RA, scleroderma, and systemic lupus erythematosus. For example, joint destruction in RA has been correlated with high GAG levels in the synovial fluid,30 and GAGs have been shown to induce the clonal expansion of several immune cell types (eg, B and T cells and macrophages). This, in turn, promotes inflammation and bone destruction.11 Infiltrating inflammatory cells also express high-affinity GAG-binding receptors, further promoting their adherence to GAG-enriched connective tissues. Studies in mice have further shown that the administration of GAGs in vivo causes autoimmune connective tissue disease.11 Interestingly, these mice had the same lesions observed in MPS patients and animals, including synovial hyperplasia, hypertrophy of synovial lining cells, and the infiltration of immune cells in various connective tissues.
Our research has focused on the MPS disorders as a model of GAG-mediated disease. Several naturally occurring MPS animal models exist,15,16,17,18 providing an excellent source of materials to study this important disease mechanism. The data presented, along with our previous studies,2,3 show that a central consequence of GAG storage in MPS animals is inflammation. Various lines of evidence also suggest that GAGs stimulate the inflammatory pathway via a mechanism similar to LPS, leading to elevated expression of LBP and TLR4 in MPS cells.
The LPS signaling pathway has been extensively studied.31,32,33 Recognition of LPS by the innate immune system leads to activation of cytokines (eg, TNF-α and IL-1β) and chemokines (eg, CXCR4 and MIP-1α) and the production of other inflammatory mediators.34 Our gene and protein expression studies in MPS cells and tissues revealed a similar inflammatory profile. In addition, LBP, CD14, CXCR4, and TLR4, molecules essential for the LPS response, were markedly up-regulated in MPS cells, further supporting the concept that GAG storage in these diseases stimulates LPS-mediated signaling. The downstream signaling adaptor molecule, MyD88, also was up-regulated in the MPS synovial cells, suggesting the regulation of this molecule by TLR4 in the synovial membrane. We also show that synoviocytes, like chondrocytes, respond to and stimulate GAG-induced inflammation, contributing to the cartilage and (ultimately) bone destruction.
Macrophages are prominent cells at sites of inflammation and can produce MMPs when activated by agents such as cytokines and LPS. Among the MMPs, the collagenase, MMP-13, plays a pivotal role in cartilage destruction.35,36 Interestingly, MMP-13 is highly overexpressed in MPS synoviocytes, likely contributing to the cartilage breakdown. This also suggests that synoviocytes may be an important source of MMP-13 in other inflammatory diseases as well. Our studies further show that MPS synovial cells proliferate at a faster rate than normal cells, likely explaining the hyperplastic synovial tissue observed in MPS patients. Hyperproliferation likely results from the increased levels of TNF-α and IL-1β, consistent with what has been previously observed in RA. In addition, MPS synovial cells have elevated levels of the prosurvival lipid, S1P, contributing to the enhanced proliferation rate. Changes in the production of the signaling lipids, ceramide and S1P, are known consequences of LPS signaling,26,27,37 supporting our central hypothesis.
Osteopenia, in addition to other bone lesions, has been observed in some of the MPS disorders, including MPS VI.13,38 Osteoclasts are essential mediators of inflammatory bone erosion, and in MPS VI this is supported by the occurrence of numerous osteoclasts and osteoclast precursors in the bone marrow. Osteoclastogenesis is dependent on the interaction of RANK and its ligand, RANKL.39 RANKL expression is up-regulated by proinflammatory cytokines such as TNF-α and IL-1β. Notably, MPS VI synoviocytes secrete RANKL, which attracts macrophages from the blood and stimulates their differentiation into osteoclasts, leading to osteopenia. However, because not all MPS disorders exhibit osteopenia, these observations on osteoclastogenesis may not be generalized to other MPS disorders.
Lastly, we provide data in this report showing for the first time abnormalities in ceramide/S1P levels in MPS cells. Ceramide is a proinflammatory and proapoptotic molecule that is up-regulated by LPS and other stress factors. S1P, on the other-hand, is a proliferative factor that counteracts the proapoptotic effects of ceramide. Regulation of the ceramide/S1P levels constitutes an important control mechanism for maintaining the proper balance between cell death and growth (for a recent review on ceramide/S1P signaling see Huwiler and Zangemeister-Wittke40). Ceramide/S1P signaling has been specifically studied with relation to synovial tissue and cartilage.41,42 In these tissues ceramide plays a dual role; it promotes apoptosis and inhibits proliferation by interfering with the MEK/ERK signaling pathway. It also blocks the activation of Akt kinase. S1P, on the other-hand, stimulates the MEK/ERK pathway and induces proliferation of chondrocytes and synoviocytes. Our data (summarized in Figure 2) are consistent with these observations and show that MPS chondrocytes, which undergo apoptosis,2 have elevated ceramide and low levels of S1P. Importantly, treatment of normal chondrocytes with dermatan sulfate also induces ceramide and represses S1P, leading to apoptosis. MPS synoviocytes, on the other hand, have an enhanced proliferation rate and elevated levels of S1P.
Thus, in this report we have further defined the mechanism of GAG-mediated disease in the MPS disorders. Our long-term goal is to develop new therapeutic strategies for the MPS and other GAG storage diseases, and several novel therapeutic targets have been identified. Although enzyme replacement therapy is available for several MPS disorders, it has limited effects on the bones and joints.43,44 Our observations suggest that anti-inflammatory treatments should be studied in these patients, alone or in combination with enzyme and gene therapies. Anti-TNF-α drugs, in particular, may be very useful in these disorders. In addition, MMP inhibitors could be evaluated. Other experimental strategies that should be studied in the MPS animal models include the use of drugs that block TLR4 signaling, and/or drugs that block sphingolipid-mediated activation. These drugs might also impact other GAG storage diseases (eg, RA), and the naturally occurring MPS animals represent excellent model systems in which to begin their evaluation.
Lastly, synovial fluid is shown for the first time to be an excellent biomarker source for the MPS disorders. Currently, the only widely used biomarker for these diseases is GAG levels in the urine. However, it is unclear how urinary GAGs reflect the state of bone, joint, brain, and other organ-specific disease. We propose that synovial fluid should be collected from MPS patients undergoing orthopedic procedures and that numerous inflammatory and other molecules should be monitored to indicate the state of joint and bone disease.
Acknowledgments
We thank Ms. Yi Ge and Ms. Patrisia Mattioli for their expert technical assistance and Dr. Tearina Chu, the Mount Sinai Microarray Shared Resource Facility, for assistance.
Footnotes
Address reprint requests to Calogera M. Simonaro, Ph.D., Associate Professor, Department of Genetics and Genomic Sciences, Mount Sinai School of Medicine, 1425 Madison Ave., New York, NY, 10029. E-mail: calogera.simonaro@mssm.edu.
Supported the National Institutes of Health (grants DK 25759 and RR02512) and the National MPS Society.
References
- Neufeld EF, Muenzer J. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill,; 2001:pp 3421–3452. [Google Scholar]
- Simonaro CM, Haskins ME, Schuchman EH. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: a possible mechanism underlying degenerative joint disease in the mucopolysaccharidoses. Lab Invest. 2001;81:1319–1328. doi: 10.1038/labinvest.3780345. [DOI] [PubMed] [Google Scholar]
- Simonaro CM, D’Angelo M, Haskins ME, Schuchman EH. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr Res. 2005;57:701–707. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- De Vries-Bouwstra JK, Goekoop-Ruiterman YP, Wesoly J, Hulsmans HJ, de Craen AJ, Breedveld FC, Dijkmans BA, Allaart CF, Huizinga TW: Ex vivo IL1 receptor antagonist production upon LPS stimulation is associated with development of RA and with greater progression of joint damage. Ann Rheum Dis 2007, Jan 12:[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring SR. Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin Rheumatol. 2002;4:406–410. doi: 10.1097/00002281-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- Auclair D, Hein LK, Hopwood JJ, Byers S. Intra-articular enzyme administration for joint disease in feline mucopolysaccharidosis VI: enzyme dose and interval. Pediatr Res. 2006;59:538–543. doi: 10.1203/01.pdr.0000203090.41012.a6. [DOI] [PubMed] [Google Scholar]
- Pastores GM, Meere PA. Musculoskeletal complications associated with lysosomal storage disorders: Gaucher disease and Hurler-Scheie syndrome (mucopolysaccharidosis type I). Curr Opin Rheumatol. 2005;17:70–78. doi: 10.1097/01.bor.0000147283.40529.13. [DOI] [PubMed] [Google Scholar]
- Parildar Z, Uslu R, Tanyalcin T, Doganavsargil E, Kutay F. The urinary excretion of glycosaminoglycans and heparan sulphate in lupus nephritis. Clin Rheumatol. 2002;21:284–288. doi: 10.1007/s100670200075. [DOI] [PubMed] [Google Scholar]
- Edward M, Fitzgerald L, Thind C, Leman J, Burden AD. Cutaneous mucinosis associated with dermatomyositis and nephrogenic fibrosing dermopathy: fibroblast hyaluronan synthesis and the effect of patient serum. Br J Dermatol. 2007;156:473–479. doi: 10.1111/j.1365-2133.2006.07652.x. [DOI] [PubMed] [Google Scholar]
- Wang JY, Roehrl MH. Glycosaminoglycans are a potential cause of rheumatoid arthritis. Proc Natl Acad Sci USA. 2002;99:14362–14367. doi: 10.1073/pnas.222536599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000;43:2523–2530. doi: 10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nuttall JD, Brumfield LK, Fazzalari NL, Hopwood JJ, Byers S. Histomorphometric analysis of the tibial growth plate in a feline model of mucopolysaccharidosis type VI. Calcif Tissue Int. 1999;65:47–52. doi: 10.1007/s002239900656. [DOI] [PubMed] [Google Scholar]
- Migita K, Eguchi K, Kawabe Y, Ichinose Y, Tsukada T, Aoyagi T, Nakamura H, Nagataki S. TNF-alpha mediated expression of membrane-type matrix metalloproteinase in rheumatoid synovial fibroblasts. Immunology. 1996;89:553–557. doi: 10.1046/j.1365-2567.1996.d01-789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezyk PF, Haskins ME, Patterson DF, Mellman WJ, Greenstein M. Mucopolysaccharidosis in a cat with arylsulfatase B deficiency: a model of Maroteux-Lamy syndrome. Science. 1977;198:834–836. doi: 10.1126/science.144321. [DOI] [PubMed] [Google Scholar]
- Haskins ME, Desnick RJ, DiFerrante N, Jezyk PF, Patterson DF. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984;18:980–998. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Noguchi J, Ikadai H, Takahashi M, Nagase S. Arylsulfatase B-deficient mucopolysaccharidosis in rats. J Clin Invest. 1993;91:1099–1104. doi: 10.1172/JCI116268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins M, Casal M, Ellinwood NM, Melniczek J, Mazrier H, Giger U. Animal models for mucopolysaccharidoses and their clinical relevance. Acta Paediatr Suppl. 2002;9:S88–S97. doi: 10.1111/j.1651-2227.2002.tb03117.x. [DOI] [PubMed] [Google Scholar]
- Kunieda T, Simonaro CM, Yoshida M, Ikadai H, Levan G, Desnick RJ, Schuchman EH. Mucopolysaccharidosis type VI in rats: isolation of cDNAs encoding arylsulfatase B, chromosomal localization of the gene, and identification of the mutation. Genomics. 1995;29:582–587. doi: 10.1006/geno.1995.9962. [DOI] [PubMed] [Google Scholar]
- Beratis NG, Turner BM, Weiss R, Hirshhorn K. Arylsulfatase B deficiency in Maroteaux Lamy syndrome: cellular studies and carrier identification. Pediatr Res. 1975;9:475–480. doi: 10.1203/00006450-197505000-00003. [DOI] [PubMed] [Google Scholar]
- He X, Chen F, Gatt S, Schuchman EH. An enzymatic assay for quantifying sphingomyelin in tissues and plasma from humans and mice with Niemann-Pick disease. Anal Biochem. 2001;293:204–211. doi: 10.1006/abio.2001.5108. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Cheon H, Yu SJ, Yoo DH, Chae IJ, Song GG, Sohn J. Increased expression of proinflammatory cytokines and metalloproteinase-1 by TGF-β1 in synovial fibroblasts from rheumatoid arthritis and normal individuals. Clin Exp Immunol. 2002;127:547–552. doi: 10.1046/j.1365-2249.2002.01785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonaro C, Haskins ME, Kunieda T, Evans SM, Visser JW, Schuchman E. Bone marrow transplantation in newborn rats with mucopolysaccharidosis type VI: biochemical, pathological, and clinical findings. Transplantation. 1997;63:1386–1393. doi: 10.1097/00007890-199705270-00003. [DOI] [PubMed] [Google Scholar]
- Cuschieri J, Billgren J, Maier RV. Phosphatidylcholine-specific phospholipase C (PC-PLC) is required for LPS-mediated macrophage activation through CD14. J Leukoc Biol. 2006;80:407–414. doi: 10.1189/jlb.1105622. [DOI] [PubMed] [Google Scholar]
- Cuschieri J, Bulger E, Billgrin J, Garcia I, Maier RV. Acid sphingomyelinase is required for lipid raft TLR4 complex formation. Surg Infect (Larchmt) 2007;8:91–106. doi: 10.1089/sur.2006.050. [DOI] [PubMed] [Google Scholar]
- Ainola MM, Mandelin JA, Liljestrom MP, Li TF, Hukkanen MV, Konttinen YT. Pannus invasion and cartilage degradation in rheumatoid arthritis: involvement of MMP-3 and interleukin-1beta. Clin Exp Rheumatol. 2005;23:644–650. [PubMed] [Google Scholar]
- Chen Q, Casali B, Pattacini L, Boiardi L, Salvarani C. Tumor necrosis factor-alpha protects synovial cells from nitric oxide induced apoptosis through phosphoinositide 3-kinase Akt signal transduction. J Rheumatol. 2006;33:1061–1068. [PubMed] [Google Scholar]
- Ortutay Z, Polgár A, Gömör B, Géher P, Lakatos T, Glant TT, Gay RE, Gay S, Pállinger E, Farkas C, Farkas E, Tóthfalusi L, Kocsis K, Falus A, Buzás E. Synovial fluid exoglycosidases are predictors of rheumatoid arthritis and are effective in cartilage glycosaminoglycan depletion. Arthritis Rheum. 2003;48:2163–2172. doi: 10.1002/art.11093. [DOI] [PubMed] [Google Scholar]
- Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor. Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diks SH, Richel DJ, Peppelenbosch MP. LPS signal transduction: the picture is becoming more complex. Curr Top Med Chem. 2004;4:1115–1126. doi: 10.2174/1568026043388141. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Receptor cluster formation during activation by bacterial products. J Endotoxin Res. 2003;9:331–335. doi: 10.1179/096805103225002557. [DOI] [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- Tardif G, Reboul P, Pelletier JP, Martel-Pelletier J. Ten years in the life of an enzyme: the story of the human MMP-13 (collagenase-3). Mod Rheumatol. 2004;14:197–204. doi: 10.1007/s10165-004-0292-7. [DOI] [PubMed] [Google Scholar]
- Sakata A, Ochiai T, Shimeno H, Hikishima S, Yokomatsu T, Shibuya S, Toda A, Eyanagi R, Soeda S. Acid sphingomyelinase inhibition suppresses lipopolysaccharide-mediated release of inflammatory cytokines from macrophages and protects against disease pathology in dextran sulphate sodium-induced colitis in mice. Cell Immunol. 2007;245:24–31. doi: 10.1111/j.1365-2567.2007.02612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigante D, Caradonna P. Secondary skeletal involvement in San Filippo syndrome. QJM. 2004;97:205–209. doi: 10.1093/qjmed/hch041. [DOI] [PubMed] [Google Scholar]
- Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS. Mechanisms of disease: the link between RANKL and arthritic bone disease. Rheumatology. 2005;1:47–54. doi: 10.1038/ncprheum0036. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Zangemeister-Wittke U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Crit Rev Oncol Hematol. 2007;63:150–159. doi: 10.1016/j.critrevonc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Migita K, Honda S, Yamasaki S, Hirai Y, Fukuda T, Aoyagi T, Kita M, Ida H, Tsukada T, Kawakami A, Kawabe Y, Eguchi K. Regulation of rheumatoid synovial cell growth by ceramide. Biochem Biophys Res Commun. 2000;269:70–75. doi: 10.1006/bbrc.2000.2239. [DOI] [PubMed] [Google Scholar]
- Kim M-K, Lee H, Kwak J-Y, Park J-I, Yun J, Bae Y-S. Sphingosine-1-phosphate stimulates rat primary chondrocyte proliferation. Biochem Biophys Res Commun. 2006;345:67–73. doi: 10.1016/j.bbrc.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Jacobs S, Kierstein J, Van Hove J. Outcome after three years of laronidase enzyme replacement therapy in a patient with Hurler syndrome. J Inherit Metab Dis. 2006;29:762. doi: 10.1007/s10545-006-0457-y. [DOI] [PubMed] [Google Scholar]
- Cox-Brinkman J, Smeulders MJ, Hollak CE, Wijburg FA. Restricted upper extremity range of motion in mucopolysaccharidosis type I: no response to one year of enzyme replacement therapy. J Inherit Metab Dis. 2007;30:47–50. doi: 10.1007/s10545-006-0490-x. [DOI] [PubMed] [Google Scholar]