Abstract
Recent findings in the animal model for multiple sclerosis (MS), experimental autoimmune encephalomyelitis, implicate a novel CD4+ T-cell subset (TH17), characterized by the secretion of interleukin-17 (IL-17), in disease pathogenesis. To elucidate its role in MS, brain tissues from patients with MS were compared to controls. We detected expression of IL-17 mRNA (by in situ hybridization) and protein (by immunohistochemistry) in perivascular lymphocytes as well as in astrocytes and oligodendrocytes located in the active areas of MS lesions. Further, we found a significant increase in the number of IL-17+ T cells in active rather than inactive areas of MS lesions. Specifically, double immunofluorescence showed that IL-17 immunoreactivity was detected in 79% of T cells in acute lesions, 73% in active areas of chronic active lesions, but in only 17% of those in inactive lesions and 7% in lymph node control tissue. CD8+, as well as CD4+, T cells were equally immunostained for IL-17 in MS tissues. Interestingly, and in contrast to lymph node T cells, no perivascular T cells showed FoxP3 expression, a marker of regulatory T cells, at any stage of MS lesions. These observations suggest an enrichment of both IL-17+CD4+ and CD8+ T cells in active MS lesions as well as an important role for IL-17 in MS pathogenesis, with some remarkable differences from the experimental autoimmune encephalomyelitis model.
Multiple sclerosis (MS) is the most common chronic inflammatory demyelinating disease of the central nervous system (CNS). Animal and human studies have shown that T cells and inflammatory cytokines play an important role in MS lesion pathogenesis.1 Therefore, pathologically distinct areas in the brain can be characterized as acute, chronic active and inactive lesions, depending on the degree of mononuclear cell infiltrates and level of destruction of myelin sheaths.
For many years, it was widely accepted that MS is a CD4+ T-helper 1 (TH1)-mediated disease.2 These TH1 cells are differentiated in response to the cytokine interleukin (IL)-12 and are themselves characterized by their expression of the proinflammatory cytokine interferon-γ. Most of this thinking originated from the results obtained from the animal model of MS, experimental autoimmune encephalomyelitis (EAE).2 However, these views were also first challenged in EAE by the finding that IL-12 knockout mice cannot generate TH1 cells, but are still susceptible to EAE, whereas IL-23 knockout mice are not.3 In addition to IL-6 and transforming growth factor-β, the cytokine IL-23 helps to expand a subpopulation of TH cells that specifically express the cytokines IL-17, IL-6, tumor necrosis factor-α,3 and IL-224 (TH17) and that have since been shown to play a crucial role in EAE.3 By contrast, naïve T cells receiving only transforming growth factor-β, IL-2, and retinoic acid signals differentiate into the distinct CD4+CD25+FoxP3+ regulatory T-cell population at the expense of TH17 cells.5,6 When the TH17 cells were depleted from the T-cell population obtained from EAE mice, the remaining T cells no longer induced EAE after adoptive transfer.3 EAE severity was also greatly reduced on treatment with a monoclonal antibody to IL-173; similarly, mice treated with an antibody against IL-23 failed to develop EAE.7 Although EAE could still occur in IL-17 knockout mice, the disease was heavily attenuated,8 and mice deficient for the key transcription factor for differentiation of TH17 cells, the orphan nuclear receptor RORγt, showed a mild EAE course and a delayed onset.9 However, although CD4+FoxP3+ regulatory T cells are present in the CNS during EAE, they are unable to contain the inflammatory damage.10
In addition, IL-17 has been implicated not only in the animal model for MS, but also in various disease models for other autoimmune diseases, such as rheumatoid arthritis,11 inflammatory bowel disease,12 psoriasis,4 and uveitis.13 Although EAE and models for various other autoimmune diseases show a dominant role of TH17 cells in disease pathogenesis, few studies have addressed their role in their human disease prototypes. In fact, human TH17 cells seem to be distinctively different from those described in the mouse. Transforming growth factor-β is not needed for IL-17 production in human T cells and even inhibits IL-17 production, whereas IL-1 is a very effective and IL-6 a poor inducer of TH17 differentiation.14,15 The role of human IL-23 in inducing IL-17-secreting cells is, however, unclear, because it has been shown to be either an ineffective inducer14 or a highly potent inducer15 of TH17 cells.
IL-17-secreting cells have been detected in MS16 and also in rheumatoid arthritis17,18 and inflammatory bowel disease.19 In MS increased numbers of mononuclear cells have been shown to express IL-17 mRNA in the peripheral blood, particularly during exacerbations, with a higher proportion of IL-17 mRNA-expressing cells in the cerebrospinal fluid.16 They have also recently been shown to migrate preferentially across a model for the blood-brain barrier.20 In addition, IL-17 levels seem to be elevated in cerebrospinal fluid samples from MS patients, in particular in opticospinal MS patients.21 Furthermore, early microarray analyses of different MS lesions noted increased levels of IL-17 mRNA transcripts, particularly in more chronic lesions,22 along with other genes for proinflammatory cytokines. However, it has not yet been investigated whether IL-17-producing cells actually invade into the CNS in MS patients and can be found in the inflammatory infiltrates.
Here, we address this question for the first time, identifying the different cell types in MS brains that express and produce IL-17. We show that mRNA expression and protein production of IL-17 is primarily restricted to the active areas of MS lesions. Not only T cells but also, surprisingly, astrocytes and oligodendrocytes produce the cytokine in inflammatory areas of different lesions. In the active areas of acute lesions and the active borders of chronic active lesions, we found higher densities of IL-17+ T cells than in inactive areas, normal-appearing white matter (NAWM), and control tissue. We also demonstrate high proportions of CD8+ as well as CD4+ T cells that are IL-17+ with a similar distribution. By contrast, we could not detect any FoxP3+ regulatory T cells in any MS brain tissues, suggesting an absence of T-cell suppression in the CNS. The predominance of both CD4+IL-17+ and CD8+IL-17+ T cells within active areas, together with the known evidence of IL-17 as a proinflammatory cytokine, supports a pathogenic role for IL-17 in MS.
Materials and Methods
Tissue Specimens
All brain tissues were obtained from the UK Multiple Sclerosis Tissue Bank, the Thomas Willis Oxford Brain Collection, or NeuroResource, University College London Institute of Neurology. Twenty-two frozen tissue blocks were used in this study, comprising five acute, six chronic active, and five inactive lesions, three tissue samples of NAWM, and four control (normal) brains. These were obtained from 14 MS patients and 4 patients with nonneurological diseases (Table 1). To characterize the lesions, oil red O (ORO), Luxol fast blue-cresyl violet, and hematoxylin and eosin stainings were performed to identify the centers and borders of the lesions, lesion activity, the hypercellularity (inflammatory cell infiltrate), and areas of hypocellularity (loss of parenchymal cells in inactive lesions). Lesions were characterized as follows: 1) active lesions by hypercellularity because of lymphocytic infiltration and high number of macrophages highly stained with ORO because of the positive lipid droplets from the breakdown of myelin; 2) chronic active lesions by hypercellular active border stained highly positive with ORO and by a hypocellular demyelinated center with a very low density of macrophages, ORO-negative; 3) inactive lesions by demyelination and a high degree of hypocellularity because of the significant loss of parenchymal cells and the limited number of inflammatory infiltrates stained negative for ORO. In all MS cases, the clinical diagnosis of MS had been made during life and confirmed by neuropathological autopsy examination. This study was approved by the Oxford Regional NHS Research Ethics Committee, UK.
Table 1.
Cryostat MS Lesions
| Case | Block | Sample type | Disease | Age (years) | Disease duration (years) | MS type | Cause of death |
|---|---|---|---|---|---|---|---|
| 1 | Acute | MS | 29 | 8 | Secondary progressive | Bronchopneumonia | |
| 2 | A | Acute | MS | 54 | 16 | Primary progressive | Myocardial infarct |
| 3 | A | Acute | MS | 40 | 9 | Secondary progressive | Bronchopneumonia |
| 4 | A | Acute | MS | NA | NA | NA | NA |
| 4 | B | Acute | MS | NA | NA | NA | NA |
| 5 | Chronic active | MS | 65 | 25 | Secondary progressive | Bronchopneumonia | |
| 6 | Chronic active | MS | 46 | 22 | Secondary progressive | Bronchopneumonia | |
| 7 | Chronic active | MS | 59 | 20 | Secondary progressive | Bronchopneumonia | |
| 8 | Chronic active | MS | 51 | 10 | Secondary progressive | Bronchopneumonia | |
| 2 | B | Chronic active | MS | 54 | 16 | Primary progressive | Myocardial infarct |
| 2 | C | Chronic active | MS | 54 | 16 | Primary progressive | Myocardial infarct |
| 9 | Inactive | MS | 46 | 22 | Secondary progressive | Bronchopneumonia | |
| 10 | Inactive | MS | 67 | 29 | Secondary progressive | Bronchopneumonia | |
| 11 | Inactive | MS | 71 | 32 | Secondary progressive | Bronchopneumonia | |
| 2 | D | Inactive | MS | 54 | 16 | Primary progressive | Myocardial infarct |
| 12 | Inactive | MS | NA | NA | NA | NA | |
| 13 | NAWM | MS | 42 | 6 | Primary progressive | Bronchopneumonia | |
| 3 | B | NAWM | MS | 40 | 9 | Secondary progressive | Bronchopneumonia |
| 14 | NAWM | MS | 39 | NA | NA | Pulmonary embolism, pneumonia | |
| 15 | Control | 75 | CVA, aspiration pneumonia | ||||
| 16 | Control | 35 | Carcinoma of the tongue | ||||
| 17 | Control | 73 | Aortic aneurysm | ||||
| 18 | Control | 49 | Myocardial infarct |
NAWM, normal appearing white matter; NA, not available.
In Situ Hybridization
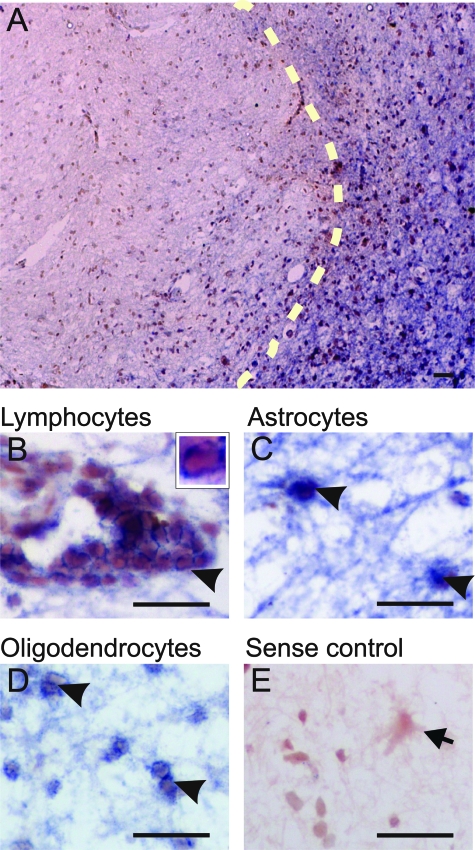
For the generation of the riboprobes, a 451-base IL-17 cDNA fragment was obtained by polymerase chain reaction from an IL-17 cDNA inserted in pDNR-dual donor vector (GenBank accession number NM_002190; Bio Techniques, Westborough, MA) and verified by sequencing. Digoxygenin-labeled sense and anti-sense riboprobes recognizing IL-17 nucleotide sequences 3 to 453 were prepared by in vitro transcription. Sections were processed for in situ hybridization cytochemistry as previously described23 using an isoform-specific riboprobe to IL-17. In brief, tissue sections were cut at 10 μm and fixed for 11 minutes with 4% paraformaldehyde followed by treatment with proteinase K (Sigma-Aldrich, Dorset, UK) for 20 minutes at room temperature under RNase-free conditions. Hybridization with the digoxigenin-labeled riboprobe (DIG RNA labeling kit; Roche Diagnostics, Mannheim, Germany) was performed by overnight incubation at 68°C. After washes, the sections were incubated with anti-digoxigenin alkaline phosphatase-conjugated Fab fragments (1:2000) (Roche Diagnostics) overnight at 4°C and visualized using nitro blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyl phosphate p-toluidine salt (Roche Diagnostics) chromogenic reaction. Positive controls of fresh frozen tonsil demonstrated specific signal in cells within the parafollicular region (T cells) (data not shown). Sense riboprobes yielded no signals in in situ hybridization (see Figure 1).
Figure 1.
IL-17 mRNA expression in chronic active MS lesions. A: Low magnification with light microscopy of IL-17 mRNA expression in a chronic active lesion visualized by in situ hybridization with a specific probe for IL-17. Dotted line denotes the edge of an active border. B–D: High-magnification images of IL-17 mRNA expression in the active area of MS lesions with perivascular cells consistent with T cells [arrowhead in B and in high magnification (see inset)], parenchymal cells with astrocytic morphology (arrowhead in C) and cells with characteristic oligodendrocyte sizes and locations (arrowheads in D), all showing evidence of IL-17 mRNA expression. E: Negative control using the sense IL-17 riboprobe on a consecutive section counterstained with toluene blue (arrow in E). The figure shows that IL-17+ cells are located predominantly at the active border of a chronic active lesion (right side of dotted line in A), and that T cells (B), astrocytes (C), and oligodendrocytes (D) express IL-17 mRNA. Scale bars = 30 μm.
Immunohistochemistry
Frozen sections (10 μm thick) were air-dried, fixed in 0.4% paraformaldehyde, and quenched in ammonium chloride (0.1 mmol/L). Endogenous peroxidase was blocked by incubation with 1 to 3% hydrogen peroxide for 45 minutes. Consecutive sections were stained with mouse anti-CD3 1/100 dilution (DAKO, Cambridge, UK) and mouse anti-IL-17 1/100 dilution (R&D Systems, Abingdon, UK) antibodies overnight at 4°C. Slides were developed with the Envision kit by incubating for 1 hour at room temperature with the horseradish peroxidase anti-rabbit/mouse complex (DAKO). Staining was performed using diaminobenzidine (DAKO), as chromogenic substrate for up to 5 minutes. We used double immunofluorescence to localize IL-17+ cells. Tissue sections were fixed as described above and then incubated with anti-IL-17 antibody (R&D Systems), 1/100 dilution overnight at 4°C, followed by staining using the tyramide signal amplification kit with Alexa Fluor 488 anti-mouse Ig (Invitrogen, Paisley, UK). After washing, slides were then incubated overnight with a second antibody: rat anti-CD3, 1/100 dilution (Serotec, Oxford, UK), rat anti-CD4, 1/100 dilution (MCA4b4G, Serotec), rat anti-CD8, 1/100 dilution (YTC 182.20, Serotec), rabbit anti-GFAP, 1/1000 dilution (DAKO), sheep anti-CAII (carbonic anhydrase isoenzyme II) 1/300 dilution (Serotec), rat anti-MHC class II, 1/100 dilution (Serotec), or rabbit anti-FoxP3 1/200 dilution (ab10563; Abcam, Cambridge, UK). Appropriate secondary antibodies included Cy3 anti-rat Ig, 1/100 dilution (Jackson Laboratory, Suffolk, UK), and Alexa Fluor 568 anti-rabbit 1/200 or anti-sheep 1/200 Ig (Invitrogen) for 4 hours. As negative controls for each second layer, the same procedure as above was followed except that the primary antibodies were omitted. No staining was seen in these sections. Positive control sections for IL-17 consisted of fresh frozen tonsillar tissue.
Data Acquisition
Areas of different activity were used for quantification, from acute, chronic active, and inactive lesions as well as NAWM, control brains, and lymphatic tissues. In active areas, hypercellular (oil red-positive) areas, or in inactive areas, hypocellular (oil red-negative) areas >900 μm away from lesion borders were quantified. Digital images of tissue sections were captured using an Olympus light microscope BX41 and a Zeiss camera (Zeiss, Thornwood, NY). To calculate the density of IL-17+ and CD3+ cells in areas of different activity, consecutive sections of all MS cases and controls were stained using diaminobenzidine immunohistochemistry. Multiple images of different perivascular cuffs were acquired and the areas calculated by the Axion Image software (Zeiss). Within the cuffs, our double-immunofluorescence data demonstrate that only cells with T-cell morphology label for IL-17. The numbers of CD3+ (T-cell marker) and IL-17+ cells with such morphology were counted by eye within the predefined area of each perivascular cuff and the density expressed as cells/mm2. In each lesion, three to six cuffs with a total area >2 × 105 μm2 were counted. A second blinded observer counted the number of CD3+ and IL-17+ cells from a representative number of lesions of various activities, differences being <4% between observers. To investigate IL-17 protein co-localization with CD4+ and CD8+ T cells, multiple images were acquired using a confocal microscope (Bio-Rad, Hercules, CA). For counting percentages of CD3+, CD4+, and CD8+ cells in lesion areas of different activity, multiple images were taken from all of the cuffs, and their cells counted using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
Statistical significance was calculated using Student’s t-test for unequal variances and analysis of variance with multiple comparisons as appropriate. Data are presented as mean ± SEM. *P < 0.05 denotes statistically significant differences.
Results
T cells, Astrocytes, and Oligodendrocytes Preferentially Express IL-17 in Active Areas of MS Lesions
To assess potential contributions of IL-17 in the pathogenesis of MS, we compared the expression of its mRNA and protein in acute, chronic active, and inactive MS lesions and in NAWM and control brain tissue. Using a specific anti-sense riboprobe, we detected increased expression of IL-17 mRNA within active areas of acute lesions and in the borders of chronic active lesions relative to inactive areas of chronic active lesions (Figure 1A) and NAWM. Interestingly, the morphological characteristics and relative distribution of the cells suggested that IL-17 mRNA was expressed in T cells (Figure 1B), astrocytes (Figure 1C), and oligodendrocytes24 (Figure 1D). Moreover, within all lesions examined, there was a consistently higher level of IL-17 expression within all cell types identified in active areas of MS lesions (acute and chronic active lesion borders) than in inactive areas or control tissue (Figure 1A and data not shown).
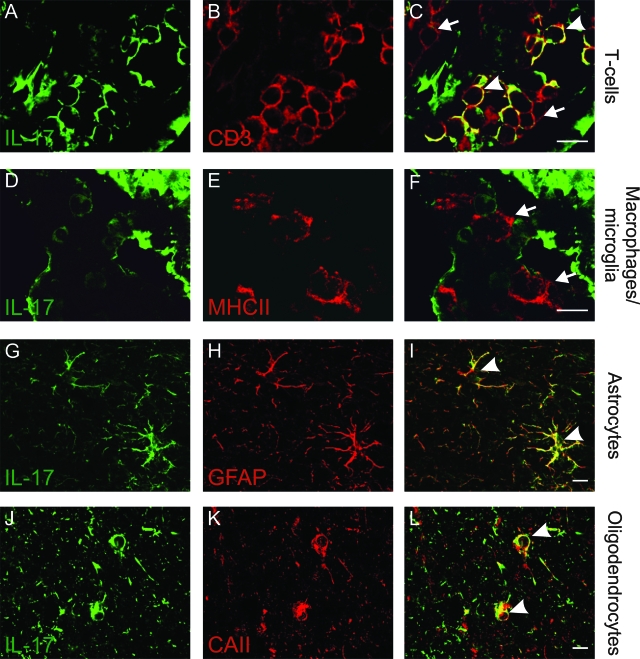
To investigate the production of IL-17 protein in immune and glial cells, we used double immunofluorescence. We found that, within acute or active borders of chronic active lesions, T cells (CD3+; Figure 2, A–C), astrocytes (GFAP+; Figure 2, G–I), and oligodendrocytes (CAII+; Figure 2, J–L) were also IL-17-positive. In contrast, microglia/macrophages (MHCII+; Figure 2, D–F) showed no detectable immunoreactivity for IL-17. In control CNS tissue, astrocytes were only marginally IL-17+ (Supplementary Figure 1A, see http://ajp.amjpathol.org), whereas their activated counterparts in NAWM from MS patients showed greater IL-17 immunoreactivity than control brains (data not shown); they were compared to robust IL-17 immunostaining in acute lesions and at chronic active lesion borders (Supplementary Figure 1, B and C; see http://ajp.amjpathol.org). In inactive lesions, no clear staining of astrocyte cell bodies was evident, but the network of astrocytic processes stained positively for IL-17 (Supplementary Figure 1D, see http://ajp.amjpathol.org).
Figure 2.
IL-17 production in inflammatory and glial cells in active areas of MS lesions. Double-immunofluorescence staining analyzed by confocal microscopy. Staining for IL-17 (A, D, G, and J; green), CD3 (B; for T cells, red), MHC class II (E; macrophages/microglia, red), GFAP (H; astrocytes, red), or CAII (K; oligodendrocytes, red) taken from active areas of an MS lesion. Overlays demonstrate expression of IL-17 in T cells (C; arrowhead demonstrates IL-17+ T cells, arrows demonstrate T cells negative for IL-17), astrocytes (I; arrowheads), and in oligodendrocytes (L; arrowheads), but not in macrophages/microglia, which are IL-17-negative (F; arrows). Scale bars = 10 μm.
IL-17+ T Cells Are Associated with Increased Activity in MS Lesions
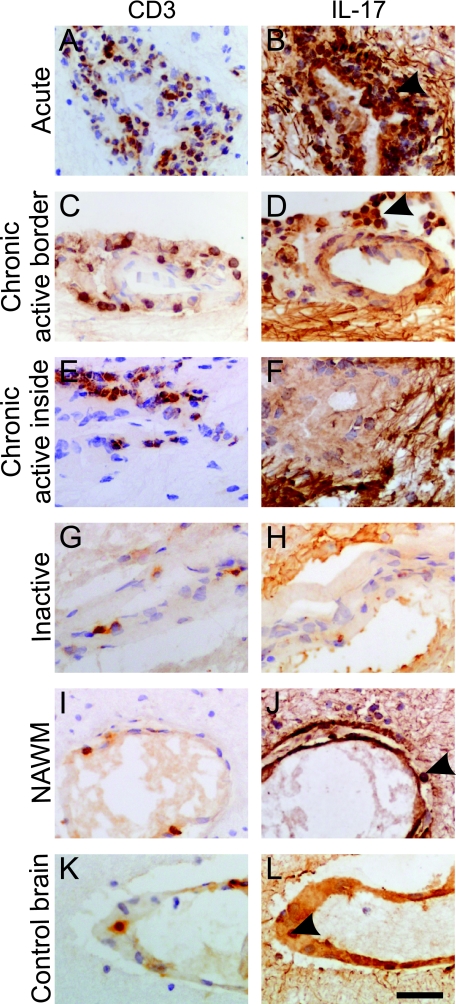
Because recent studies in animal models suggest an important role for the highly proinflammatory TH17 cells in the development of autoimmunity, we next focused on their distribution within MS lesions. As in previous studies,25,26,27,28 we found significantly higher densities of T cells especially within perivascular areas of acute lesions (Figure 3A) and active borders of chronic active lesions (Figure 3C) (2631 ± 521 cells/mm2), but also within inactive lesions (Figure 3G) and inactive areas of chronic active lesions (Figure 3E) (1080 ± 208 cells/mm2), than in NAWM (Figure 3I) and control tissue (Figure 3K) (495 ± 143 cells/mm2, P = 0.01; summarized in Figure 4A).
Figure 3.
IL-17 immunohistochemistry in perivascular cuffs from lesions with different activity. Light microscopic analysis of A, C, E, G, I, and K: CD3 immunostaining on consecutive sections. B, D, F, and H: IL-17 immunostaining in perivascular cells in MS lesions of different activity. A and B: Acute lesion. C and D: Active border of a chronic active lesion. E and F: Inactive center of a chronic active lesion. B, D, and F: The fibrillary staining beyond the perivascular spaces represents immunostaining for IL-17 in the processes of astrocytes. G and H: An inactive lesion is shown. I and J: CD3 and IL-17 immunostaining in NAWM from a case of MS. K and L: CD3 and IL-17 immunostaining in control brain. The figure shows more IL-17+ perivascular cells in the active (B and D) than the inactive (F and H) areas of the lesions. Arrowheads in B and D indicate abundance of IL-17 cells, whereas J and L indicate sparse IL-17+ cells. All sections were counterstained with hematoxylin. Scale bar = 30 μm.
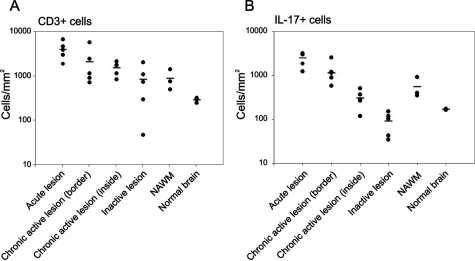
Figure 4.
Density of IL-17+ T cells in MS lesions of different activity. Quantitative analysis of perivascular CD3+ (A) and IL-17+ (B) T-cell density in areas of MS lesions with different activity, in NAWM, and in control brain. Histograms demonstrate a significantly higher density of T cells (A) and IL-17+ T cells (B) within perivascular active areas (acute lesions/active border of chronic active lesions) than in inactive lesions/inactive areas of chronic active lesions or NAWM and control tissue (n = 4). In contrast, there is no significant difference in the density of IL-17+ T cells within inactive areas and control tissue. The proportion of T cells that are IL-17+ was significantly higher in active than inactive areas of chronic active lesions (P = 0.0005).
Consistent with this observation, we noted significantly higher densities of IL-17+ T cells within acute lesions (Figure 3B) and active borders of chronic active lesions (Figure 3D) (1687 ± 284 cells/mm2) than in NAWM (Figure 3J) and control tissue (Figure 3L) (333 ± 105 cells/mm2, P = 0.001). However, IL-17+ T cells were nearly 10× less frequent within inactive lesions (Figure 3H) and inactive regions of chronic active lesions (Figure 3F) (198 ± 92 cells/mm2) and in controls (Figure 3L) (P = 0.2). Again these analyses are summarized in Figure 4B. Interestingly, there was a significantly higher proportion of IL-17+ T cells in acute lesions and active regions of chronic active lesions (71.2 ± 5%) than in inactive lesions and inactive areas of chronic active lesions (24 ± 9%, P = 0.0005). Examination of NAWM and control brain tissue demonstrated 69% and 63% of T cells were IL-17+; however, the relative intensity of IL-17+ immunostaining was weaker compared to IL17+ T cells in active MS lesions. In contrast to the abundance of IL-17+ T cells in active MS tissues, in nonreactive control lymphatic tissue in T-cell areas, we found only 6 ± 1.2% IL-17+ cells (we observed similar observations in tonsil and lymph node tissue). We quantified proportions of T cells expressing comparably high amounts of IL-17 to those found in active MS lesions.
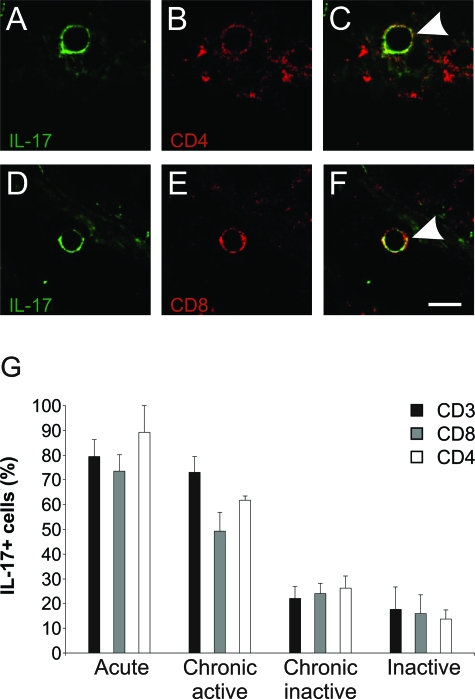
IL-17+CD4+ and IL-17+CD8+ T-Cell Subsets Are Present in Active Regions of MS Lesions
We investigated IL-17 labeling of total (CD3+) T cells and their CD4+ and CD8+ subsets in different MS lesions by double immunofluorescence (Figure 5). Specifically, we detected IL-17 in 79 ± 7% of the T cells in acute lesions, 73 ± 7% in active areas of chronic active lesions, 22 ± 5% in inactive areas of chronic active lesions and 17 ± 9% of the T cells in inactive lesions (Figure 5G). Interestingly, not only CD4+ (Figure 5, A–C) but also CD8+ T cells labeled for IL-17 in active MS lesions (Figure 5, D–F). Moreover, consistently with its important proinflammatory roles, we observed significantly higher frequencies of both CD4+IL-17+ (89.2 ± 10.8%) and CD8+IL-17+ (73.5 ± 6.5%) T cells in acute than inactive lesions (13.6 ± 3.7%, P = 0.02) (16.0 ± 7.4%, P = 0.001) (Figure 5G). Likewise, we noted a similar difference between active borders and inactive central areas within chronic active lesions (Figure 4B). We detected that active areas are associated with a significantly increased proportion of CD4+IL-17+ (61.7 ± 1.8%) and CD8+IL-17+ (49.25 ± 7.5%) T cells compared to the number of CD4+IL-17+ (13.6 ± 3.7%, P = 0.002) and CD8+IL-17+ (16.0 ± 7.4%, P = 0.03) T cells in inactive lesions. In control, nonreactive lymphatic tissue 7.5 ± 1.1% of the CD3+ cells were detected as IL-17+ (Supplementary Figure 2, see http://ajp.amjpathol.org).
Figure 5.
IL-17+ in CD4+ and in CD8+ T cells. Confocal microscopy, high-magnification image from an inflammatory cuff, demonstrating a single cell. A and D: IL-17 staining (green); B: CD4 staining (red); E: CD8 staining (red). C and F: Overlays show production of IL-17 in CD4+ T cells (arrowhead in C, yellow) and in CD8+ T cells (arrowhead in F, yellow). G: Bars demonstrate higher frequency of IL-17+ both in CD4+ and CD8+ T-cell subsets within active lesions and in active borders of chronic active lesions. In contrast, in inactive areas of inactive and chronic active lesions there is a very low frequency of IL-17+ CD4+ (P = 0.002) and CD8+ (P = 0.03) T cells. Scale bar = 10 μm.
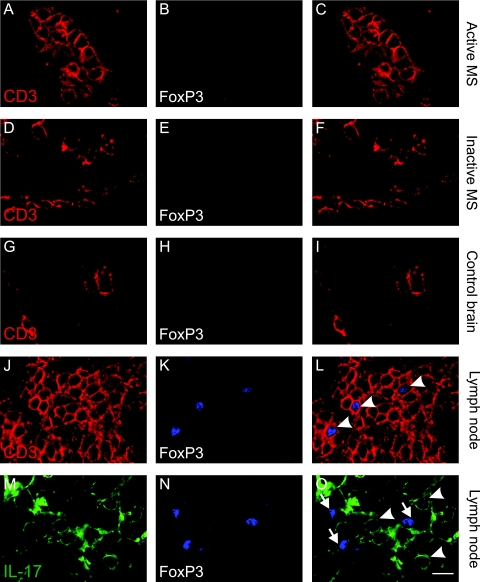
FoxP3+ T Cells Are Absent in MS Lesions
Taking into account recent data10 that FoxP3+ T cells accumulate in the CNS during EAE, in combination with the fact that IL17+ and FoxP3+ have so far been shown to exclude each other in T cells, we hypothesized that FoxP3+ T cells may be part of the IL-17− T-cell population in inactive lesions. Interestingly, we did not detect any FoxP3+ T cells in either active (Figure 6, A–C) or inactive areas of MS brains (Figure 6, D–F) nor in control brains (Figure 6, G–I). However, we observed intense FoxP3 staining in 9.2 ± 2.45% of total T cells in nonreactive lymph node tissue in T-cell areas (Figure 6, J–L), which did not show any overlap with IL-17+ cells (Figure 6, M–O).
Figure 6.
FoxP3+ T cells are absent in MS lesions. Double-immunofluorescent staining, analyzed by confocal microscopy. A, D, G, and J: staining for CD3 (red) and B, E, H, and K: FoxP3 (blue). C, F, and I: Overlays demonstrate that FoxP3 is not expressed in CD3+ cells in perivascular cuffs from acute (C) and inactive (F) MS lesions and control brain (I). L: Overlay demonstrates in lymph nodes that some T cells express FoxP3. M: IL-17+ (green) and N: FoxP3+ (blue) cells. O: Overlay demonstrates absence of FoxP3 expression from IL-17+ cells (arrowheads demonstrate IL-17+ cells and arrows demonstrate FoxP3+ cells). Scale bar = 10 μm.
Discussion
We show for the first time increased labeling for IL-17 in both CD4+ and CD8+ T cells in active areas in MS lesions, as well as in astrocytes and oligodendrocytes. The clear correlation with IL-17 mRNA detected by in situ hybridization argues strongly for active synthesis of IL-17 by all of these cell types (rather than mere uptake). Our findings also suggest that there is a climate even in the normal human CNS that favors IL-17 production, which may influence pathogenetic processes in MS and may also have implications for therapies.
The earlier literature on T cells in MS lesions showed that both CD8+ and CD4+ T cells were more numerous in lesions than in either control brains or NAWM in MS brains, being especially frequent in acute lesions and in the active margins of chronic active lesions than in inactive lesions.25,26,27,28 This finding is confirmed in the present study. The relative proportions of CD8+ and CD4+ T cells differ between studies, some describing a predominance of CD8+ T cells25,27,28 and others of CD4+ cells.26,29 These differences may reflect differing locations of cells because CD4+ cells are mainly found within perivascular spaces and meninges and CD8+ cells in both perivascular spaces and the parenchyma.25,27,30,31
The present study casts new light on the nature of the T cells populating MS lesions. One main finding is the abundance of IL-17+ T cells in perivascular spaces in acute and chronic active MS lesions, where they accounted for the majority of CD3+ cells (Figure 3, A–D). In contrast, in inactive lesions, they constituted a much smaller percentage of the less frequent CD3+ cells (Figure 3, G and H; and Figure 5G). The large numbers of IL-17+ T cells we found in both active and chronic active lesions implicate the cytokine IL-17 not only in initiating lesions but also in their persistence.
A second important finding is that, in double-labeling experiments, IL-17+ cells are found to constitute a rather high proportion of both CD4+ and CD8+ T cells (Figure 5). This is in contrast to EAE in which IL-17+ cells are reported to be mainly CD4+ (ie, TH17).3 In fact, in cultured human cells, IL-23 polarized more naïve CD8+ than CD4+ T cells into IL-17+ cells.32 However, in healthy human peripheral blood,33 only CD4+ and not CD8+ T cells have been shown to produce IL-17. Possibly, production of IL-17 in CD8+ T cells may be a distinctive feature of the human brain microenvironment. Because CD8+ T cells are predominantly found in the CNS parenchyma whereas CD4+ T cells remain primarily in the perivascular regions,31 these findings support a dominant action of CD8+ T cells in actual tissue destruction in contrast with an initiating role for CD4+ T cells.34
The high proportion of T cells with IL-17 in NAWM and control brains suggests either preferential homing or induction in the CNS, and may reflect a bias in the inductive cytokines normally available there. Indeed, there are several known factors in the MS lesion environment that might be expected to favor such recruitment and/or differentiation of IL-17+ T cells. They include the cytokine IL-23, which is expressed by dendritic cells and macrophages/microglia cells in MS lesions.35 In addition, after CNS injury or inflammation, astrocytes respond with hypertrophy, hyperplasia, and increased production of IL-6 and IL-1β, which are also found to be increased in MS lesions.36,37 In combination with additional inflammatory cytokines released by macrophages/microglia (IL-6, IL-1β, and tumor necrosis factor-α), they could not only divert the differentiation of activated or naïve T cells entering the inflamed CNS into an IL-17-producing phenotype but could also alter the astrocytes themselves in an autocrine/paracrine manner in the active MS lesions.38,39,40
This leads to the third major finding in the present study; unexpectedly, many astrocytes and even some oligodendrocytes were found to express IL-17 mRNA and protein in active MS lesions (Figure 2, I and L). Indeed, IL-17 immunostaining was abundant in the highly activated astrocytes in acute lesions and in the borders of chronic active lesions (Supplementary Figure 1, B and C; see http://ajp.amjpathol.org), but was barely detectable in astrocytes in control brain. In inactive lesions, staining of astrocyte cell bodies was difficult to detect but there was a network of astrocytic processes stained positively for IL-17. The fact that in inactive parts of the lesions we could detect IL-17 immunoreactivity in astrocytic processes but not in cell bodies could be attributable to the ability of astrocytes to act as scavenger cells.41
Expression of IL-17 in astrocytes may explain the marked up-regulation of the IL-17 gene noted in microarray studies of chronic MS lesions.22 Indeed, not only was IL-17 mRNA detected in an earlier study in astrocytes cultured from adult donors in a gene array analysis,37,42 but it was also up-regulated after treatment with tumor necrosis factor-α or IL-1β, but not, interestingly, with interferon-γ.43 Again, this agrees with the known polarization of TH17 cells by such proinflammatory cytokines as IL-6, tumor necrosis factor-α, and IL-1β.14,15 We propose that similar combinations of cytokines can also induce astrocytes (or even oligodendrocytes) to produce IL-17, which might amplify chronic inflammatory responses in the CNS.
Recently, IL-17-producing human CD4+ T cells have been found to express the chemokine receptors CCR4 and CCR6.33 Interestingly, the CCR6 agonist CCL20 has been implicated in priming pathogenic T cells in EAE.44 In addition, CCL20 is up-regulated by IL-17.45 In fact, CCL20 has been shown to be elevated in peripheral blood mononuclear cells46 in MS patients and also in astrocytes in MS lesions.47 Thus, IL-17 produced by CNS-infiltrating CD4+ and CD8+ T cells and activated astrocytes may trigger a positive feedback that further attracts not only TH17 but also TH1 cells.48
Furthermore, in EAE, it has been shown that FoxP3+ regulatory T cells play a critical role in controlling immune responses. Although they are present in the CNS of EAE animals, they are unable to inhibit pathogenic T cells at the site of inflammation.10 This finding is again in contrast to what we detected in MS because we were unable to detect any FoxP3+ T cells in MS brains (Figure 6). In MS, regulatory CD4+CD25+ T cells are present in normal numbers in the peripheral blood, but they show impaired function in comparison with control individuals.49 Therefore, if functionally impaired regulatory T cells are relevant to MS pathogenesis they must be exerting their influence outside the CNS.
Although there are significant differences between MS and EAE,50 our findings now implicate IL-17-producing T cells in human MS just as others have shown in EAE. In addition, we find IL-17 production by many CD8+ T cells and astrocytes and some oligodendrocytes, which spotlight IL-17 as a critical cytokine in MS. Exactly how it exerts any pathogenic effects, and how well the mechanisms are modeled in EAE, will require further studies. However, it might be possible that IL-17+ T cells are particularly attracted to the CNS (in contrast to FoxP3+ T cells), or are expanded in the CNS, and that they release products that are neurotoxic and/or lead to demyelination. Studying the arsenal of molecules released by IL-17+ T cells in MS patients will shed more light onto the complex scene of MS pathology and might have major implications for potential therapeutic intervention.
Supplementary Material
Acknowledgments
We thank the UK Multiple Sclerosis Tissue Bank, the Thomas Willis Oxford Brain Collection, and NeuroResource of the University College London Institute of Neurology for providing human tissues; Mrs. Carolyn Sloan for technical help; and Professor Nick Willcox for his critical reading of the manuscript.
Footnotes
Address reprint requests to Professor Margaret M. Esiri, Department of Neuropathology, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DS, UK. E-mail: margaret.esiri@clneuro.ox.ac.uk.
See related Commentary on page 8
Supported by the Medical Research Council (UK and DK; to L.F.), the European Commission (Descartes prize grant to L.F.), the Danish MS Society (to L.F), The Lundbeck Foundation (to L.F), and the Deutsche Forschungsgemeinschaft (DFG FR1720/1-1 to M.A.F.).
J.S.T. and M.A.F. contributed equally to this study.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Kim HY. Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol Cells. 2005;19:180–184. [PubMed] [Google Scholar]
- Nielsen OH, Kirman I, Rudiger N, Hendel J, Vainer B. Upregulation of interleukin-12 and -17 in active inflammatory bowel disease. Scand J Gastroenterol. 2003;38:180–185. doi: 10.1080/00365520310000672. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human T(H)17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, Minohara M, Murai H, Mihara F, Taniwaki T, Kira J. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128:988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Kataoka Y, Lo AC, Black JA, Baker D, Waxman SG. Temporal course of upregulation of Na (v) 1.8 in Purkinje neurons parallels the progression of clinical deficit in experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 2003;62:968–975. doi: 10.1093/jnen/62.9.968. [DOI] [PubMed] [Google Scholar]
- Morris CS, Esiri MM, Sprinkle TJ, Gregson N. Oligodendrocyte reactions and cell proliferation markers in human demyelinating diseases. Neuropathol Appl Neurobiol. 1994;20:272–281. doi: 10.1111/j.1365-2990.1994.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Nyland H, Mork S, Matre R. In-situ characterization of mononuclear cell infiltrates in lesions of multiple sclerosis. Neuropathol Appl Neurobiol. 1982;8:403–411. doi: 10.1111/j.1365-2990.1982.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, Weiner HL. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986;19:578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- Woodroofe MN, Bellamy AS, Feldmann M, Davison AN, Cuzner ML. Immunocytochemical characterisation of the immune reaction in the central nervous system in multiple sclerosis. Possible role for microglia in lesion growth. J Neurol Sci. 1986;74:135–152. doi: 10.1016/0022-510x(86)90100-0. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Hafler DA, Castro EE, Morimoto C, Weiner HL. Immunohistochemical analysis of suppressor-inducer and helper-inducer T cells in multiple sclerosis brain tissue. Ann NY Acad Sci. 1988;540:306–308. doi: 10.1111/j.1749-6632.1988.tb27082.x. [DOI] [PubMed] [Google Scholar]
- Booss J, Esiri MM, Tourtellotte WW, Mason DY. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci. 1983;62:219–232. doi: 10.1016/0022-510x(83)90201-0. [DOI] [PubMed] [Google Scholar]
- Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain. 1997;120:1461–1483. doi: 10.1093/brain/120.8.1461. [DOI] [PubMed] [Google Scholar]
- Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur J Immunol. 2005;35:469–475. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130:490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- Schönrock LM, Gawlowski G, Bruck W. Interleukin-6 expression in human multiple sclerosis lesions. Neurosci Lett. 2000;294:45–48. doi: 10.1016/s0304-3940(00)01543-3. [DOI] [PubMed] [Google Scholar]
- Woodroofe MN, Cuzner ML. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993;5:583–588. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Kuhlmann T, Da Costa C, Bunkowski S, Polak T, Bruck W. Tumour necrosis factor alpha mRNA expression in early multiple sclerosis lesions: correlation with demyelinating activity and oligodendrocyte pathology. Glia. 2000;29:366–375. doi: 10.1002/(sici)1098-1136(20000215)29:4<366::aid-glia7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–253. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- Kohler RE, Caon AC, Willenborg DO, Clark-Lewis I, McColl SR. A role for macrophage inflammatory protein-3 alpha/CC chemokine ligand 20 in immune priming during T cell-mediated inflammation of the central nervous system. J Immunol. 2003;170:6298–6306. doi: 10.4049/jimmunol.170.12.6298. [DOI] [PubMed] [Google Scholar]
- Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- Furlan R, Rovaris M, Martinelli Boneschi F, Khademi M, Bergami A, Gironi M, Deleidi M, Agosta F, Franciotta D, Scarpini E, Uccelli A, Zaffaroni M, Kurne A, Comi G, Olsson T, Filippi M, Martino G. Immunological patterns identifying disease course and evolution in multiple sclerosis patients. J Neuroimmunol. 2005;165:192–200. doi: 10.1016/j.jneuroim.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese MA, Montalban X, Willcox N, Bell JI, Martin R, Fugger L. The value of animal models for drug development in multiple sclerosis. Brain. 2006;129:1940–1952. doi: 10.1093/brain/awl083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.