Abstract
Neointima formation after vascular injury is exaggerated in ovariectomized (OVX) human C-reactive protein transgenic mice (CRPtg) compared to nontransgenic mice (NTG). We tested the hypothesis that this CRP-mediated exacerbation requires IgG Fc receptors (FcγRs). OVX NTG, CRPtg, and CRPtg lacking FcγRI, FcγRIIb, FcγRIII, or the common γ chain (FcRγ) had their common carotid artery ligated. Twenty-eight days later neointimal thickening in CRPtg/FcγRI−/− and CRPtg/FcRγ−/− was significantly less than in CRPtg and no worse than in NTG, whereas in CRPtg/FcγRIIb−/− and CRPtg/FcγRIII−/− neointimal thickness was equal to or greater than in CRPtg. Immunohistochemistry revealed human CRP in the neointima of CRPtg, but little or none was observed in those lacking FcγRI or FcRγ. Real-time reverse transcriptase-polymerase chain reaction demonstrated that FcγR types I to III were expressed in the CRPtg arteries, with FcγRI expression increasing by threefold after ligation injury. Levels of serum complement (C3), neointimal deposition of complement (C3d), and cellular composition (monocytes, macrophages, lymphocytes) in the neointima did not differ among the different CRPtg genotypes. However, by immunofluorescence a neointimal population of F4/80+CRP+ cells was revealed only in OVX CRPtg. The exaggerated response to vascular injury provoked by CRP in OVX CRPtg depends on FcγRI and probably requires its expression by F4/80+ cells.
C-reactive protein (CRP) blood level has served as a useful biomarker of generalized injury and inflammation for decades,1 and the recent introduction of practical and high-sensitivity assays has led to accumulation of vast amounts of clinical data suggesting serum CRP levels can also predict risk of cardiovascular disease.2 At the same time mechanistic studies performed using isolated cells have provided evidence that CRP might initiate and/or accelerate the atherosclerotic/atherothrombotic process. For example in vitro experiments show that CRP can induce endothelial cells to express adhesion molecules and monocyte chemoattractant protein-1 (MCP-1), both thought to be essential for the initiation of atherogenesis.3,4 CRP was also shown to decrease nitric oxide5 and prostacyclin6 and increase endothelin-17 production by endothelial cells, effects predicted to contribute to endothelial dysfunction. Despite these compelling data, controversy remains whether CRP has a causal pathogenic role in any vascular disease process or if it is just an innocent bystander.8,9
Although there is no direct proof that CRP has a pathogenic role in humans, evidence from a variety of animal models has shown that human CRP can accelerate the developments of vascular disease. Notably, using various CRP transgenic (CRPtg) mouse models, we have shown that mice expressing human CRP experience much more rapid and higher rates of complete thrombotic occlusion after vascular injury than their nontransgenic (NTG) counterparts10 and that transgenic expression of human CRP accelerates aortic atherosclerotic lesion progression in apolipoprotein E knockout mice.11 Most recently we demonstrated that when ovariectomized (OVX) mice were subjected to ligation of the common carotid artery, CRPtg had nearly twofold greater neointima formation than wild type.12 Interestingly this was associated with extensive human CRP mRNA expression and protein deposition in the injured vessels of CRPtg, despite the fact that blood levels of the protein did not substantially rise.12
Not all of the published CRPtg findings have been in complete agreement. For example Hirschfield and colleagues13 reported that human CRP was not proatherogenic in a second strain of CRPtg apolipoprotein E knockout mice, and we showed that transgenic human CRP did not influence atherogenesis in the apolipoprotein E*3-Leiden mouse.14 Importantly, however, in the study by Hirschfield and colleagues,13 despite the fact that the difference was not statistically significant, there was a reported trend toward worsened disease in CRPtg until 20 weeks of age, and human CRP was present in CRPtg vascular lesions. In our study in CRPtg apolipoprotein E*3-Leiden mice,14 human CRP was not detected in the developing atheromas. The combined results are therefore consistent with the interpretation that human CRP is proatherogenic in atherosclerosis-prone mice if CRP is present in the developing lesion. These studies thus provide compelling direct evidence that human CRP can have prothrombotic and atherogenic effects and that it facilitates the adverse remodeling seen after acute arterial injury, at least in some CRPtg models. The hypothesis that CRP deposition/expression in the vasculature may have pathogenic significance in the clinical setting is in line with the protein’s clinical association with heart disease risk.
Expression of the human transgene in CRPtg faithfully mimics that of the authentic gene in humans,15 and human CRP binds to the IgG Fc receptors FcγRI (CD64) and FcγRII (CD32) and perhaps also FcγRIII (CD16), in both mouse and man.16,17,18 Because many of the biological effects of CRP on human and mouse monocytes, neutrophils, and endothelial cells are mediated by binding to FcγRs,16,17,18,19,20,21,22 we tested the hypothesis that FcγRs are required for human CRP-mediated exacerbation of the vascular injury response in CRPtg. To do so we used our well established OVX/carotid artery ligation model.12
Materials and Methods
Animals
Mutant mice generated by breeding CRPtg10,12,15 with congenics deficient in the FcR common γ chain23 (CRPtg/FcRγ−/−), FcγRI24 (CRPtg/FcγRI−/−), FcγRIIb25 (CRPtg/FcγRIIb−/−), and FcγRIII26 (CRPtg/FcγRIII−/−), and CRPtg and NTG wild-type mice, were studied at 10 to 12 weeks of age. All mutant mice were backcrossed at least 10 generations onto the C57BL/6 background. FcγR-deficient versus -sufficient CRPtg and NTG progeny were obtained in the expected Mendellian ratios. Each of the genotypes appeared phenotypically normal, and no remarkable differences in lifespan have been noted. Wild-type mice were from The Jackson Laboratory (Bar Harbor, ME).
Details of the human CRP transgene and its human-like expression in CRPtg have been fully described.15,27 Importantly, the flanking sequences of the transgene include all of the human cis-acting regulatory elements (ie, the entire human promoter) responsible for both tissue specificity and acute phase inducibility of human CRP, and all of the trans-acting factors required for correct regulation of the transgene are conserved from mice to humans.15,27 Thus in CRPtg, human CRP is present in the blood at concentrations relevant to human disease, ie, low levels (<3 μg/ml) under steady-state conditions and at high levels (>500 μg/ml) during an endotoxemia- or infection-induced acute phase response.15 Despite this neither OVX nor carotid artery ligation leads to appreciable elevation of human CRP in the blood of CRPtg,12 and blood levels of human CRP in CRPtg are unaffected by deletion of FcγRs (data not shown). Endogenous mouse CRP is present at low levels in CRPtg but itself is not an acute phase protein.28
All mice were fed a standard mouse pellet diet (Ralston Purina Diet, Vevey, Switzerland) and maintained at constant humidity (60 ± 5%) and temperature (24 ± 1°C) with a 12-hour light cycle (6 a.m. to 6 p.m). All protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and were consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 96-01, revised 1996).
Carotid Artery Injury
Mice were anesthetized with ketamine (80 mg/kg i.p.; Abbott Laboratories) and xylazine (5 mg/kg i.p., Rompun; Bayer Corp., Morristown, NJ) and subjected to OVX. Each of the six experimental groups included at least 4 and as many as 12 mice. Five days after OVX, mice were anesthetized and, to stimulate neointima formation, the right common carotid artery was exposed through a midline cervical incision and ligated with an 8-0 silk suture just proximal to the bifurcation, as described.12,29 The left common carotid artery was also exposed but not ligated and thus served as an internal control (sham injured). In a separate series of animals not subjected to ligation injury, the right carotid artery was removed and served as a second (uninjured) control.
Morphometric Analysis
Twenty-eight days after injury, mice were euthanized with an overdose of pentobarbital. The vasculature was immediately flushed with 0.01 mol/L sodium phosphate buffer (pH 7.4) and perfused with 10% formalin. Both carotid arteries were excised, fixed in 10% formalin, embedded in paraffin, and sectioned. The ligation site was identified by inspecting representative serial sections stained with hematoxylin and eosin (H&E). Additional serial sections of vessels 200, 500, and 700 μm distal to the ligation site were then treated with Verhoeff’s elastin stain to enhance the elastic laminae. Serial sections of the unligated contralateral vessels were obtained and processed in the same manner.
Computer-assisted morphometric analysis of digitized images captured from each arterial section was performed with image analysis software (Scion Image; Scion Corp., Frederick, MD). Measurements of the four serial sections obtained for each vessel were averaged for statistical purposes. The cross-sectional area of the media, ie, the zone bounded by the external elastic lamina and the internal elastic lamina, and the area of the intima, ie, the zone between the internal elastic lamina and the lumen, was calculated. Neointima formation in the occluded carotid arteries is expressed as intimal area or as the intimal area/medial area ratio. All measurements were performed by a single examiner blinded to the genotype and treatment of the mice.
Immunochemistry and Immunofluorescence
For immunochemistry representative arteries were harvested, fixed in paraformaldehyde, and paraffin-embedded before preparation of thin sections (5 μm). To detect human CRP and mouse C3d, immunochemical analysis was performed as described11,12 using anti-human CRP and anti-mouse C3d antibodies and the Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Additional arteries were embedded directly in frozen O.C.T. compound (Sakura Fineteck U.S.A. Inc., Torrance, CA) and serial sections (5 μm) were cut on a cryostat microtome at −20°C. Frozen sections were mounted on glass slides, air-dried, acetone-fixed, and processed to visualize mouse blood cells using Vectastain and antibodies against the mouse cell surface markers F4/80 (expressed primarily by tissue macrophages), CD45R/B220 (primarily B cells), and CD4/CD8 (primarily T cells) (eBiosciences, San Diego, CA). Hematoxylin was used as a counterstain.
For immunofluorescence, thin sections (5 μm) of frozen arteries were processed with affinity-purified goat anti-human CRP antibody conjugated with fluorescein isothiocyanate (Bethyl Laboratories, Inc., Montgomery, TX) and with biotin-conjugated antibodies (mouse anti-actin from Research Diagnostics, Inc., Concord, MA; rat anti-mouse F4/80 from eBiosciences) followed by Texas Red-conjugated streptavidin (Rockland, Gilbertsville, PA). Each antibody was used according to the manufacturers’ instructions. Sections were washed and mounted in Fluormount G (Southern Biotechnology Associates, Birmingham, AL) and viewed with a Nikon TE2000-U inverted epifluorescence microscope equipped with appropriate filters (Chroma Technology Corp., Brattleboro, VT). The excitation filters were housed in a DG4 filter changer/illumination unit (Sutter Instruments, Novato, CA), and to ensure perfect spectral separation of green and red fluorescence, a green emission filter (D535/40M, bandpass 515 to 555 nm; Chroma Technology) was placed in the available space of the DIC analyzer slider underneath the objective turret. Images were acquired with a Coolsnap HQ charge-coupled device camera (Photometrics, Tucson, AZ) and processed with Adobe Photo Shop and IP LAB Spectrum software (Signal Analytics Software, Vienna, VA). Texas Red fluorescence (red) and fluorescein isothiocyanate fluorescence (green) produced a yellow-orange color where the two fluorochromes coincided.
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay was used to measure mouse serum C3. Microtiter plates were coated overnight at 4°C with 25 μg of polyclonal goat anti-mouse C3 (Cappel Organon Teknika, Durham, NC) per ml diluted in 15 mmol/L Na2CO3 and 30 mmol/L NaHCO3, pH 9.6. The next day, the antibody mixture was removed, and the plate was rinsed and blocked for 1 hour with 100 μl of 1% bovine serum albumin in phosphate-buffered saline containing 10 mmol/L ethylenediaminetetraacetic acid, pH 7.5. Plates were subsequently washed with the same buffer containing 0.05% Tween 20. Normal mouse serum (200 ng of mouse C3/ml) and sera collected from mice on the day of vessel harvest were diluted in washing buffer containing 0.05% bovine serum albumin and added to the wells. The plates were then incubated at room temperature for 2 hours. At the end of this time, wells were washed and 50 μl of goat anti-mouse C3 peroxidase-conjugated antibody was added to each well. The plates were incubated for 1 hour and washed twice in wash buffer, and 100 μl of ABTS (Sigma, St. Louis, MO) substrate buffer was added. After 30 minutes of substrate conversion, the plates were scanned at 405 nm with a Labsystems Multiskan MS scanner (Scientific Consultant Inc., Gaithersburg, MD).
Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Real-time quantitative RT-PCR was used to assess local expression of FcγRs by the injured arteries. Separate groups of OVX CRPtg were subjected to right common carotid artery ligation or sham artery operations. Carotid arteries were harvested 1 day after injury. RNA was then extracted with TRIzol, treated with DNase I to remove genomic DNA, and then purified with the use of an RNA purification kit (all from Invitrogen, Carlsbad, CA). For real-time quantitative RT-PCR, protein- and DNA-free RNA was reverse-transcribed to cDNA using the SYBR Green RT-PCR kit (Applied Biosystems, Foster City, CA) and specific primers (for ribosomal protein S9, 5′-GCTGGATGAGGGCAAGAT-3′ and 5′-CGAACAATGAAAGATGGGAT-3′; for FcγRI, 5′-CACAAATGCCCTTAGACCAC-3′ and 5′-ACCCTAGAGTTCCAGGGATG-3′; FcγRIIb, 5′-CTGTCACCATCACTGTCCAA-3′ and 5′-TAATAACAATGGCTGCGACA-3′; FcγRIII, 5′-AGAATGCACACTCTGGAAGC-3′ and 5′-ATGTCTTCCTTGAGCACCTG-3′). cDNA was amplified by PCR in the iCycler for 40 cycles, and relative RNA level was calculated with iCycler software (Applied Biosystems). Unknowns were normalized against mRNA for the housekeeping gene ribosomal protein S9 (Rp), whose expression has been shown to remain stable in carotid injury models, and then standardized to the uninjured value.
Statistical Analysis
Results of morphometric analyses, RT-PCR, and C3 enzyme-linked immunosorbent assay are expressed as the mean ± SEM without transformation. All statistical analyses were performed using the SigmaStat software package (SigmaStat; Jandel Scientific, San Rafael, CA). Comparisons among experimental groups were performed with one-way analysis of variance followed by pair-wise multiple comparisons using the Student-Newman-Keul’s test. Differences were considered significant when the associated P value was <0.05.
Results
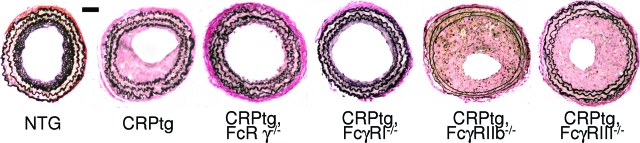
The architecture of injured carotid arteries was examined 28 days after ligation injury. Neointimae in CRPtg, CRPtg/FcγRIIb−/−, and CRPtg/FcγRIII−/− mice were thicker than in NTG, whereas in CRPtg/FcRγ−/− and CRPtg/FcγRI−/− neointimal thickening was comparable to that in NTG (Figure 1). Morphometric analysis confirmed a significant twofold to threefold increase in the neointima area in CRPtg, CRPtg/FcγRIIb−/−, and CRPtg/FcγRIII−/− compared to NTG (Figure 2A). Remarkably, CRPtg mice lacking FcγRI and CRPtg mice lacking the common γ chain (CRPtg/FcγRI−/− and CRPtg/FcRγ−/−, respectively) both had nearly patent vessels with neointimal thickening no worse than that seen in wild-type mice not expressing human CRP (NTG) (Figure 2A). The area of the media was not significantly different among the six genotypes (Figure 2B), thus the resultant neointima/media ratio was also increased significantly and by twofold in CRPtg compared to NTG mice (Figure 2C). Neointima/media ratio for CRPtg/FcγRIIb−/− and CRPtg/FcγRIII−/− mice was not significantly different from that for CRPtg but was slightly worsened, indicating that deletion of these receptors did not prevent the deleterious impact of CRP on the injury response. On the other hand, CRPtg/FcγRI−/− and CRPtg/FcRγ−/− mice had a neointima/media ratio that was lower than their CRPtg counterparts and similar in magnitude to that seen in the NTG, indicating that deletion of FcγRI or the common γ chain abolished the exacerbating effect of CRP on vascular remodeling (Figure 1 and Figure 2C).
Figure 1.
Representative light micrographs of Verhoeff’s elastin-stained sections of carotid arteries harvested 28 days after ligation injury from OVX wild-type (NTG), CRPtg, and CRPtg FcγR-deficient mice. Note the narrow neointima in vessels from NTG, CRPtg/FcRγ−/−, and CRPtg/FcγRI−/− mice. Scale bar = 50 μm. Original magnifications, ×20.
Figure 2.
Cross-sectional areas of neointima (A), media (B), and the resultant neointima/media ratio (C) of carotid arteries harvested 28 days after ligation injury from OVX wild-type (NTG), CRPtg, and CRPtg FcγR-deficient mice. Note that neointima area in vessels from CRPtg/FcRγ−/− and CRPtg/FcγRI−/− is comparable to that in NTG. Data shown are mean + SEM. Sample size (number of mice) is indicated. *P < 0.05 versus CRPtg.
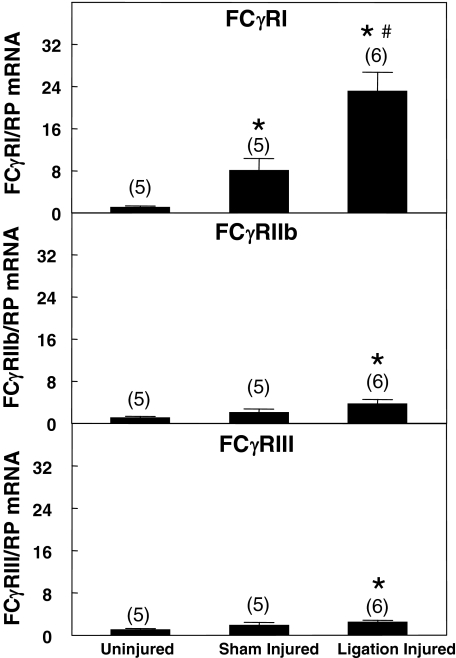
Immunohistochemical staining of paraffin-embedded tissue revealed substantial amounts of human CRP in the neointima of injured vessels in CRPtg, CRPtg/FcγRIIb−/−, and CRPtg/FcγRIII−/− (Figure 3). Consistent with CRP’s ability to bind the high-affinity Fc receptor FcγRI,17 little or no human CRP could be detected in the neointimal area in the CRPtg/FcγRI−/−. The scant deposits of human CRP in the neointima of CRPtg/FcRγ−/− variant is consistent with residual expression of the FcγRI α chain in FcRγ−/− (Figure 3).24 Using real-time RT-PCR we confirmed that FcγR types I, IIb, and III were all expressed in arteries of CRPtg (Figure 4). Only FcγRI expression was up-regulated in response to sham injury. Importantly, only FcγRI expression was up-regulated in response to ligation injury to an extent significantly (threefold) greater than that seen in sham-injured (surgically exposed and manipulated but not ligated) arteries (Figure 4).
Figure 3.
Immunoreactive human CRP in carotid arteries 28 days after carotid artery ligation injury. Human CRP (brown-red staining) is evident in injured arteries of CRPtg (A), CRPtg/FcγRIIb−/− (D), and CRPtg/FcγRIII−/− (E) but little or no human CRP is detected in CRPtg/FcRγ−/− (B) and CRPtg/FcγRI−/− (C). Original magnifications, ×20.
Figure 4.
FcγR expression (RT-PCR) in carotid arteries of OVX CRPtg. FcγRI was up-regulated after ligation injury compared to sham injury. Data are standardized to mean mRNA levels in uninjured arteries and are expressed as the mean ± SEM. *P < 0.05 versus uninjured; #P < 0.05 versus sham injured.
Because variation in complement deposition and/or the community of inflammatory cells within the neointima could account for the disparate pathology we attribute to perturbation of CRP→FcγRI interaction, we compared these parameters in ligated arteries from CRPtg versus CRPtg/FcγR-deficient mice. We observed robust deposition of immunoreactive C3d in the injured vessels of each genotype (Figure 5) and no significant difference in their serum C3 levels (data not shown). In addition, although the balance between the numbers of circulating and tissue resident cells changed as the degree of occlusion varied, F4/80+ monocytes/macrophages, B220+ B cells, and CD4/CD8+ T cells were present in lesions from both CRPtg and CRPtg/FcγRI−/− genotypes (Figure 6). Furthermore, by image analysis we confirmed that the percentage of the neointimal areas stained with antibodies specific for F4/80 (7.79 to 7.84%), B220 (1.53 to 3.37%), and CD4/CD8 (0.91 to 1.17%) did not differ between CRPtg versus CRPtg/FcγRI−/− mice (ranges given are minima and maxima for three representative CRPtg and three representative CRPtg/FcγRI−/− tissue sections).
Figure 5.
Complement deposition in ligation-injured carotid arteries. Despite widely variable neointima thickness, deposition of immunoreactive C3d (brown-red staining) is evident in injured arteries from all CRPtg genotypes. Original magnifications, ×20.
Figure 6.
Cell types and their distribution in ligation-injured carotid arteries. Arteries were harvested from CRPtg (top) versus CRPtg/FcγRI−/− mice (bottom) 28 days after ligation injury, processed, and stained for immunoperoxidase-based detection of mouse F4/80 (monocytes/macrophages), B220 (B-cells), and CD4/CD8 (T cells). Mostly circulating monocytes and lymphocytes (punctuate staining) are seen in the patent vessels of CRPtg/FcγRI−/−, whereas in the more occluded vessels of CRPtg, these were fewer in number, and there were more cells within the proliferative area (stellate/diffuse staining). Importantly all three cell types were found in both genotypes. Original magnifications, ×20.
Because no evidence was found for variation in complement deposition or the types and numbers of inflammatory cells within the neointima, we reasoned that human CRP interacting with an FcγRI-bearing cell type(s) might promote neointimal hyperplasia in CRPtg and not CRPtg/FcγRI−/−. Using confocal immunofluorescence microscopy we identified a population of F4/80+ cells that also reacted with anti-human CRP antibody, in a pattern that suggested human CRP was membrane-bound (Figure 7, third and fourth panel from top of left column). In stark contrast human CRP did not co-localize with smooth muscle actin-positive cells (Figure 7, right column of panels). As already mentioned, in CRPtg/FcγRI−/− there is little human CRP detected in the neointimal area (Figure 3) despite no apparent diminution of the number of F4/80+ cells (Figure 6): this is consistent with an expected loss of CRP binding to F4/80+ cells in the absence of FcγRI expression.
Figure 7.
Human CRP is detected on monocytes/macrophages in the neointima of ligation-injured carotid arteries. Arteries were harvested from CRPtg mice 28 days after ligation injury, processed, and stained for immunofluorescence-based detection of human CRP, mouse F4/80, and mouse smooth muscle actin. The elastic laminae, which autofluoresce in the green channel, were used as a landmark. Left: Tissue sections were stained for human CRP (green) and mouse F4/80 (red); co-localization of CRP and F4/80 signals appears yellow-orange in the merged image (third panel from top). In the merged image the arrow points to a cell that stains for CRP only, whereas the box surrounds several F4/80-positive cells that also stain positive for human CRP. The area within the box is rotated and magnified and presented at the bottom of the left column. Right: Tissue sections were stained for human CRP (green) and mouse smooth muscle actin (red). Note that there is no evidence of co-localization in the merged image (third panel from the top). In the merged image the box surrounds a CRP-positive stellate cell, which is magnified and reproduced in the bottom panel. Original magnifications, ×20.
Discussion
Many of the biological activities of CRP are probably propagated by binding of FcγRs, and a cleft directly opposite the ligand-binding site on the CRP subunit is believed to accommodate them.30,31,32 FcγRs are a family of receptors of which most mammals express three main types: FcγRI, FcγRII, and FcγRIII. A fourth type, FcγRIV,33,34 was recently identified in mice and other species. FcγRI, FcγRIII, and FcγRIV are each comprised of a ligand-binding α chain and a common γ chain (∼8 kDa) essential to propagate cell signals. FcγRI, FcγRIII, and FcγRIV thus compete for a limiting supply of γ chain. The γ chain has five extracellular amino acids, a membrane-spanning region, and a cytoplasmic tail of 43 residues that encodes an immunoreceptor tyrosine-based activation motif (ITAM).34 Importantly in FcRγ−/− mice, surface expression of the α chains of FcγRI, FcγRIII, and FcγRIV is essentially abolished. In contrast to these activating FcγRs, FcγRIIb is comprised of a single α chain that carries a cytoplasmic tyrosine-based inhibitory motif (ITIM).34 Because of the varied signaling capacity of different FcγRs, in theory at least, CRP binding could potentially promote either an inflammatory state by binding the activating receptors FcγRI, FcγRIII, or FcγRIV, an inhibitory one by binding the inhibitory receptor FcγRIIb, or one of intermediate phenotype by co-ligation of FcγRIIb with either of the three activating receptors. Use of CRPtg mice with selective deletion of FcγRs allowed us to address specifically the role of each of the FcγR subtypes in the acute vascular injury response.
The results of this study reinforce our initial findings12 that human CRP exacerbates the vascular injury response in CRPtg and provide compelling new evidence that this deleterious action depends on CRP binding to the α chain of the activating receptor FcγRI and requires cell signaling by its accessory common γ chain. Thus CRPtg mice lacking FcγRI or the FcR common γ chain exhibit a greatly attenuated neointimal response to carotid artery ligation compared to CRPtg that express these FcRs. In fact, the neointimal response of FcγRI−/− and FcRγ−/− that express human CRP is not significantly different from that of wild-type mice with a complete FcγR repertoire that do not express human CRP. In contrast, the CRP-associated exaggeration of the neointima response was either unaffected or slightly worsened in CRPtg mice lacking FcγRIIb and FcγRIII. The former is consistent with removal of a potentially protective interaction of CRP with the inhibitory FcγRIIb receptor, whereas the latter is consistent with stronger FcγRI-mediated signaling in the absence of FcγRIII because of reduced competition for the common γ chain.
We have previously reported12 that expression of human CRP protein (immunohistochemical analysis) and mRNA (laser microdissection followed by real-time quantitative RT-PCR) can be detected in the neointima of ligation-injured arteries from CRPtg, but not in uninjured arteries or in the media of injured arteries, and that the accompanying blood CRP level is consistently low. In the current study we did not re-evaluate CRP mRNA expression, but we did document CRP protein deposition and we again recorded no appreciable elevation of human CRP blood levels in CRPtg or their FcγR mutant counterparts after OVX and carotid artery ligation; the highest serum CRP concentration recorded here being 2.1 μg/ml. A new finding in the current study was the demonstration that human CRP was virtually absent from the neointima of CRPtg lacking FcγRI, that in CRPtg there was a rapid eightfold increase in expression of FcγRI in the carotid artery after its surgical exposure and manipulation, and that beyond that level there was a threefold increase after ligation injury. The combined results are in concert with the position that the detrimental effect of human CRP in the setting of vascular injury in the CRPtg mouse depends directly on FcγRI expression. Furthermore, our immunofluorescence studies provide convincing indirect evidence that F4/80+ cells (mature monocytes, inflammatory macrophages, dendritic cells) are probably the FcγRI-bearing effector cell type that links CRP to neointimal hyperplasia. Indeed, although the dramatic difference in neointima thickness in the two genotypes made the comparisons difficult, we saw no differences in complement deposition or the inflammatory cell community in the injured arteries that could reasonably account for the different vascular responses of CRPtg versus CRPtg/FcγRI−/−. We thus propose that CRP→ FcγRI interaction is a crucial trigger for neointima thickening and that this event takes place on cells expressing the F4/80 antigen. Consistent with our earlier proposal,12 these new data also suggest that locally expressed CRP could be a crucial contributor to the vascular remodeling process. The same may be true in humans.35,36
Our findings in the ligation injury model are in line with several recent reports made by others. Hernandez-Vargas and colleagues37 reported that apolipoprotein E knockout mice lacking the FcR common γ chain are protected from the development of atherosclerosis. Granted, their model and ours are very different, but the fact that those atherosclerosis-prone mice did not express human CRP does not mean the γ chain effect was CRP-independent because mice do express their own CRP28 and mouse CRP binds mouse FcγRI.16,17,18,19 Our findings are also in alignment with the very recent realization that the affinity of human CRP toward FcγRI is ∼30-fold enhanced in the presence of the γ chain,38 and that CRP up-regulation of monocyte-endothelial adhesion is partly through engagement of FcγRI.39 Although Mineo and colleagues40 showed that CRP→FcγRIIb interaction on endothelial cells mediates inhibition of eNOS, this observation too is not at odds with our findings. Indeed CRP probably binds both FcγRIIb (an inhibitory receptor) and FcγRI (an activating one) in both Mineo and colleagues’40 model (administration of human CRP to acetylcholine-treated mice) and ours (transgene expression of human CRP in mice with carotid artery ligation). In the model of Mineo and colleagues,40 CRP→FcγRIIb is the main effector pathway but importantly, in FcγRIIB−/− mice, acetylcholine-induced increases in vascular conductance were reportedly enhanced by CRP. This suggests that in the absence of inhibitory CRP→FcγRIIb interaction (because FcγRIIb is missing in FcγRIIb−/− mice), a second activating CRP→FcγR pathway is revealed. Mineo’s group40 postulated that CRP→FcγRIII interaction was at work. Likewise in our model of acute injury, although the remodeling process is altered mainly by CRP→FcγRI interaction, we saw that in CRPtg lacking FcγRIIb the neointimal response is further worsened (although this was not statistically significant worsening, see Figure 2). This suggests that CRP binding FcγRI exacerbates neointima formation, but this action is tempered by CRP binding to the inhibitory FcγRIIb. Thus despite the major differences in the two models, there is consensus that CRP interacts with FcγRIIb and at least one activating FcγR to achieve its function in both situations.
Compelling evidence has been presented by others that monocytes and macrophages can transform into vascular elements including endothelial cells, myofibroblasts, and smooth muscle cells.41,42,43,44 Efforts now underway in our laboratory seek to determine whether CRP→FcγRI interaction is a trigger for such transformations. That the exaggerated response to vascular injury is dependent on FcγRI has not yet been demonstrated in humans, but if future studies show that this is the case, then targeting the CRP→FcγRI→neointimal hyperplasia pathway offers a promising new therapeutic strategy for patients suffering from vascular diseases.
Acknowledgments
We thank Michal Mrug, M.D., and Juling Zhou, Ph.D., for their assistance with C3d immunochemistry; and Albert Tousson of the University of Alabama at Birmingham High Resolution Imaging Facility for his help with confocal immunofluorescence.
Footnotes
Address reprint requests to Fadi G. Hage, M.D., 306 Lyons-Harrison Research Building, 1530 3rd Ave. S, Birmingham, AL 35294-0007. E-mail: fadihage@uab.edu.
Supported, in part, by the National Heart, Lung, and Blood Institute (grants RO1 HL75211, T32 HL07457, and R43 HL076929); the American Heart Association (grant in aid 0655258B and postdoctoral fellowship 0425455B); and the Vascular Biology Working Group (cardiovascular medicine fellowship research award).
Editorial note: a guest editor acted as editor-in-chief for this article. No person at the University of Alabama at Birmingham was involved in the peer review process or final disposition for this article.
References
- van Leeuwen MA, van Rijswijk MH. Acute phase proteins in the monitoring of inflammatory disorders. Baillieres Clin Rheumatol. 1994;8:531–552. doi: 10.1016/s0950-3579(05)80114-1. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–493. [PubMed] [Google Scholar]
- Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–2534. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–1441. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- Venugopal SK, Devaraj S, Jialal I. C-reactive protein decreases prostacyclin release from human aortic endothelial cells. Circulation. 2003;108:1676–1678. doi: 10.1161/01.CIR.0000094736.10595.A1. [DOI] [PubMed] [Google Scholar]
- Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, Dhillon B, Mickle DA. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890–1896. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- Scirica BM, Morrow DA. Is C-reactive protein an innocent bystander or proatherogenic culprit? The verdict is still out. Circulation. 2006;113:2128–2151. doi: 10.1161/CIRCULATIONAHA.105.611350. [DOI] [PubMed] [Google Scholar]
- Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44:6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- Danenberg HD, Szalai AJ, Swaminathan RV, Peng L, Chen Z, Seifert P, Fay WP, Simon DI, Edelman ER. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation. 2003;108:512–515. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–655. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- Wang D, Oparil S, Chen YF, McCrory MA, Skibinski GA, Feng W, Szalai AJ. Estrogen treatment abrogates neointima formation in human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:2094–2099. doi: 10.1161/01.ATV.0000179602.85797.3f. [DOI] [PubMed] [Google Scholar]
- Hirschfield GM, Gallimore JR, Kahan MC, Hutchinson WL, Sabin CA, Benson GM, Dhillon AP, Tennent GA, Pepys MB. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 2005;102:8309–8314. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trion A, de Maat MP, Jukema JW, van der Laarse A, Maas MC, Offerman EH, Havekes LM, Szalai AJ, Princen HM, Emeis JJ. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-Leiden/human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:1635–1640. doi: 10.1161/01.ATV.0000171992.36710.1e. [DOI] [PubMed] [Google Scholar]
- Szalai AJ, McCrory MA. Varied biologic functions of C-reactive protein: lessons learned from transgenic mice. Immunol Res. 2002;26:279–287. doi: 10.1385/IR:26:1-3:279. [DOI] [PubMed] [Google Scholar]
- Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell LL, Mold C, Volzer MA, Burlingame RW, Du Clos TW. C-reactive protein binds to Fc gamma RI in transfected COS cells. J Immunol. 1995;155:2185–2193. [PubMed] [Google Scholar]
- Stein MP, Mold C, Du Clos TW. C-reactive protein binding to murine leukocytes requires Fc gamma receptors. J Immunol. 2000;164:1514–1520. doi: 10.4049/jimmunol.164.3.1514. [DOI] [PubMed] [Google Scholar]
- Mold C, Gresham HD, Du Clos TW. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J Immunol. 2001;166:1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- Heuertz RM, Schneider GP, Potempa LA, Webster RO. Native and modified C-reactive protein bind different receptors on human neutrophils. Int J Biochem Cell Biol. 2005;37:320–335. doi: 10.1016/j.biocel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Khreiss T, Jozsef L, Potempa LA, Filep JG. Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. 2004;109:2016–2022. doi: 10.1161/01.CIR.0000125527.41598.68. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Du Clos TW, Jialal I. Binding and internalization of C-reactive protein by Fcgamma receptors on human aortic endothelial cells mediates biological effects. Arterioscler Thromb Vasc Biol. 2005;25:1359–1363. doi: 10.1161/01.ATV.0000168573.10844.ae. [DOI] [PubMed] [Google Scholar]
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, van de Winkel JG, Verbeek JS. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- Murphy C, Beckers J, Ruther U. Regulation of the human C-reactive protein gene in transgenic mice. J Biol Chem. 1995;270:704–708. doi: 10.1074/jbc.270.2.704. [DOI] [PubMed] [Google Scholar]
- Bodmer B, Siboo R. Isolation of mouse C-reactive protein from liver and serum. J Immunol. 1977;118:1086–1089. [PubMed] [Google Scholar]
- Tolbert T, Thompson JA, Bouchard P, Oparil S. Estrogen-induced vasoprotection is independent of inducible nitric oxide synthase expression: evidence from the mouse carotid artery ligation model. Circulation. 2001;104:2740–2745. doi: 10.1161/hc4701.099581. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Shrive AK, Greenhough TJ, Volanakis JE. Topology and structure of the C1q-binding site on C-reactive protein. J Immunol. 2001;166:3998–4004. doi: 10.4049/jimmunol.166.6.3998. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Volanakis JE. Probing the C1q-binding site on human C-reactive protein by site-directed mutagenesis. J Immunol. 1994;152:5404–5410. [PubMed] [Google Scholar]
- Szalai AJ, Agrawal A, Greenhough TJ, Volanakis JE. C-reactive protein: structural biology, gene expression, and host defense function. Immunol Res. 1997;16:127–136. doi: 10.1007/BF02786357. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–45. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcg receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Haider DG, Leuchten N, Schaller G, Gouya G, Kolodjaschna J, Schmetterer L, Kapiotis S, Wolzt M. C-reactive protein is expressed and secreted by peripheral blood mononuclear cells. Clin Exp Immunol. 2006;146:533–539. doi: 10.1111/j.1365-2249.2006.03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Turu MM, Martinez-Gonzalez J, Carvajal A, Juan-Babot JO, Iborra E, Slevin M, Rubio F, Badimon L. Endogenous expression of C-reactive protein is increased in active (ulcerated noncomplicated) human carotid artery plaques. Stroke. 2006;37:1200–1204. doi: 10.1161/01.STR.0000217386.37107.be. [DOI] [PubMed] [Google Scholar]
- Hernández-Vargas P, Ortiz-Munoz G, Lopez-Franco O, Suzuki Y, Gallego-Delgado J, Sanjuan G, Lazaro A, Lopez-Parra V, Ortega L, Egido J, Gomez-Guerrero C. Fcgamma receptor deficiency confers protection against atherosclerosis in apolipoprotein E knockout mice. Circ Res. 2006;99:1188–1196. doi: 10.1161/01.RES.0000250556.07796.6c. [DOI] [PubMed] [Google Scholar]
- Röcker C, Manolov DE, Kuzmenkina EV, Tron K, Slatosch H, Torzewski J, Nienhaus GU. Affinity of C-reactive protein toward Fc{gamma}RI Is strongly enhanced by the {gamma}-chain. Am J Pathol. 2007;170:755–763. doi: 10.2353/ajpath.2007.060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Davis B, Simon SI, Jialal I. CRP promotes monocyte-endothelial cell adhesion via Fcg receptors in human aortic endothelial cells under static and shear flow conditions. Am J Physiol. 2006;291:H1170–H1176. doi: 10.1152/ajpheart.00150.2006. [DOI] [PubMed] [Google Scholar]
- Mineo C, Gormley AK, Yuhanna IS, Osborne-Lawrence S, Gibson LL, Hahner L, Shohet RV, Black S, Salmon JE, Samols D, Karp DR, Thomas GD, Shaul PW. FcγRIIB mediates C-reactive protein inhibition of endothelial NO synthase. Circ Res. 2005;97:1124–1131. doi: 10.1161/01.RES.0000194323.77203.fe. [DOI] [PubMed] [Google Scholar]
- Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38:113–119. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML, Adamkiewicz J, Elsasser HP, Muller R, Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, Shibuya M. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]