Abstract
In the present studies the role of stem cell factor (SCF) in mediating eosinophil and fibroblast activation during their interaction was investigated. SCF was significantly higher in fibroblasts grown from lungs of chronic allergen-challenged mice compared to fibroblasts grown from normal mice. When eosinophils were layered onto fibroblasts from allergic mice, a significant increase in SCF was detected compared to fibroblasts from nonallergic mice. The interaction of fibroblasts with eosinophils also increased the production of asthma-associated chemokines, CCL5 and CCL6, was dependent on cell-to-cell interaction, and was observed only with fibroblasts derived from lungs of chronic allergen-challenged mice and not from those derived from unchallenged normal mice. Chemokine production was significantly decreased when anti-SCF antibodies were added during eosinophil-fibroblast interaction. The interaction of fibroblasts from chronic allergen-challenged mice with eosinophils also increased α-smooth muscle cell actin and procollagen I expression as well as induced transforming growth factor-β. The changes in myofibroblast activation were dependent on SCF-mediated pathways because anti-SCF antibody treatment reduced the expression of all three of these latter fibrosis-associated markers. Thus, our data suggest that SCF mediates an important activation pathway for fibroblasts during chronic allergic responses on interaction with recruited eosinophils and suggest a potential mechanism of airway remodeling during chronic disease.
Allergic airway inflammation is characterized by peribronchial eosinophil accumulation accompanied by mucus production and airway changes. Structural changes in the asthmatic airway include an increase in smooth muscle mass and deposition of extracellular matrix proteins. These changes correlate with airway hyperresponsiveness, reduced lung function, and an increase in fibroblast and myofibroblast numbers. These debilitating effects are not specifically targeted by current therapeutic agents, presenting a clinical challenge that might be better addressed by an enhanced understanding of the mechanisms.1
In addition to contributing to airway remodeling, lung fibroblasts may also play a role in local regulation of immune and inflammatory responses. Fibroblasts are a potential source of granulocyte monocyte-colony stimulating factor (GM-CSF) and stem cell factor (SCF), known to promote differentiation, activation, and survival of eosinophils and mast cells.2 Eosinophils have been shown to highly express c-kit (SCF receptor) on their surface.3,4 We have previously shown that SCF is an important eosinophil-activating factor4 that influences eosinophil recruitment in allergic airway inflammation.5 SCF-induced activation of eosinophils has been shown to up-regulate expression of fibroblast growth factors, such as FGF5 and FGF7 (KGF).4 Eosinophils also express at least two potent mediators [interleukin (IL)-1β and transforming growth factor (TGF)-β] that induce a myofibroblast phenotype. The emerging role for the eosinophil in airway remodeling might be important in future anti-asthma strategies.6,7,8 IL-5 has been a primary target for this therapy9,10; however, additional eosinophil-altering agents other than anti-IL-5 may be required before the definitive role of this cell type in asthma airway pathophysiology can be established. Altogether these data support a role for the eosinophil in the regulation of extracellular matrix homeostasis, tissue remodeling, and fibrosis in eosinophil-associated diseases.
Multiple studies indicate an important role of SCF in murine models of asthma. SCF can directly induce a dose-dependent increase in airway hyperresponsiveness11 via mast cell activation.12 Examination of SCF-mutant mice, which were deficient in both SCF and pulmonary mast cells, demonstrated significant reduction in the allergen-induced airway hyperresponsiveness responses.11 Neutralization of SCF in vivo was very beneficial in murine models of asthma, attenuating Th2 responses, eosinophilia, mucus production, airway remodeling, and collagen deposition.11,13,14 In the present study we investigated SCF-dependent cross talk between eosinophils and lung fibroblasts derived from chronic allergen-challenged animals (CRA fibroblasts) compared to those from control animals (naïve fibroblasts). The results from the present studies indicate that SCF has an important role in eosinophil-induced fibroblast activation.
Materials and Methods
Animals
Female BALB/c mice, 6 to 8 weeks of age, were purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained under standard pathogen-free conditions. All experiments involving the use of animals were approved by the University of Michigan care and use of animals committee.
Mouse Chronic Cockroach Allergen (CRA) Asthma Model
Normal female BALB/c mice were sensitized intraperitoneally and subcutaneously with 1000 protein nitrogen units of CRA (Hollister Stier, Toronto, Canada) 1/1 in IFA (Sigma-Aldrich, St. Louis, MO). Then mice were challenged intranasally with 150 protein nitrogen units of CRA on days 14, 18, 22, and 26 after initial sensitization to localize the response to the lung. The final two allergen challenges were given by intratracheal injection 4 days apart on days 30 and 34. On day 38, 4 days after the final allergen challenge, animals were sacrificed and lungs were removed.
Mouse Lung Fibroblast Isolation and Culture
Mouse lung fibroblasts were isolated from lung tissue by mincing and enzymatic digestion with 0.2% collagenase IV in RPMI 1640 (Mediatech, Inc., Herndon, VA) supplied with 2% fetal calf serum (Atlas, Fort Collins, CO) for 30 minutes at 37°C with continuous agitation. After dispersion with collagenase, isolated cells were centrifuged, washed, and cultured in complete medium composed of Dulbecco’s modified Eagle’s medium (Mediatech, Inc.) supplement with 15% fetal calf serum, 1% of l-glutamine, and 100 U/ml of penicillin, streptomycin, and fungizone (Cambrex, Walkersville, MD). Cells were used for experiments after three passages.
Antigen-Elicited Peritoneal Eosinophil Purification
Eosinophils were elicited by injection of thioglycollate plus soluble egg antigen into the peritoneum of Schistosoma mansoni-infected mice. Soluble egg antigen was prepared in our laboratory by grinding isolated eggs from heavily infected S. mansoni mice as previously described.15 The injection of soluble egg antigen into infected mice induces a pool of circulating eosinophils recruited into the peritoneum in an antigen-specific manner. After 48 hours the mice were peritoneal lavaged and the cells collected. The initial population that was isolated from the peritoneum was ∼50% eosinophils with only 2 to 5% neutrophils and ∼35 to 45% mononuclear cells (lymphocytes and macrophages). Adherent cell populations were removed from the population by plastic adherence in tissue culture dishes for 1.5 hours using standard media (RPMI 1640 supplemented with 2 mmol/L l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% heat-inactivated fetal bovine serum). The nonadherent cells were washed and resuspended in phosphate-buffered saline (PBS)/bovine serum albumin (90 μl of PBS/bovine serum albumin per 107 cells), and eosinophils were purified by negative immunomagnetic bead-coupled antibodies to exclude contaminating immune cells using the MACS system (Miltenyi Biotec, Auburn, CA). The antibodies used were anti-Thy1 (for T cells), anti-B220 (for B cells), and anti-MHC class II (for APCs) (Miltenyi Biotec). After the plate adherence and MACS separation, the population of cells contained >97% eosinophils contaminated with neutrophils (∼1%) and mononuclear cells (1 to 2%).
Culture of Purified Eosinophils
Freshly isolated murine eosinophils (3 × 106 per well) were cultivated overnight in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 2 mmol/L l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin (BioWhittaker, Walkersville, MD), and 10% heat-inactivated fetal bovine serum (Atlas) with 5 ng/ml of IL-5.
Fibroblast-Eosinophil Cell Co-Culture
Lung fibroblasts cultured to 60% confluence in six-well plates were overlaid with 1 × 106 murine eosinophils in our standard media (RPMI 1640 supplemented with 2 mmol/L l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% heat-inactivated fetal bovine serum). After 24 hours, eosinophils were pelleted, and supernatants were harvested and frozen at −20°C. In some cultures, cell populations were separated by a 3-μm membrane in a trans-well culture dish (Costar, Kennebunkport, ME) or co-incubated with 50 μg of anti-SCF antibodies or whole rabbit serum control antibodies.
To determine whether there were more eosinophils adherent to fibroblasts from allergic mice compared to those from naive animals, we performed co-culture experiments using an eight-well Lab-Tek (Nalge Nunc International, Rochester, NY) cell culture system. Fibroblasts were grown to ∼60% confluence, and eosinophils (1 × 105/well) were added to the fibroblasts. After 24 hours slides were washed to remove nonadherent cells, stained using Diff-Quick stain (Dade Behring Inc., Newark, DE), and eosinophils adhered to fibroblasts counted by light microscopy. The data were collected using 40 high-power fields (×400 magnification) per group and counted in a blinded manner.
Western Blot
Similar protocols to the ones described here were reported elsewhere.16,17 Cells were lysed in 1% Nonidet P-40 buffer containing complete protease inhibitor cocktail (Roche, Indianapolis, IN) for 30 minutes on ice with frequent vortexing and centrifuged (1200 × g, 5 minutes at 4°C). Equal amounts of protein from a detergent-soluble fraction were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrotransferred onto polyvinylidene difluoride membranes and probed with monoclonal anti-α-smooth muscle actin (α-SMA) antibodies (1:1000; Sigma, St. Louis, MO) and corresponding horseradish peroxidase-conjugated secondary IgG antibodies (1:2000). Proteins were visualized by chemiluminescence (Super Signal West Pico chemiluminescent substrate; Pierce, Rockford, IL) using a Kodak photo imager (Eastman-Kodak, Rochester, NY). GAPDH (1:2000; Cell Signaling Technology, Inc., Danvers, MA) was detected as a loading control.
Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
Total RNA was purified from isolated eosinophils using TRIzol reagent (Life Technologies, Inc., Grand Island, NY) and chloroform. RNA was quantified by measuring absorbance at 260 nm. Samples were then standardized to 5 μg/ml with diethyl pyrocarbonate water. RNA was then reverse-transcribed to cDNA and 2 μl of this cDNA was used in the TaqMan reaction mixture as we described before.13 The specific primer/probe sets for real-time PCR were predeveloped by Applied Biosystems, Foster City, CA, except mouse procollagen I and SCF primers. The sequence of the primers used are as follows: 5′-TCGTGACCGTGACCTTGCG-3′; reverse: 5′-GAGGCACAGACGGCTGAGTAG-3′ for procollagen, 5′-GTGGGGCGCCCCAGGCACCA-3′ (sense) and 5′-GCTCGCCCGTGGTGGTGAAGC-3′ (antisense) for β-actin (350 bp), 5′-CACTCAGCTTGACTACTCTT-3′ (sense) and 5′-GTCATTCCTAAGGGAGCTGG-3′ (antisense) for soluble SCF (358 bp), and 5′-CACTCAGCTTGACTACTCTT-3′ (sense) and 5′-TTGCGGCTTTCCCTTTCTCG-3′ (antisense) for transmembrane SCF (302 bp). The mixture was first incubated for 5 minutes at 94°C and then cycled 40 times at 95°C for 30 seconds, followed by 58°C for 45 seconds, and elongated at 72°C for 75 seconds, as described.18 This format allowed optimal amplification with little or no nonspecific amplification of contaminating DNA as checked using nonreverse-transcribed mRNA. After amplification, the sample (20 μl) was separated on a 2% agarose gel containing 0.3 mg/ml (0.003%) of ethidium bromide. The bands were visualized and quantified using a Molecular Imager FX scanner and Quantity One software (Bio-Rad, Hercules, CA).
Enzyme-Linked Immunosorbent Assays
Cytokines were quantified from supernatants of overnight cultured cells using a sandwich enzyme-linked immunosorbent assay system, as we described before.13 The murine enzyme-linked immunosorbent assays were designed using standardized antibodies that were purchased from R&D Systems (Minneapolis, MN) that detect protein at concentrations greater than 10 pg/ml, are specific, and do not cross-react with any other cytokines.
Measurement of SCF from Pulmonary Fibroblasts
After co-culture of fibroblasts and eosinophils for 24 hours, eosinophils were washed out and fibroblasts lysed in 0.1% Nonidet P-40 containing complete protease inhibitors cocktail (Roche Diagnostics Corp., Indianapolis, IN) to allow quantitation of all SCF, membrane and soluble. To clear out nonadherent cells, collected supernatants were centrifuged at 400 × g for 5 minutes at + 4°C and transferred to a fresh tube. In the case of soluble SCF measurements in the supernatant, we did not add the Nonidet P-40 before collection of the supernatant.
Statistical Analyses
Statistical significance was determined using analysis of variance with P values less than 0.05 followed by Student-Neuman-Keuls post-test when appropriate.
Results
Fibroblasts and Eosinophil Interaction Activates Membrane SCF Production
Previous studies demonstrated that neutralization of SCF during chronic allergen responses reduced peribronchial remodeling and collagen deposition.14 Although lung fibroblasts produce significant levels of the transmembrane form of SCF (mSCF),18 soluble SCF may also contribute to the cellular activation profile.19,20 However, when investigating the expression of the soluble form of SCF in our studies, no significant level of soluble SCF expression (mRNA or protein) in fibroblasts cultured alone or with eosinophils was found (data not shown). Thus our studies have focused on the transmembrane form of SCF. Figure 1 illustrates that fibroblasts from chronic allergen-challenged animals (CRA fibroblasts) express significantly higher mSCF mRNA levels compared to naïve fibroblasts (Figure 1). Because previous studies have demonstrated altered fibroblast function when they interact with eosinophils,21,22,23,24 we were interested in how eosinophils influenced mSCF production from fibroblasts. Increased mSCF production was observed when eosinophils were combined with CRA fibroblasts compared to naïve fibroblasts, which displayed no increase in SCF mRNA or protein when combined with eosinophils (Figure 1). Although the levels of SCF mRNA and protein appeared to be additive in the co-cultures with CRA fibroblasts, the difference compared to the naive fibroblast cultures was significant and noteworthy.
Figure 1.
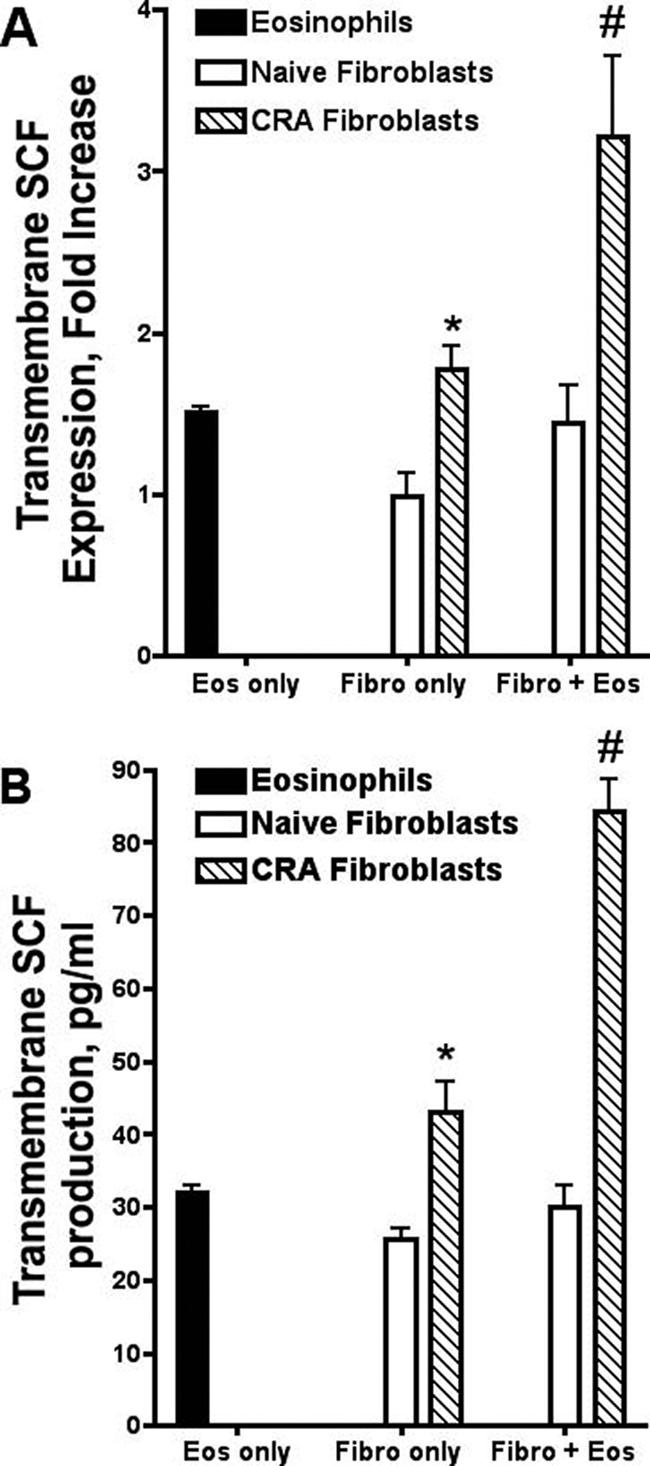
CRA fibroblasts exhibit higher constitutive and eosinophil-induced SCF levels compared to control fibroblasts. CRA fibroblasts have higher mRNA (mRNA) SCF expression (A) and protein production levels (B) compared to control fibroblasts. CRA but not control fibroblasts also have increased SCF mRNA expression (A) and protein production (B) in co-culture with eosinophils (1 × 106). Naïve, lung fibroblasts from control unchallenged animals; CRA, lung fibroblasts from chronic CRA allergen-challenged animals; Fibro, lung fibroblasts; Eos, murine eosinophils. Data represent the means ± SE from 3 to 12 repeated experiments. *P < 0.05 compared with naive fibroblasts only; #P < 0.05 compared with CRA fibroblasts only.
Because earlier studies indicated that SCF contributes to mast cell and eosinophil adherence3,25,26 the numbers of eosinophils adhered to the fibroblasts were examined. To determine whether there was a general increase in eosinophil adherence to the fibroblasts from lungs of allergic mice, co-cultures were washed free of nonadherent eosinophils, fixed, and differentially stained. The morphometric enumeration of adhered eosinophils in the co-cultures demonstrated an increase in the number of eosinophils adhered to the fibroblasts in allergic versus naive cultures (CRA fibroblasts 178 ± 28 versus naive fibroblasts 128 ± 10), but this was not statistically significant. In additional studies when cultures were treated with anti-SCF, no reduction in eosinophil adherence could be observed compared to control antibody-treated fibroblasts from allergic mice (control Ab,169 ± 58 versus anti-SCF,152 ± 38). Thus, although SCF may contribute to cellular adhesion, its role in these static cultures was not evident.
CCL5 and CCL6 Production Requires Contact between Fibroblasts and Eosinophils and Is Dependent on SCF
SCF is an eosinophil degranulator and activator that may play a number of roles during an inflammatory/immune response, including production of CC chemokines from eosinophils.4 In particular, CCL6 can be produced at significant levels by eosinophils, whereas CCL5 levels are more modest but significantly increased. When the two populations were combined, a significant increase in both CCL5 and CCL6 could be observed in the production of both chemokines (Figure 2, A and B). To determine whether cell-to-cell contact was required for production of chemokines, we used transwell barrier plates (3 μm pores) to perform co-cultures of naïve or CRA fibroblasts with eosinophils. The transwell separation of the two cell populations inhibited CCL5 and CCL6 production (Figure 2). We conclude that the observed increase in chemokine production in fibroblast and eosinophil co-cultures requires cell-to-cell contact.
Figure 2.

Increased CCL6 and CCL5 production in fibroblast-eosinophil co-culture requires cell-to-cell contact. Eosinophils (1 × 106) were layered onto a semiconfluent monolayer of lung fibroblasts or trans-well barrier plate. Elimination of fibroblast-to-eosinophil contact prevents the observed CCL6 and CCL5 increase in co-cultures. Fibro, lung fibroblasts; Eos, murine eosinophils; T, transwell. Data represents the means ± SE from three repeated experiments. *P < 0.05 compared with naïve fibroblasts only; #P < 0.05 compared with CRA fibroblasts only; σP < 0.05 compared with naive fibroblasts and eosinophils; ΔP < 0.05 compared with CRA fibroblasts and eosinophils.
In the next set of experiments, we examined whether cell-to-cell contact-induced chemokine production involved SCF-mediated activation. CRA fibroblasts produced significantly higher levels of CCL6 compared to naïve fibroblasts (Figure 3A). After eosinophils were overlaid onto naive or CRA fibroblasts, CCL6 production was significantly elevated. The increase in CCL6 production was much more dramatic in CRA fibroblast-eosinophil co-cultures, and anti-SCF antibodies significantly reduced CCL6 production in only the CRA fibroblast-eosinophil co-cultures. The CCL5 level was also significantly increased after naïve and CRA fibroblasts were combined with eosinophils (Figure 3B). Similar to CCL6 production, anti-SCF antibody treatment was not effective in blocking CCL5 production in naive fibroblast-eosinophil co-culture, whereas the treatment significantly reduced CCL5 in CRA fibroblast-eosinophil co-culture. The antibody treatments had no effect in cultures with eosinophils alone. Together, these data suggest that lung fibroblasts from chronic CRA-challenged animals induced higher chemokine production during eosinophil interaction and depended on SCF-mediated mechanisms.
Figure 3.
Anti-SCF antibody administration blocks CCL6 and CCL5 synergistic increase in CRA fibroblast-eosinophil co-culture. Eosinophils (1 × 106) were layered onto a semiconfluent monolayer of lung fibroblasts. Anti-SCF antibodies (50 μg) were added, and cells were co-cultured and incubated for 24 hours (A and B) or 4 hours (C) at 37°C. Fibro, lung fibroblasts; Eos, murine eosinophils. Data represent the means ± SE from three experiments. *P < 0.05 compared with naïve fibroblasts only; #P < 0.05 compared with CRA fibroblasts only; ΔP < 0.05 compared with CRA fibroblasts and eosinophils. Treatment of fibroblast only with anti-SCF had no effect on the level of chemokine produced.
A final aspect of these investigations was whether the interactions of eosinophils with fibroblasts led to a degranulation event by the eosinophils. To this end, we assessed the level of eosinophil peroxidase in supernatants of 4-hour cultures as previously performed.4 The eosinophil peroxidase assay demonstrated that a significant degranulation event occurred equally as well when eosinophils were incubated with fibroblasts either from nonallergic mice or from mice that had received chronic allergen exposures (Figure 3C). When anti-SCF antibody was added to the co-culture, there was a slight but insignificant reduction in the amount of eosinophil peroxidase released into the culture supernatant. Thus, a general degranulation event could be observed in the co-cultures, but this was neither dependent on the source of the fibroblast nor the level of SCF expressed.
Eosinophil-Induced Fibroblast Activation and Phenotypic Change Correlates with TGF-β Expression
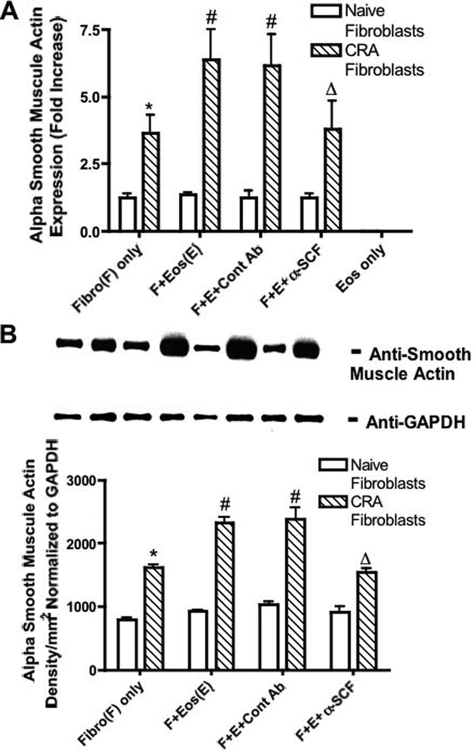
In addition to chemokine production, eosinophils have also been suggested to induce development of myofibroblasts.27,28 To examine this aspect we assessed α-SMA, which is the primary indication for myofibroblast differentiation. At baseline CRA fibroblasts exhibited significantly higher α-SMA mRNA expression level (Figure 4A) and protein production (Figure 4B) compared to fibroblasts from naive animals. We did not observe any change in mRNA expression or protein production in fibroblasts from naive mice when cultured with eosinophils. In contrast, CRA fibroblasts co-cultured with eosinophils resulted in significantly increased α-SMA mRNA expression (Figure 4A) and protein production (Figure 4B). Administration of anti-SCF antibodies inhibited eosinophil-induced α-SMA mRNA expression and protein production in fibroblasts from chronic allergen-challenged animals.
Figure 4.
Anti-SCF antibodies inhibited eosinophil-induced α-SMA production by CRA fibroblasts. Eosinophils (1 × 106) were layered onto a monolayer of lung fibroblasts, and anti-SCF or control antibodies (50 μg) were added. A: α-SMA mRNA expression was assayed by real-time PCR in 1-hour culture. B: α-SMA protein production was assayed by Western blot in 24-hour culture. Fibro, lung fibroblasts; Eos, murine eosinophils. Data represents the means ± SE from three repeated experiments. * P < 0.05 compared with naïve fibroblasts only; #P < 0.05 compared with CRA fibroblasts only; ΔP < 0.05 compared with CRA fibroblasts and eosinophils. Treatment of fibroblast only with anti-SCF had no effect on the level of α-SMA produced.
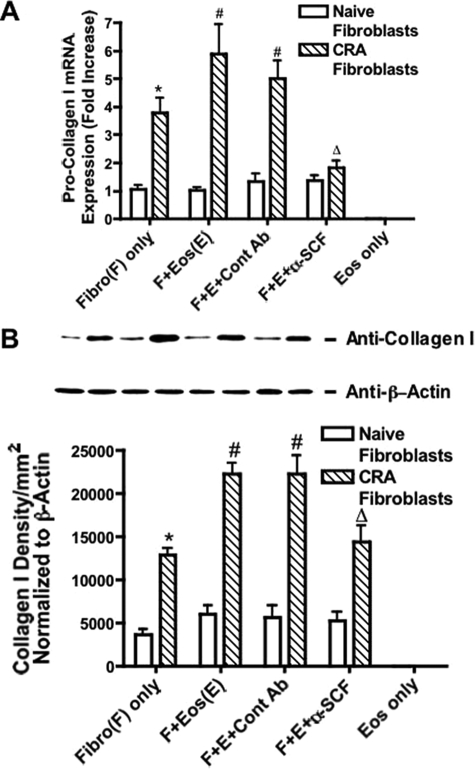
A second aspect of myofibroblast activation is collagen production. To investigate the regulation of collagen production during the fibroblast-eosinophil interaction, we examined procollagen type I expression. CRA fibroblasts maintained a significantly higher level of procollagen type I expression compared to naïve fibroblasts (Figure 5). When eosinophils were added to the fibroblast cultures, CRA fibroblasts were much more responsive to eosinophil-induced activation and demonstrated significantly higher procollagen I expression than the naive fibroblasts (Figure 5). Addition of anti-SCF antibodies significantly suppressed collagen I mRNA expression in the fibroblast-eosinophil co-cultures (Figure 5A). We also assessed the protein expression using Western blot analysis and found a similar up-regulation of protein in only the CRA fibroblast co-cultures and a dependence on SCF, as addition of specific antibodies reduced the amount of type I collagen protein (Figure 5B). These observations indicate that SCF is involved in the activation process during fibroblast-eosinophil interaction.
Figure 5.
Anti-SCF antibodies inhibited eosinophil-induced type I procollagen expression by CRA fibroblasts. Eosinophils (1 × 106) were layered onto a monolayer of lung fibroblasts and anti-SCF or control antibodies (50 μg) were added. A: Collagen I mRNA expression was assayed by real time PCR in 1-hour culture. B: Collagen I protein production was assayed by Western blot in 24-hour culture. Fibro, lung fibroblasts; Eos, murine eosinophils. Data represent the means ± SE from three repeated experiments. *P < 0.05 compared with naïve fibroblasts only; #P < 0.05 compared with CRA fibroblasts only; ΔP < 0.05 compared with CRA fibroblasts and eosinophils. Treatment of fibroblast only with anti-SCF had no effect on the level of collagen produced.
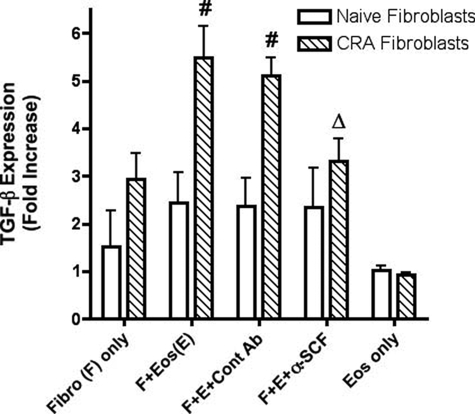
As indicated above, fibroblasts from chronic allergen-challenged lungs displayed a myofibroblast phenotype with increased collagen and α-SMA when combined with eosinophils. TGF-β is a primary stimulus for differentiation of fibroblasts into myofibroblasts.1,29 When we assessed the expression of TGF-β during the cellular interaction, we observed an increased expression when eosinophils were co-cultured with CRA fibroblasts but not with naive fibroblasts (Figure 6). When anti-SCF was added to the co-cultures, a significant reduction in the expression of TGF-β was observed. Altogether, these data suggest that fibroblasts from the lungs of chronic allergen-challenged animals maintained a more fibrotic phenotype and could be further driven toward myofibroblast differentiation via SCF-mediated TGF-β expression presumably derived from c-kit+ eosinophils after interaction with the fibroblasts.
Figure 6.
Anti-SCF antibodies inhibited eosinophil-induced TGF-β expression by CRA fibroblasts. Eosinophils (1 × 106) were layered onto a monolayer of lung fibroblasts, and anti-SCF or control antibodies (50 μg) were added. TGF-β mRNA expression was assayed by real-time PCR in 1-hour culture. Fibro, lung fibroblasts; Eos, murine eosinophils. Data represent the means ± SE from three repeated experiments; #P < 0.05 compared with CRA fibroblasts only; ΔP < 0.05 compared with CRA fibroblasts and eosinophils. Treatment of fibroblast only with anti-SCF had no effect on the level of TGF-β produced.
Discussion
Multiple mediators have been implicated and targeted during chronic allergic disease. Increases in SCF and c-Kit mRNA have been identified in the airway epithelial cell layer of asthmatic patients compared with controls.30,31,32 In addition, significant differences in the number of SCF- and c-Kit-expressing cells was observed by in situ hybridization and immunohistochemistry, suggesting a coordinated role for SCF in the pathophysiological changes that occur in asthma.32 Evidence for a role of SCF in human airway disease was supported by studies demonstrating increased SCF expression in nasal polyps of asthmatics and allergic rhinitis patients.33,34 Additionally, SCF has been implicated as a potential mediator in fibrotic diseases in the kidney and intestine.34,35,36,37 Recently, the role of SCF has been verified using a chronic mouse model of allergic disease characterized by airway hyperresponsiveness, mucus overproduction, eosinophil accumulation, and peribronchial collagen deposition.11,13,14 Thus, SCF is up-regulated in inflammatory conditions and appears to be associated with chronic remodeling disease.
Numerous cell populations have been shown to express SCF, including epithelial cells, smooth muscle cells, macrophages, mast cells, eosinophils, and fibroblasts.5,18,31,32,38,39,40,41,42,43 SCF production by pulmonary fibroblasts has previously been characterized in several end-stage diseases.30,38,44 SCF activates eosinophils to promote adhesion to matrix and VCAM-1 via VLA4,45 while also having the ability to promote degranulation and production of inflammatory and profibrotic factors.4 Furthermore, a number of publications have outlined that fibroblasts express SCF and can promote the activation, adhesion, and survival of mast cells, which also have a detrimental role in the development of allergic disease.18,41,46 By evaluating mouse lung fibroblasts derived from chronic allergen-treated animals (CRA fibroblasts) or those from control, unsensitized animals, we demonstrate that fibroblasts from chronic disease have elevated SCF expression and protein production. Fibroblasts grown from the lungs of chronically challenged mice also produced additional mediators, including CCL5 and CCL6, that were further up-regulated on contact with eosinophils. The increased chemokines were abrogated by treatment with anti-SCF antibodies during the co-culture period. These observations correspond with previous data that demonstrated that SCF can promote chemokines from mast cells and eosinophils4,5 and suggest a potential mechanism for SCF activation during development of chronic allergic disease. These findings at least in part explain the beneficial role of anti-SCF treatment in vivo that resulted in reduced airway inflammation and remodeling during chronic CRA-induced mouse asthma.13,14,47
One of the primary considerations in our studies was to assess the impact of eosinophils on fibroblast maturation/differentiation to a myofibroblast phenotype. Previous studies have indicated that eosinophils can contribute significantly to myofibroblast differentiation.27,28 Eosinophils store and release several fibrogenic factors, such as TGF-β, that can stimulate fibroblast activation and collagen synthesis.48 In the present study CRA fibroblasts displayed significantly enhanced expression of type I collagen and α-SMA, two important markers of myofibroblast differentiation. In addition, the expression of TGF-β was also markedly increased when eosinophils were combined with fibroblasts, and this was SCF-dependent. The data indicate that fibroblasts from chronic allergen-challenged animals have an altered phenotype compared to that observed in fibroblasts from unchallenged animals. The data further indicate that interaction with eosinophils via an SCF-mediated mechanism enhances the inflammatory response and myofibroblast phenotype. Our overall hypothesis, illustrated in Figure 7, suggests that 1) the increased expression of SCF by fibroblasts in allergic airway provides a significant stimulus for eosinophil activation during an allergic response and 2) this interaction leads to TGF-β production that 3) subsequently causes myofibroblast differentiation. At the same time the overexpression of chemokines during the allergic response increases inflammatory cell influx. We would suggest that targeting the SCF/c-kit activation pathway in chronic inflammatory diseases would have a beneficial effect on the control of the disease process. This has recently been supported by studies using a c-kit tyrosine kinase inhibitor, imatinib, that by itself can block the development of chronic allergen responses in animal models.13,14 Thus, local airway application of SCF- and/or c-kit-specific inhibitors may provide significant attenuation of clinical disease during periods of chronic inflammation and remodeling.
Figure 7.
SCF-c-kit activation mediates a profibrotic environment and differentiation of myofibroblasts.
Footnotes
Address reprint requests to Vladislav Dolgachev, Ph.D., Department of Pathology, University of Michigan, 109 Zina Pitcher, Ann Arbor, MI 48109-2200. E-mail: vdolgach@umich.edu.
References
- Howell JE, McAnulty RJ. TGF-beta: its role in asthma and therapeutic potential. Curr Drug Targets. 2006;7:547–565. doi: 10.2174/138945006776818692. [DOI] [PubMed] [Google Scholar]
- Laberge S, El Bassam S. Cytokines, structural cells of the lungs and airway inflammation. Paediatr Respir Rev. 2004;5(Suppl A):S41–S45. doi: 10.1016/s1526-0542(04)90009-7. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Austen KF, Friend DS, Heidtman M, Boyce JA. Human peripheral blood eosinophils express a functional c-kit receptor for stem cell factor that stimulates very late antigen 4 (VLA-4)-mediated cell adhesion to fibronectin and vascular cell adhesion molecule 1 (VCAM-1). J Exp Med. 1997;186:313–323. doi: 10.1084/jem.186.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SH, Taub DD, Nagel J, Smith R, Hogaboam CM, Berlin A, Lukacs NW. Stem cell factor induces eosinophil activation and degranulation: mediator release and gene array analysis. Blood. 2002;100:4291–4297. doi: 10.1182/blood.V100.13.4291. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Lincoln PM, Brownell E, Pullen DM, Schock HJ, Chensue SW, Taub DD, Kunkel SL. Stem cell factor (c-kit ligand) influences eosinophil recruitment and histamine levels in allergic airway inflammation. J Immunol. 1996;156:3945–3951. [PubMed] [Google Scholar]
- Levi-Schaffer F, Weg VB. Mast cells, eosinophils and fibrosis. Clin Exp Allergy. 1997;27(Suppl 1):S64–S70. doi: 10.1111/j.1365-2222.1997.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Munitz A, Levi-Schaffer F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy. 2004;59:268–275. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25:477–482. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Ichinose M. Agents against cytokine synthesis or receptors. Eur J Pharmacol. 2006;533:289–301. doi: 10.1016/j.ejphar.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Campbell E, Hogaboam C, Lincoln P, Lukacs NW. Stem cell factor-induced airway hyperreactivity in allergic and normal mice. Am J Pathol. 1999;154:1259–1265. doi: 10.1016/S0002-9440(10)65377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SH, Hogaboam CM, Berlin A, Lukacs NW. SCF-induced airway hyperreactivity is dependent on leukotriene production. Am J Physiol. 2001;280:L1242–L1249. doi: 10.1152/ajplung.2001.280.6.L1242. [DOI] [PubMed] [Google Scholar]
- Berlin AA, Lincoln P, Tomkinson A, Lukacs NW. Inhibition of stem cell factor reduces pulmonary cytokine levels during allergic airway responses. Clin Exp Immunol. 2004;136:15–20. doi: 10.1111/j.1365-2249.2004.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin AA, Hogaboam CM, Lukacs NW. Inhibition of SCF attenuates peribronchial remodeling in chronic cockroach allergen-induced asthma. Lab Invest. 2006;86:557–565. doi: 10.1038/labinvest.3700419. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Standiford TJ, Chensue SW, Kunkel RG, Strieter RM, Kunkel SL. C-C chemokine-induced eosinophil chemotaxis during allergic airway inflammation. J Leukoc Biol. 1996;60:573–578. doi: 10.1002/jlb.60.5.573. [DOI] [PubMed] [Google Scholar]
- Dolgachev V, Oberley LW, Huang TT, Kraniak JM, Tainsky MA, Hanada K, Separovic D. A role for manganese superoxide dismutase in apoptosis after photosensitization. Biochem Biophys Res Commun. 2005;332:411–417. doi: 10.1016/j.bbrc.2005.04.141. [DOI] [PubMed] [Google Scholar]
- Dolgachev V, Farooqui MS, Kulaeva OI, Tainsky MA, Nagy B, Hanada K, Separovic D. De novo ceramide accumulation due to inhibition of its conversion to complex sphingolipids in apoptotic photosensitized cells. J Biol Chem. 2004;279:23238–23249. doi: 10.1074/jbc.M311974200. [DOI] [PubMed] [Google Scholar]
- Hogaboam C, Kunkel SL, Strieter RM, Taub DD, Lincoln P, Standiford TJ, Lukacs NW. Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. J Immunol. 1998;160:6166–6171. [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Wershil BK. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am J Pathol. 1993;142:965–974. [PMC free article] [PubMed] [Google Scholar]
- Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037–1051. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116:796–804. doi: 10.1016/j.jaci.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Takafuji S, Shoji S, Ito K, Yamamoto K, Nakagawa T. Eosinophil degranulation in the presence of lung fibroblasts. Int Arch Allergy Immunol. 1998;117(Suppl 1):S52–S54. doi: 10.1159/000053572. [DOI] [PubMed] [Google Scholar]
- Sabatini F, Silvestri M, Sale R, Scarso L, Defilippi AC, Risso FM, Rossi GA. Fibroblast-eosinophil interaction. Modulation of adhesion molecules expression and chemokine release by human fetal lung fibroblasts in response to IL-4 and TNF-alpha. Immunol Lett. 2002;84:173–178. doi: 10.1016/s0165-2478(02)00183-9. [DOI] [PubMed] [Google Scholar]
- Rochester CL, Ackerman SJ, Zheng T, Elias JA. Eosinophil-fibroblast interactions. Granule major basic protein interacts with IL-1 and transforming growth factor-beta in the stimulation of lung fibroblast IL-6-type cytokine production. J Immunol. 1996;156:4449–4456. [PubMed] [Google Scholar]
- Dastych J, Metcalfe DD. Stem cell factor induces mast cell adhesion to fibronectin. J Immunol. 1994;152:213–219. [PubMed] [Google Scholar]
- Kinashi T, Springer TA. Steel factor and c-kit regulate cell-matrix adhesion. Blood. 1994;83:1033–1038. [PubMed] [Google Scholar]
- Phipps S, Ying S, Wangoo A, Ong YE, Levi-Schaffer F, Kay AB. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604–4612. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- Zhang S, Mohammed Q, Burbidge A, Morland CM, Roche WR. Cell cultures from bronchial subepithelial myofibroblasts enhance eosinophil survival in vitro. Eur Respir J. 1996;9:1839–1846. doi: 10.1183/09031936.96.09091839. [DOI] [PubMed] [Google Scholar]
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- Al-Muhsen SZ, Shablovsky G, Olivenstein R, Mazer B, Hamid Q. The expression of stem cell factor and c-kit receptor in human asthmatic airways. Clin Exp Allergy. 2004;34:911–916. doi: 10.1111/j.1365-2222.2004.01975.x. [DOI] [PubMed] [Google Scholar]
- Da Silva CA, Frossard N. Regulation of stem cell factor expression in inflammation and asthma. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):S145–S151. doi: 10.1590/s0074-02762005000900025. [DOI] [PubMed] [Google Scholar]
- Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 533:327–340. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Kowalski ML, Lewandowska-Polak A, Wozniak J, Ptasinska A, Jankowski A, Wagrowska-Danilewicz M, Danilewicz M, Pawliczak R. Association of stem cell factor expression in nasal polyp epithelial cells with aspirin sensitivity and asthma. Allergy. 2005;60:631–637. doi: 10.1111/j.1398-9995.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- El-Koraie AF, Baddour NM, Adam AG, El Kashef EH, El Nahas AM. Role of stem cell factor and mast cells in the progression of chronic glomerulonephritides. Kidney Int. 2001;60:167–172. doi: 10.1046/j.1523-1755.2001.00783.x. [DOI] [PubMed] [Google Scholar]
- Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol. 2005;289:G2–G7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- El Kossi MM, El Nahas AM. Stem cell factor and crescentic glomerulonephritis. Am J Kidney Dis. 2003;41:785–795. doi: 10.1016/s0272-6386(03)00026-x. [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183–C201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- Fireman E, Kivity S, Shahar I, Reshef T, Mekori YA. Secretion of stem cell factor by alveolar fibroblasts in interstitial lung diseases. Immunol Lett. 1999;67:229–236. doi: 10.1016/s0165-2478(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Kassel O, Schmidlin F, Duvernelle C, Gasser B, Massard G, Frossard N. Human bronchial smooth muscle cells in culture produce stem cell factor. Eur Respir J. 1999;13:951–954. doi: 10.1034/j.1399-3003.1999.13e04.x. [DOI] [PubMed] [Google Scholar]
- Kim YK, Nakagawa N, Nakano K, Sulakvelidze I, Dolovich J, Denburg J. Stem cell factor in nasal polyposis and allergic rhinitis: increased expression by structural cells is suppressed by in vivo topical corticosteroids. J Allergy Clin Immunol. 1997;100:389–399. doi: 10.1016/s0091-6749(97)70254-9. [DOI] [PubMed] [Google Scholar]
- Koma Y, Ito A, Watabe K, Hirata T, Mizuki M, Yokozaki H, Kitamura T, Kanakura Y, Kitamura Y. Distinct role for c-kit receptor tyrosine kinase and SgIGSF adhesion molecule in attachment of mast cells to fibroblasts. Lab Invest. 2005;85:426–435. doi: 10.1038/labinvest.3700231. [DOI] [PubMed] [Google Scholar]
- Linenberger ML, Jacobson FW, Bennett LG, Broudy VC, Martin FH, Abkowitz JL. Stem cell factor production by human marrow stromal fibroblasts. Exp Hematol. 1995;23:1104–1114. [PubMed] [Google Scholar]
- Lukacs NW, Kunkel SL, Strieter RM, Evanoff HL, Kunkel RG, Key ML, Taub DD. The role of stem cell factor (c-kit ligand) and inflammatory cytokines in pulmonary mast cell activation. Blood. 1996;87:2262–2268. [PubMed] [Google Scholar]
- Da Silva CA, Kassel O, Lebouquin R, Lacroix EJ, Frossard N. Paradoxical early glucocorticoid induction of stem cell factor (SCF) expression in inflammatory conditions. Br J Pharmacol. 2004;141:75–84. doi: 10.1038/sj.bjp.0705598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierke CT, Ballmaier M, Werner U, Manns MP, Welte K, Bischoff SC. Human endothelial cells regulate survival and proliferation of human mast cells. J Exp Med. 2000;192:801–811. doi: 10.1084/jem.192.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Katayama I, Nishioka K. Expression of stem cell factor in basal cell carcinoma. Br J Dermatol. 1997;137:709–713. [PubMed] [Google Scholar]
- Finotto S, Buerke M, Lingnau K, Schmitt E, Galle PR, Neurath MF. Local administration of antisense phosphorothioate oligonucleotides to the c-kit ligand, stem cell factor, suppresses airway inflammation and IL-4 production in a murine model of asthma. J Allergy Clin Immunol. 2001;107:279–286. doi: 10.1067/mai.2001.113049. [DOI] [PubMed] [Google Scholar]
- Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]