Abstract
Globoid cell leukodystrophy, or Krabbe’s disease, is a severe disorder of the central and peripheral nervous system caused by the absence of galactocerebrosidase (GALC) activity. Herein, we describe the clinical, neuropathological, histochemical, and immunohistological features observed in rhesus macaques affected with Krabbe’s disease. Clinical signs included pronounced muscle tremors of head and limbs, difficulty ambulating, ataxia, hypermetria, proprioceptive deficits, and respiratory abnormalities. Histopathologically, all animals presented with evidence of demyelination in the peripheral and central nervous systems and accumulation of mononuclear and multinuclear globoid cells in the cerebral and cerebellar white matter associated with severe gliosis. Using immunohistochemistry and multi-label confocal microscopy, it was determined that globoid cells were CD68+, HAM56+, LN5+, CD163+, IBA-1+, and Glut-5+, suggesting that both peripheral blood-derived monocytes/macrophages and resident parenchymal microglia gave rise to globoid cells. Interestingly, many of the globoid cells and parenchymal microglia with a more ameboid morphology expressed HLA-DR, indicating immune activation. Increased expression of iNOS, TNF-α, and IL-1β were observed in the affected white matter, colocalizing with globoid cells, activated microglia, and astrocytes. Cytokine mRNA levels revealed markedly increased gene expression of CCL2 in the brain of affected macaques. CCL2-expressing cells were detected throughout the affected white matter, colocalizing with GFAP+ cells and astrocytes. Collectively, these data suggest that dysregulation of monocyte/macrophage/microglia and up-regulation of certain cytokines may contribute to the pathogenesis of Krabbe’s disease.
Globoid cell leukodystrophy (GLD; also known as Krabbe’s disease) is a rapidly progressing hereditary autosomal recessive neurological disease.1,2,3,4,5,6,7 GLD is one of a group of approximately 40 diseases known as lysosomal storage diseases (LSDs) that share common clinical and biochemical properties. The LSDs are a direct consequence of mutation(s) in the gene(s) involved in the generation of enzymes responsible for the turnover or transport of normal cellular metabolic components. As a consequence, the substrate of the defective enzyme accumulates within lysosomes of cells that use it in their metabolic processes and disrupts the normal biological pathway(s). Although they are individually rare, LSDs such as Tay-Sachs disease, Krabbe’s disease, various mucopolysaccharidoses, and sphingolipidoses collectively occur in approximately 1 in 5000 live births, with an average life expectancy across the diseases of about 15 years. The incidence of Krabbe’s disease, specifically, in the United States is 1 in 100,000 live births.
GLD is defined by the deficiency of the lysosomal enzyme galactocerebrosidase (GALC). GALC is responsible for lysosomal hydrolysis of several galactolipids, including galactosylceramide, a major sphingolipid of the white matter of the central nervous system, galactosylsphingosine (psychosine), and galactosyldigluceride.4,8,9,10 GALC deficiency results in the accumulation of psychosine, which results in apoptotic death of oligodendrocytes in the central nervous system and Schwann cells of the peripheral nervous system, and a markedly shortened life span in humans and animals with this disease.3,11
The clinical manifestations of each lysosomal storage disease depend on the nature of the storage products and the organs affected. The pathogenesis of GLD is usually severe and rapidly progressive. Infants present with pronounced irritability, hypersensitivity to external stimuli, hypotonicity, blindness, and deafness.12,13,14,15 Human infants affected by Krabbe’s disease show a rapid deterioration in a variety of mental and neuromotor skills. Krabbe-affected infants deteriorate rapidly, and survival beyond two years is uncommon. However, there is phenotypic variability in the age of onset and clinical signs in infants affected with GLD. The histopathological hallmark of this disease is the appearance of globoid cells in the white matter of the central nervous system, located predominantly around blood vessels. Globoid cells are composed of macrophages that have accumulated large amounts of glycolipids in their cytoplasm. In addition to the formation of globoid cells, there is extensive loss of myelin and astrocytosis in the white matter of the central nervous system. In peripheral nerves, axonal degeneration, fibrosis, and macrophage infiltration are often present.16
Several animal models of Krabbe’s disease have been described in species such as the mouse, dog, cat, sheep, and rhesus monkey.4,6,11,17,18,19,20,21 The rhesus monkey model represents the first reported observation of a lysosomal storage disease in any nonhuman primate species. The mutation causing the disease in the rhesus monkey has been identified as a 2-bp deletion at positions 387 and 388 in exon 4 that results in a frameshift and preliminary stop codon.9 Newborn macaques homozygous for the gene defect appear normal at birth but decline rapidly. Clinical signs observed in affected animals include moderate to severe muscle tremors of head and limbs, ataxia, and hypermetria. Many of the affected animals develop pronounced tremors and difficulty ambulating, as well as respiratory difficulties including a double inspiratory effort. All of the affected animals have marked decreases in nerve conduction velocities.22 The survival times of affected animals varies from less than one week to as long as 22 months.
The focus of our ongoing research in the nonhuman primate model is the development of a link between the observed clinical signs of disease and pathological alterations. Of particular interest is the origin and role, if any, of the globoid cells in disease pathogenesis. A number of neurodegenerative diseases, such as Alzheimer’s disease, that were originally thought to have no inflammatory component, have been shown to be associated with activated macrophages/microglia that produce cytokines and chemokines that may play a role in disease pathogenesis.23,24,25,26,27,28,29 Discerning a possible role of brain macrophages/microglia is dependent on an ability to differentiate populations of brain macrophages. Perivascular macrophages and parenchymal microglia are the two brain macrophage populations most commonly involved in response to CNS lesions. Perivascular macrophages include blood-derived monocytes that recently immigrated into the brain from the blood. Parenchymal microglia are the resident macrophages of the brain. They are derived from monocyte/macrophage lineage cells that enter the CNS early in development.30 Perivascular macrophages and parenchymal microglia may be differentiated based on differential expression of myeloid markers in which expression of CD11b is shared, but perivascular macrophages uniquely express CD14 and CD45.31,32,33,34,35,36
In this study a detailed neuropathologic and immunohistologic characterization of the CNS lesions in the context of the neuromotor and behavioral deficits that occur in animals affected with Krabbe’s disease is described. The origin of the globoid cells is defined, and it was demonstrated that activation of microglia/macrophages play a role in the progression of Krabbe’s disease in the nonhuman primate model.
Materials and Methods
Animals and Clinical and Neurological Evaluation
All animals with Krabbe’s disease and the controls were Indian-origin rhesus macaques (Macaca mulatta). Two of the affected macaques were males (EJ72, DH31) and one was female (EA75). Infants were kept with their mothers in appropriate caging equipped with various enrichment devices until disease progression warranted euthanasia. A commercial nonhuman primate biscuit was provided twice daily. Water was available ad libitum. Supplemental fruits and forage were provided throughout the week. The Tulane National Primate Research Center’s (TNPRC) Institutional Animal Care and Use Committee approved all studies and procedures.
Animal weights were obtained, and a veterinarian performed a complete physical examination monthly. Nerve conduction studies and structural magnetic resonance images (MRIs) were collected monthly on all affected animals.22 Motor maturity items on neurobehavioral assessment scales standardized for use with infant rhesus macaques37,38,39 were used to assess affected infants through 10 months of age, provided their health status permitted testing. Scores were compared with scores obtained from 24 unaffected, normal rhesus infants at the TNPRC.
Tissue Collection
Animals affected with Krabbe’s disease were euthanized on the advice of the attending veterinarian once the disease progressed to the point that the animal had difficulty swallowing/eating, significant and consistent weight loss, and/or respiratory difficulties. Macaques were humanely euthanized with an i.v. overdose of pentobarbital. Age-matched tissues from three unaffected macaques of 2, 20, and 27 months of age were examined in parallel.
A complete necropsy was performed, and all tissues were collected immediately after euthanasia. Tissues were fixed in 10% neutral buffered formalin, processed routinely, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Selected neural tissues were also stained with periodic acid-Schiff, Luxol Fast blue/PAS, Alcian Blue (pH 2.5) and Safranin O. Tissues adjacent to those fixed in formalin were embedded in optimal cutting temperature compound (O.C.T.; Miles Inc., Elkhart, IN), snap frozen by immersion in 2-methylbutane in dry ice, and cut into sections 6 to 8 μm thick for immunohistochemistry and confocal microscopy, and at 100 μm for RNA extraction for PCR.
Immunophenotype of Cells in Krabbe-Damaged Brain
To evaluate neural lesions in affected animals and define the immunophenotype of cells involved, we used immunohistochemistry and multilabel confocal microscopy using specific cell markers. Single-label immunohistochemistry for myelin-producing cells (anti-CNPase, IgG1; Sigma, Saint Louis, MO), microglia (anti-IBA 1, polyclonal; Wako Pure Chemical Industries, Richmond, VA), astrocytes (anti-glial fibrillary acidic protein, IgG1; Sigma; and anti-peripherin, IgG2b; Novocastra Laboratories Ltd., Newcastle, UK), and neurons (anti-neuronal nuclei, IgG1, Chemicon, Temecula, CA) was performed as previously described by Borda et al40 Tissue sections were incubated sequentially with the primary, cell-type-specific antibody for 60 minutes (monoclonal) or 30 minutes (polyclonal) at room temperature followed by biotinylated horse anti-mouse or goat anti-rabbit (Vector Laboratories, Burlingame, CA) secondary antibodies, respectively. Finally, sections were incubated with avidin-biotin complex for 30 minutes, and the reaction was visualized with 3,3′-diaminobenzidine (DAB; Dako, Carpinteria, CA) as the chromogen.41 As a negative control, serial sections were processed identically using equivalent concentrations of irrelevant primary antibodies of the same isotype or preimmmune rabbit serum.
To evaluate more rigorously the immunophenotype of cells involved in neural lesions of affected animals, multilabel immunofluorescence and confocal microscopic analyses on formalin-fixed tissues and tissue samples embedded in OCT were performed as described previously by Williams and colleagues.36 Tissues were cut into sections 6 to 8 μm thick and fixed in 4% paraformaldehyde for 20 minutes. The monoclonal and polyclonal antibodies used for this analysis are listed in Table 1.
Table 1.
Antibodies Used in Immunohistochemistry and Immunofluorescence
| Antigen | Cell type | Source | Antibody type* | Dilution working
|
|
|---|---|---|---|---|---|
| IFA | IHC | ||||
| CD68 | Macrophage | Dako | IgG1, kappa | 1:20 | 1:125 |
| HAM56 | Macrophage | Dako | IgM | 1:10 | 1:25 |
| LN5 | Macrophage | Zymed (South San Francisco, CA) | IgM | 1:10 | 1:200 |
| CD163 | Macrophage | Novocastra | IgG1 | 1:50 | 1:100 |
| MAC 387 | Mono/macrophage | Dako | IgG1, kappa | 1:20 | 1:100 |
| MRP8 | Mono/macrophage | Accurate Chemical & Scientific Corp. | IgG1 | 1:20 | |
| 1:100 | |||||
| IBA-1 | Resting microglia | BD PharMingen (San Diego, CA) | IgG1,Kappa | 1:100 | |
| HLA-DR | Activation | Zymed | IgG2b | 1:20 | 1:50 |
| Glut-5 | Microglia | Chemicon | Polyclonal | 1:500 | |
| CD11b | Microglia/macrophage | Novocastra | IgG1 | 1:10 | |
| Neu-N | Neuron | Chemicon | IgG1 | 1:10 | 1:200 |
| GFAP | Astrocyte | Sigma | IgG1, labeled with CY3 | 1:300 | 1:3000 |
| Peripherin | Astrocyte | Novocastra | IgG2b | 1:20 | 1:100 |
| S-100 | Glial, ependymal, Schwann cells | Sigma | Polyclonal | 1:100 | |
| CNPase | Oligodendrocyte | Sigma | IgG1 | ||
| Schwann cells | 1:100 | ||||
| Glut-1 | Endothelial cells | Chemicon | Polyclonal | 1:20 | |
| iNOS/NOS Type II | BD PharMingen | IgG2a | 1:25 | 1:125 | |
| CCL2 (MCP-1) | Abcam | Polyclonal | 1:20 | 1:100 | |
For monoclonal antibodies the isotype is indicated. All polyclonal antibodies were made in rabbit.
For immunohistochemistry, brain sections were washed for 15 minutes in phosphate-buffered saline containing 0.2% fish skin gelatin (PBS-FSG), blocked with Dako protein block, and incubated with the primary antibody for 1 hour at room temperature. Sections were washed for 15 minutes with PBS-FSG incubated with the fluorescently labeled secondary antibody for 30 minutes at room temperature. Secondary antibodies were diluted in Dako antibody diluent at 1:1000 and included anti-mouse or anti-rabbit Alexa 488 (green), Alexa 568 (red), and Alexa 633 (far red) (all from Invitrogen, Carlsbad, CA). After antibody treatment, sections were washed twice for 15 minutes in PBS-FSG. Finally, the sections were rinsed in double distilled water (ddH2O) and mounted on coverslips with aqueous mounting medium. If a second primary antibody and/or a third primary antibody was used (double-label and triple-label) they were applied sequentially after the first primary antibody, and both or all were developed simultaneously. Some sections were stained with Topro-3 (Invitrogen) to delineate the nuclei of cells by incubation for 5 minutes at 1 μg/ml followed by a PBS wash.
iNOS and CCL2 Staining
To identify the inducible nitric oxide synthase (iNOS)- and CCL2-expressing cells, a complete examination of affected and normal control brain was performed using immunohistochemistry and multilabeled confocal microscopy. To define the immunophenotype of CCL2-producing cells, we used a rabbit polyclonal to CCL2 (Abcam Inc., Cambridge, MA) combined with GFAP (anti-glial fibrillary acidic protein, IgG1 conjugated to Cy3; Sigma) and Peripherin (IgG2b, Novocastra Laboratories), both markers for intermediate filaments expressed by astrocytes in the CNS of macaques.42
To identify the iNOS-expressing cells, we used iNOS/NOS type II monoclonal antibody (IgG2a; BD Biosciences, San Jose, CA) combined with the following markers for microglia/macrophage CD11b (IgG1; Novocastra Laboratories) and CD68 (IgG1; Dako Corporation), neurons NeuN (anti-neuronal nuclei, IgG1; Chemicon), astrocytes (anti-glial fibrillary acidic protein, IgG1, conjugated to Cy3; Sigma), and endothelial cells Glut1 (polyclonal; Chemicon).
iNOS Quantification
To evaluate and quantify the presence of iNOS, brain sections of affected animals and matched tissues from unaffected macaques were stained for iNOS using immunohistochemistry (iNOS/NOS type II, IgG2a; BD Biosciences). Sections were incubated with the primary antibody for 1 hour at room temperature, followed by biotinylated anti-mouse secondary antibodies43 for 30 minutes. Finally, sections were incubated with avidin-biotin complex for 30 minutes, and the reaction was visualized with DAB43 as the chromogen. For quantification of iNOS expression, images of ten randomly selected areas of 0.5 mm2 were collected for each slide from each animal; five areas from gray matter and five from white matter. The images were collected using a Leica DM50 microscope with a SPOT Insight digital camera (Diagnostics Instruments Inc., Sterling Heights, MI). The light, exposure time, and magnification were kept constant to allow comparison among the tissues. Image analysis of the tissues was performed by measuring area and intensity of labeling for iNOS in the images with the NIH Image software (version 1.63), as we have described previously.44
Confocal Microscopy
Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Individual optical slices represent 0.2 μm, and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (version 1.63) and Adobe Photoshop (version 7.0; Adobe Systems, San Jose, CA) were used to assign colors to the four channels collected: Alexa 568 is red, Alexa 488 is green, Alexa 633 appears blue, and the unlabeled tissue was visualized by differential interference contrast (DIC) and appears in gray scale. The four channels were collected simultaneously. In some tissues, to differentiate between individual cells, Topro-3 staining was performed to detect the nuclei. Colocalization of antigens was demonstrated by the addition of colors as indicated in the figure legends.
RNA Extraction and Real-Time SYBR Green One-Step RT-PCR
For mRNA quantification we used brain tissue (frontal, parietal, occipital cortices, and brain stem) collected from three Krabbe-affected and five control macaques. The levels of expression of TNF-α (tumor necrosis factor-α), IL-1β (interleukin 1β), iNOS, and CCL2 (monocyte chemotactic protein-1) were evaluated and quantified to assess their role in disease progression using total RNA extracted from ten (100-μm-thick) cryosections from the cerebral cortex embedded in OCT using the RNeasy lipid tissue mini kit (QIAGEN Inc., Valencia, CA). DNA contamination was removed using the TURBO DNA-free Kit (PE Applied Biosystems, Foster City, CA). Gene expression for TNF-α, IL-1β, iNOS, and CCL2 was evaluated by quantitative real-time SYBR Green one-step RT-PCR assay (qRT-PCR). Each qRT-PCR (25 μl) contained the following: 2× Master mix without uracil-N-glycosylase (12.5 μl), reverse transcriptase (0.25 μl), target forward and reverse primer, and total RNA (200 ng) quantitated in a spectrophotometer based on A260/A280 ratios. The PCR amplification was performed in the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) using ∼400 nmol/L concentration of the following primers listed in Table 2. PCR conditions were 50°C for 30 minutes, 95°C for 15 minutes followed by 40 repetitive cycles of 95°C for 15 seconds, 55°C for 30 seconds, 72°C for 30 seconds. As a normalization control for RNA loading, parallel reactions in the same multiwell plate were performed using β-actin mRNA.
Table 2.
Primer Sequence, Concentration, and Product Size Used for qRT-PCR
| Gene | Primer sequence | Product size (bp) | Primer concentration (nmol/L) |
|---|---|---|---|
| TNF-α | For-5′-TACCAGACCAAGGTCAACCTCCTC-3′ | 141 | 500 |
| Rev-5′-GCTGAGTCGATCACCCTTCTCCA-3′ | |||
| IL-1β | For-5′-TGGCATCCAGCTACAAATCTCCCA-3′ | 150 | 500 |
| Rev-5′-AAGGGAATCAAGGTGCTCAGGTCA-3′ | |||
| iNOS | For-5′-AGCCCTTTACTTGACCTCCTACCA −3′ | 137 | 500 |
| Rev-5′-ACGGGTTCCATCTTTCACCCACTT-3′ | |||
| CCL2 (MCP-1) | For-5′-TAGGAAGATCTCAGTGCAGAGGCT-3′ | 145 | 500 |
| Rev-5′-GTCCATGGAATCCTGAACCCACTT-3′ | |||
| span style=’font-family:Symbol’b-Actin | For-5′-AGGCTCTCTTCCAACCTTCCTT-3′ | 108 | 300 |
| Rev-5′-CGTACAGGTCTTTACGGATGTCCA-3′ |
Quantification of gene amplification following RT-PCR was made by determining the threshold cycle (CT) number for SYBR Green fluorescence within the geometric region of the semilog plot generated during PCR. Within this region of the amplification curve, each difference of one cycle is equivalent to a doubling of the amplified product of the PCR. The relative quantification of target gene expression across treatments was evaluated using the comparative CT method. The ΔCT value was determined by subtracting the β-actin CT value for each sample from the target CT value of that sample. Calculation of ΔΔCT involved using the highest sample ΔCT value (ie, sample with the lowest target expression) as an arbitrary constant to subtract from all other ΔCT sample values. Fold changes in the relative gene expression of target was determined by evaluating the expression 2−ΔΔ CT.
Results
Clinical and Neurological Evaluation and Disease Progression
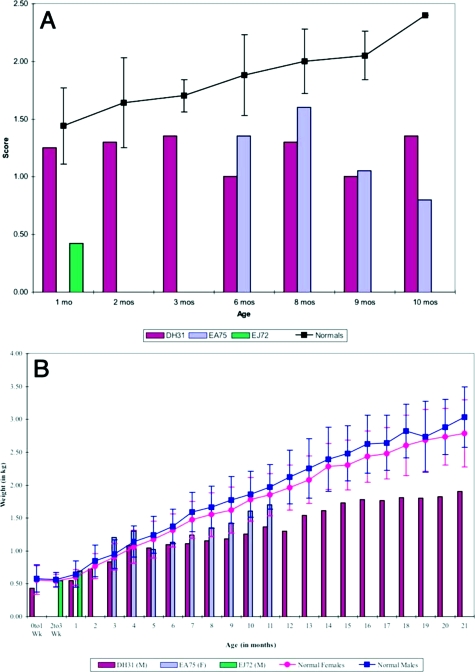
The onset of clinical signs and disease progression in Krabbe-affected rhesus macaques varied considerably despite the fact that all of the animals possess the identical genetic mutation. Clinical signs were first noted in animal EJ72 at 19 days of age. He was unable to cling to his mother with his legs. EJ72 showed the most rapid disease progression, resulting in his death at 52 days of age. Animal DH31 was noted to have very mild tremors with slowed nerve conductivity on EMG studies at 38 days of age. DH31 showed a much slower disease progression, surviving until 642 days of age. At 140 days of age, EA75 was noted to have mild to moderate tremors with difficulty grasping items. She too had a slow progression, surviving until 611 days of age.
EMG analysis of all affected animals revealed a steady decline in nerve conductivity over time.22 However, there were no remarkable findings on structural MRI studies. Affected animals consistently scored below age-matched, unaffected peers on the motor items on Infant Neurobehavioral Assessment and Modified Bayley Scales (Figure 1A). Krabbe-affected monkeys exhibited progressive loss of use of their hind limbs and became dependent on their upper body to ambulate. Hands and feet were often kept clenched. Unlike either normal age-matched animals or the other affected animals, EJ72 never made any age-appropriate vocalizations, and disease progression was very rapid. In addition, he did not exhibit the typical continuous body tremors but, rather, presented with moderate to severe clonic spasms. All affected animals struggled to maintain adequate body weights and fell below those of age-matched control animals throughout their lives (Figure 1B). As the disease progressed, one animal, EA75, was noted to have a left-sided deviation of the nose, suggestive of facial nerve paralysis. Likewise, DH31 presented with a right-sided deviation of the nose, as well as exophthalmia of the left eye shortly before death. It is important to note that the clinical signs and disease progression observed in these animals have been observed in every Krabbe-affected (homozygous for mutated GALC) animal born in our colony.
Figure 1.
Comparison of composite scores for motor maturity and body weight between Krabbe-affected and normal infants. A: Infants identified as affected in the first month after birth received the Infant Neurobehavioral Assessment. Thereafter they were assessed using the Modified Bayley Scales of Infant Development. Motor maturity for each Krabbe-affected subject was plotted (colored bars), and the mean and SD for normal, unaffected infant rhesus macaques (n = 24) from the TNPRC are presented as a line graph with error bars. B: Comparison of the Krabbe-affected subjects’ weights. Weight of Krabbe-affected animals was monitored monthly throughout their lives and compared with that of the mean and SD of normal, unaffected rhesus macaques (n = 24) from the TNPRC.
Gross Pathology
The brain from Krabbe-affected macaque EA75 compared with EJ72 and DH31 was slightly atrophic and firm when sectioned. The gray matter was normal, and the white matter appeared reduced in volume. The ventricles were slightly dilated, and there was no evidence of cerebellar hypoplasia or atrophy. This finding indicates that there are variable patterns of abnormalities in the brain of affected macaques and that morphological changes were more prominent in EA75, one of the slow progressor macaques, compared with EJ72 and DH31. The MRI report of macaque EA75 also indicated white matter changes characterized by increased T2 signal associated with the posterior horns of the lateral ventricles, more prominent on the left side than on the right side.
Histopathology
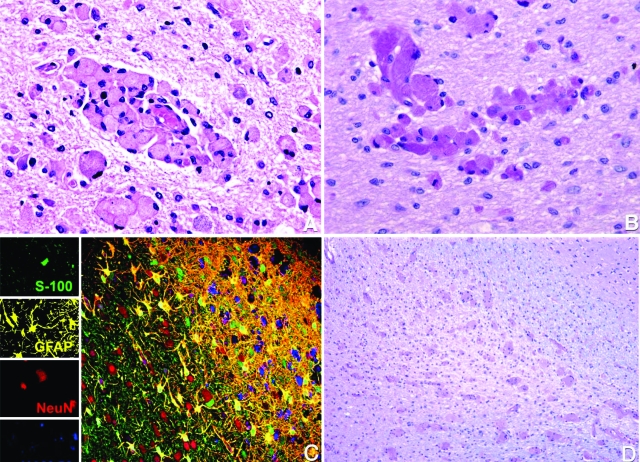
The histological examination of the brain of all affected macaques revealed moderate to severe accumulation of globoid cells in the white matter throughout the CNS, pathognomonic of globoid cell leukodystrophy. The lesions were most severe in the rapidly progressing animal (EJ72) but were identical in character among all animals. The globoid cells were primarily in the white matter of the brain, spinal cord (cervical, thoracic, lumbar, and sacral), and optic nerves and tended to cluster around blood vessels (Figure 2A). Many of these cells were mononuclear, whereas others were multinucleated with fine, granular periodic acid-Schiff-positive cytoplasm (Figure 2B). In addition to an accumulation of globoid cells, the white matter was characterized by gliosis and demyelination (Figure 2, C and D). In contrast to the lesions in the white matter, the gray matter appeared normal in all animals.
Figure 2.
Detection of globoid cells, gliosis, and demyelination in Krabbe-affected brain. A: Histological examination of the brain revealed massive accumulation of globoid cells in the white matter throughout the brain, principally around the microvasculature. Hematoxylin and eosin. Original magnification ×400. B: Globoid cells with fine granular periodic acid-Schiff-positive cytoplasm. Original magnification ×400. C: Multilabel confocal microscopy showing normal gray matter (left side) and extensive gliosis, loss of oligodendrocytes, demyelination, and numerous globoid cells in the white matter (right side). Images for individual channels (neurons with NeuN/Alexa 568, red; astrocytes with GFAP/CY3, yellow; oligodendrocytes with S-100/Alexa 488, green; and globoid cells with HAM56/Alexa 633, blue) are shown on the left with the larger merged image on the right. D: Extensive demyelination of the white matter. Luxol Fast blue. Original magnification ×100.
Immunophenotypic Characterization of Krabbe Brain
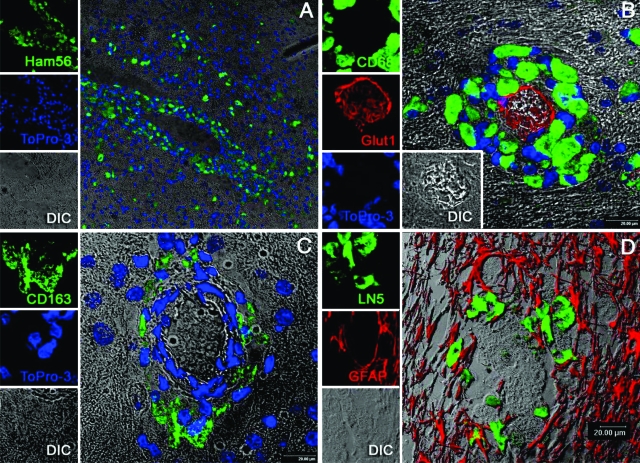
To define the cell types involved in the neuropathology of Krabbe’s disease, multilabel confocal microscopy was performed using multiple cell-type specific markers for monocyte/macrophages, parenchymal microglia, neurons, endothelial cells, astrocytes, and oligodendrocytes (Table 1). The globoid cells around the microvasculature of the white matter demonstrated uniform immunoreactivity for multiple monocyte/macrophage markers: HAM56, CD68, CD163, and LN5 (Figure 3, A–D). The globoid cells also showed strong expression for IBA-1 and weak expression of Glut-5 (Figure 4, A and B), two antigens thought to be primarily on parenchymal microglia rather than blood-derived monocyte/macrophages.45,46 CD163+ and LN5+ cells were also observed in perivascular, choroid plexus and meningeal spaces, representing resident macrophages of these specific compartments. IBA-1 and CD11b were also expressed by ramified and ameboid microglia (Figure 4, C and D). The ameboid microglia and globoid cells were also found to express both CD68 and HLA-DR, indicating that they were activated (Figure 5, A and B). MAC 387- and MRP8-positive cells, although rare, were identified in the lumen of capillaries in both white matter and gray matter, but no such cells were observed in the brain parenchyma (data not shown).
Figure 3.
Immunophenotype of globoid cells in the rhesus CNS. Multilabel confocal microscopy showing the immunoreactivity of globoid cells with multiple monocyte/macrophage markers. Images for individual channels are shown on the left with the larger merged image on the right. A: HAM56 labeled with Alexa 488 (green) and nuclear staining with Topro-3. B: CD68 labeled with Alexa 488 (green); Glut-1 labeled (endothelial cells) with Alexa 568 (red) plus nuclear staining with Topro-3. Note that nuclei of the globoid cells are eccentrically located. C: CD163 with Alexa 488 (green); nuclear staining with Topro-3 and DIC. D: LN5 with Alexa 488 (green); GFAP-labeled (astrocytes) with CY3 (shown in red) and DIC.
Figure 4.
Immunophenotype of globoid cells in the rhesus CNS: Iba-1, Glut-5, and CD11b. Strong expression of Iba-1 (A) and weak expression of Glut-5 (B) were observed in globoid cells. Original magnification ×200. C: Intense expression of IBA-1 in quiescent and reactive microglia. Original magnification ×200. D: CD11b expression was observed in quiescent microglia. Original magnification ×200.
Figure 5.
Expression of HLA-DR by microglia, macrophages, and globoid cells in Krabbe-affected brain. A: Triple-label confocal microscopy showing reactive microglia. Images for individual channels are shown on the left with a larger merged image on the right. Brain sections were stained for HLA-DR with Alexa 488 (green); CD11b with Alexa 568 (red) plus nuclear staining with Topro-3. B: Detection of HLA-DR+ globoid cells. Sections were stained for HLA-DR with Alexa 488 (green); CD68 with Alexa 568 (red) plus nuclear staining with Topro-3. Colocalization of both markers is observed as yellow.
The presence of ameboid microglia and expression of HLA-DR by parenchymal microglia, macrophages, and globoid cells at levels easily discernible by routine immmunohistochemistry was strongly suggestive of immune activation. Therefore, we examined iNOS expression using a specific antibody to iNOS/NOS type II. Although there are several different types of nitric oxide synthase, expression of the inducible form (iNOS) occurs after cells are activated by inflammatory cytokines such as TNF-α, IFN-γ, and IL-1.47,48 In the Krabbe-affected animals, immunohistochemical detection of iNOS revealed intense expression in affected areas (white matter) but not in normal brain areas (gray matter). The iNOS-expressing cells were morphologically compatible with globoid cells, macrophages, microglia, and astrocytes. Double-label immunofluorescence demonstrated that iNOS protein colocalized with CD11b+, CD68+, and GFAP+ cells (Figure 6A–C). No colocalization was observed with NeuN+ and Glut1+ cells (neuron and endothelial cell markers, respectively).
Figure 6.
Expression of iNOS by microglia, globoid cells, and astrocytes in Krabbe-affected brain. A: Intense expression of iNOS in activated microglia. iNOS with Alexa 488 (green); CD11b with Alexa 568 (red); colocalization of both markers in yellow is observed in microglia. B: Expression of iNOS in globoid cells; iNOS with Alexa 488 (green); CD68 with Alexa 568 (red); colocalization of both markers in yellow is observed in globoid cells. C: Expression of iNOS in astrocytes; iNOS with Alexa 488 (green); GFAP conjugated with Cy3 (red); colocalization of both markers in yellow is observed in astrocytes. D: Quantification of the expression of iNOS. The levels of expression of iNOS in affected rhesus macaques (red) and age-matched control animals. iNOS was detected by immunohistochemistry and quantified by densitometry (integrated density). Bars represent averages of an 0.5-mm2 area of gray (2m_G = 2 months gray matter; 20m_G = 20 months gray matter; 21m_G = 21 months gray matter; 27m_G = 27 months gray matter) and white matter (2m_W = 2 months white matter; 20m_W = 20 months white matter; 21m_W = 21 months white matter; 27m_W = 27 months white matter) per slide from three affected macaques and three age-matched controls plus one extra control.
The quantification of iNOS expression using image analysis revealed that the Krabbe-affected macaque brains contained as much as 60 times more (fold range 2–60) iNOS than did the age-matched normal counterpart. The integrated density (product of the area of positive iNOS stain multiplied by the intensity minus background) was used in all comparisons. The iNOS expression in affected macaques was detected mainly in the white matter and was elevated, on average, 7.5-fold compared with levels in gray matter. In addition, the expression levels of iNOS were determined to be 2.5 times higher in the white matter of the rapidly progressing animal compared with the iNOS production in the white matter of the two slower-progressing animals (Figure 6D).
Multiple reports indicated that chemokines such as CCL2 (MCP-1), CCL3 (MIP1α), CCL4 (MIP1β), and CCL5 (RANTES) are involved in the regulation of transendothelial migration of monocytes into the brain during inflammatory diseases such as HIV encephalitis and multiple sclerosis, a chronic demyelinating disease.49,50,51 In this study immunohistochemical staining for CCL2 was detected in the affected white matter throughout the demyelinating area, in cells morphologically consistent with astrocytes. The area of the lesion also contained a large number of globoid cells, macrophages, and astrocytes. Double-labeled immunofluorescence using CCL2 and GFAP confirmed the astrocytic phenotype of some of these cells, but many others with the same morphology were negative for GFAP (Figure 7A). To define the immunophenotype of the CCL2+ GFAP− cells, we used antibodies to CCL2 combined with antibodies to peripherin. Peripherin is a type III intermediate filament that is inversely expressed with GFAP in astrocytes and has previously been demonstrated to be expressed by astrocytes in macaques in areas of inflammation.42 This process confirmed that most of the CCL2+ cells were peripherin-positive astrocytes (Figure 7B). No CCL2+ cells were observed in the white matter of normal brain.
Figure 7.
Expression of CCL2 by astrocytes in Krabbe-affected brain. A: Double-label confocal microscopy using CCL2 and GFAP showing scattered CCL2-expressing cells throughout the demyelinating area in the affected white matter (left). A minority of the CCL2+ cells are also GFAP+ and appear yellow. A higher magnification image is shown on the right, demonstrating colocalization (yellow) in one of several GFAP+ cells in the field. Images for individual channels are shown in the center of panel A CCL2 with Alexa 488 (green); GFAP conjugated with Cy3 (red); and DIC. B: Multiple CCL2-expressing cells also express the astrocyte marker peripherin and appear yellow (left). A higher magnification image is shown on the right, demonstrating clear colocalization (yellow) between CCL2 and peripherin in several cells. Images for individual channels are shown in the center of panel B. CCL2 with Alexa 488 (green); peripherin with alexa 488 (red); and DIC. C: CCL2 gene expression in the brain of affected rhesus macaques. A statistically significant (P < 0.0001) increase (∼221-fold) in CCL2 gene expression was observed in the brain of affected macaques compared with control macaques.
Gene Expression in the Brain of Krabbe-Affected Macaques
To explore further a possible relationship between immune activation and neuropathology associated with Krabbe’s disease, gene expression levels of selected cytokines and chemokines generated by macrophages including TNF-α, IL-1β, iNOS, and CCL2 were examined using qRT-PCR. Individual fold changes in gene expression, calculated as described in Materials and Methods for TNF-α, IL-1β, iNOS, and CCL2, are shown in Table 3. Figure 8 shows averaged group-wise fold differences for TNF-α, IL-1β, iNOS, and CCL2, in the frontal, parietal, and occipital cortices and brain stem of affected and control macaques.
Table 3.
Individual Fold Differences in TNF-α, IL-1β, iNOS, and CCL2 Gene Expression
| Group | Animal Number | TNF-α | IL-1β | iNOS | CCL2 |
|---|---|---|---|---|---|
| Krabbe macaques | DH31 | 17 | 16 | 6 | 313 |
| V539 | 4 | 14 | 2 | 213 | |
| EA75 | 16 | 7 | 21 | 119 | |
| Control macaques | J491 | 12 | 13 | 1 | 18 |
| DE20 | 9 | 14 | 6 | 17 | |
| BH51 | 3 | 1.4 | 9 | 2 | |
| BI79 | 5 | 2 | 1.2 | 5 | |
| FC72 | 1 | 1 | 3 | 1 | |
| Statistical significance | Krabbe vs controls | P < 0.05 | P < 0.05 | NSD | P < 0.05 |
NSD, no significant difference.
Figure 8.
Gene expression of TNF-α, IL-1β, and iNOS in Krabbe-affected and normal brain. Relative abundance in gene expression for TNF-α, IL-1β, and iNOS detected using quantitative real-time SYBR Green one-step RT-PCR detected in the brain of affected and control macaques. The fold differences in gene expression were calculated as described in Materials and Methods. The relative fold increases for each target gene were summed and averaged to obtain a single figure that is shown on top of each bar. The error bars represent average fold difference for each group ± SEM. Both TNF-α and IL-1β were found to be statistically different between the groups (P < 0.05, indicated by asterisk). The blue, red, and yellow bars represent TNF-α, IL-1β, and iNOS, respectively.
In affected macaques, a statistically significant (P < 0.0001) increase (∼221-fold) in CCL2 gene expression was observed in the brain of Krabbe-affected macaques compared with control macaques (Figure 7C). In addition, the gene expression of TNF-α and IL-1β in the brain of Krabbe-affected animals differed significantly from that of control animals (P < 0.0038). Although iNOS levels were increased in the Krabbe-affected animals, the observed difference was not statistically significant from that in control macaques (Table 3).
Discussion
The TNPRC has previously documented a genetic galactocerebrosidase (GALC) deficiency in a colony of rhesus macaques, an animal model that is essentially genetically equivalent to the human disease.9 The rhesus macaque model of GLD has a high degree of clinical similarity to the human disease; however, a complete characterization is necessary to address unanswered questions regarding disease pathogenesis and to determine whether there are any species-specific differences in the characteristics of lysosomal hydrolases, their distribution in tissues, the nature of stored metabolites, and the organs affected.4,9,52
GALC is a lysosomal hydrolase that normally degrades galactolipids involved in myelin production. Therefore, in the absence of functional GALC, there is an accumulation of galactosphingolipids including psychosine, which is highly toxic to oligodendrocytes.
In other species with GALC deficiency, such as the human, dog, cat, mouse, and sheep, psychosine has been shown to accumulate during active myelination, resulting in toxicity and death of oligodendrocytes and scarcity of myelin.
In these Krabbe-affected macaques the clinical manifestations of disease were characterized by continuous body tremors, spasticity, hypermetria, proprioceptive deficits, and weakness with progressive demyelination of the PNS and neurodegeneration of the CNS. Affected peripheral nerves were abnormally thick, showing histological evidence of a severe demyelinating polyneuropathy characterized by an absence of myelin, demonstrated by Luxol Fast blue, and anti-myelin/oligodendrocyte-specific protein and reduced nerve conductivity previously reported by Weimer.22
In the CNS of most species affected with Krabbe’s disease, including macaques, the histological hallmark is the accumulation of globoid cells. In macaques the accumulation of globoid cells is very prominent around the microvasculature and is associated with demyelination, severe gliosis (confirmed by overexpression of GFAP), and, as shown here, the presence of activated macrophages and microglia along with overexpression of iNOS and CCL2.
The cellular origin of the globoid cells is open to debate. Some authors support a hematogenous origin, describing the presence of perivascular mononuclear cells that are CD68-ferritin- and periodic acid-Shiff (PAS)positive, containing undigested galactosylceramide.8,53 Other investigators suggest that the multinucleated globoid cells are derived from perivascular microglia.54 Perivascular macrophages have relatively short half-lives in the CNS and, as such, are continuously repopulated by blood-derived monocyte/macrophages. Parenchymal microglia are the resident macrophages of the brain. They are derived from monocyte/macrophage lineage cells that enter the CNS early in development and are replaced from the periphery very slowly if ever.30
In this study, globoid cells around the microvasculature of the white matter were positive for both monocyte/macrophage markers (CD68, HAM56, LN5, CD163) and microglial markers (IBA-1 and Glut-5) suggesting that the origin of globoid cells is from both perivascular macrophages and resident microglia.
The data also indicate that CD11b+ cells (microglia and macrophages) show strong expression of HLA-DR in affected areas, suggesting that the macrophages and microglia are activated in Krabbe-affected brain. This finding suggests a role for activated macrophages/microglia in the pathogenesis of globoid cell leukodystrophy. It is possible that this activation contributes to, or exacerbates, myelin pathology and perhaps neuronal loss, as has been reported in other CNS diseases (eg, AIDS).55,56,57 Microglia/macrophages may be activated after myelin breakdown, perhaps due to the role of macrophages/microglia in the clearance of myelin breakdown products.58,59
TNF-α, IFN-γ, and IL-1β have been demonstrated to induce iNOS expression, and CCL2 is a potent chemokine for recruitment of monocytes.47,48,49,60 TNF-α, IFN-γ, and IL-1β can also facilitate the recruitment of leukocytes. The culture of rat astrocytes with psychosine resulted in the production of the proinflammatory cytokines IL-1β, TNF-α, and IL-6.10 In the twitcher mouse model of Krabbe’s disease, TNF-α, IL-6, and the chemokines have been shown to be expressed at high levels and may indicate the involvement of an inflammatory process in disease pathogenesis.61,62
Multiple reports also suggest that macrophages/microglia may contribute to white matter injury by releasing toxic molecules such as TNF-α, complement, and nitric oxide,63,64,65,66,67,68 which are known oligodendrocyte toxins. Normally, iNOS is undetectable in brain tissue; in contrast, high levels of iNOS have been observed in the CNS of animals under experimental pathological conditions, such as experimental hepatic encephalopathy (HE) or experimental meningitis in rat.69,70,71 Immunohistochemical analysis of brain tissue of human Krabbe patients established that iNOS-expressing cells in CNS were astrocytes.72 However, data from the studies presented in this article indicate that the iNOS-producing cells were CD11b+, CD68+, and GFAP+ (microglia, macrophages, globoid cells, and astrocytes). iNOS is a potent vasoactive molecule and, similar to other neurodegenerative disease processes, seems to be a major instigator of disease progression.60 Moreover, a strong association between the massive accumulation of globoid cells, increased expression of HLA-DR and iNOS, and the up-regulation of CCL2 in brain tissue of Krabbe-affected macaques was detected in these studies. The expression of CCL2 was exclusively observed in astrocytes expressing GFAP or peripherin but predominantly observed in peripherin-positive astrocytes. Previous work has demonstrated that peripherin expression is not observed in the brains of normal macaques but is inversely expressed with GFAP in astrocytes in close proximity to inflammatory lesions.42 The expression of CCL2 predominantly by peripherin-positive astrocytes provides further support to the notion that the pathogenesis of Krabbe’s disease includes an inflammatory component. The production of CCL2 (as well as other possible chemotactic stimuli) from astrocytes may be responsible for the recruitment and accumulation of monocytes/macrophages in the affected brain contributing to the disease pathogenesis and variable disease progression observed in rhesus macaques, despite all affected animals having the identical defined genetic defect in GALC.
The findings of severe peripheral neuropathy and progressive neurodegeneration in conjunction with the pathological and immunohistological findings (demyelination, gliosis, cellular immune activation, and high expression of iNOS) in the Krabbe-affected macaques, all of which are similar to those found in CNS and PNS of affected humans, suggest that this primate model serves as an invaluable tool for studies of this rare human genetic disorder.
Unlike the twitcher mouse model, Krabbe-affected rhesus macaques showed increased expression of the cytokines TNF-α and IL-1β and the chemokine CCL2 (Table 3, and Figures 7C and 8). No statistically significant increases in gene expression were observed for iNOS. The discordance between gene expression and immunostaining observed for iNOS can be explained based on the high protein expression of iNOS observed in the rapidly progressing macaque (EJ72). However, this animal was not used for iNOS gene expression studies because of the lack of brain tissue preserved in a manner compatible for RNA extraction. It is important to note that results found with the limited numbers of end-stage Krabbe-affected animals analyzed in this study may not be representative of the results that would be obtained from performing a longitudinal study of a large number of animals. It is plausible to conclude that the levels of the inflammatory cytokines and chemokines may decline as the animal progresses into late-stage Krabbe’s disease. Thus, the data presented here represent only a snapshot of cytokine levels observed at a single stage of disease progression in the macaques.
The value of the rhesus macaque model of Krabbe’s disease lies in the fact that it has been shown from genetic, morphological, physiological, hematopoietic, immunological, and developmental perspectives that monkeys are most similar, when compared, to humans and are, therefore, an essential and appropriate animal model of human development and disease. These similarities include spatial and temporal pattern of organ development, placental structure, length of gestation, growth characteristics, and hematopoietic and immune system ontogeny. In addition, the CNS of non-human primates, in which therapeutic interventions for Krabbe’s disease would be tested, has proven to be an ideal model for neurological studies in humans. In terms of the development of disease pathology, the timing of myelination during development is very similar between rhesus macaques and humans. Large animal models such as the dog or monkey permit insight into the pathophysiology of disease because of their ability to be analyzed using magnetic resonance imaging (MRI). Similarities have been found between the MRI T-1 and T-2 values of the human and non-human primate, specifically macaque, brain.73,74 As such, we can study the pathophysiology of Krabbe’s disease from onset in the fetus through the establishment of severe disease. In the characterization of both the dog and monkey models of Krabbe’s disease, MRI has been a central tool for analysis.4,6 Moreover, the high degree of similarity between the human and macaque CNS may make MRI analysis more relevant in the rhesus model of Krabbe’s disease.
As the numbers of animals in the colony increase, a more complete analysis of the pathogenesis and the role of immune activation in progression of Krabbe’s disease in the rhesus macaque model will be performed. The rhesus model will also provide the opportunity to assess the role of accessory genes (eg, CCL2 or iNOS) in the variable disease progression of disease despite the identical genetic defect in all animals.
Acknowledgments
We thank Dorothy Kuebler for excellent technical assistance with immunohistochemical methods and Robin Rodriguez for assistance with the images.
Footnotes
Address reprint requests to Juan T. Borda, DVM, PhD, Division of Comparative Pathology, Tulane National Primate Research Center, 18703 Three Rivers Road, Covington, LA 70433. E-mail: jborda@tulane.edu.
Supported in part by Public Health Service Grants RR00164, RR016930, RR022826, and RR022826.
References
- Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat. 1997;10:268–279. doi: 10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status. Neurochem Res. 1998;23:251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Globoid cell leukodystrophy (Krabbe disease): update. J Child Neurol. 2003;18:595–603. doi: 10.1177/08830738030180090201. [DOI] [PubMed] [Google Scholar]
- Wenger DA. Murine, canine and non-human primate models of Krabbe disease. Mol Med Today. 2000;6:449–451. doi: 10.1016/s1357-4310(00)01800-1. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Sattler M, Hiatt W. Globoid cell leukodystrophy: deficiency of lactosyl ceramide beta-galactosidase, Proc Natl Acad Sci USA. 1974;71:854–857. doi: 10.1073/pnas.71.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger DA, Victoria T, Rafi MA, Luzi P, Vanier MT, Vite C, Patterson DF, Haskins MH. Globoid cell leukodystrophy in Cairn and West Highland white terriers. J Hered. 1999;90:138–142. doi: 10.1093/jhered/90.1.138. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Suzuki K, Suzuki Y, Suzuki K. Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. New York: McGraw-Hill; Galactosylceramide lipidosis: globoid cell leukodystrophy (Krabbe disease). The Metabolic and Molecular Bases of Inherited Disease. 2001:pp 3669–3687. [Google Scholar]
- Jesionek-Kupnicka D, Majchrowska A, Krawczyk J, Wendorff J, Barcikowska M, Lukaszek S, Liberski PP. Krabbe disease: an ultrastructural study of globoid cells and reactive astrocytes at the brain and optic nerves. Folia Neuropathol. 1997;35:155–162. [PubMed] [Google Scholar]
- Luzi P, Rafi MA, Victoria T, Baskin GB, Wenger DA. Characterization of the rhesus monkey galactocerebrosidase (GALC) cDNA and gene and identification of the mutation causing globoid cell leukodystrophy (Krabbe disease) in this primate. Genomics. 1997;42:319–324. doi: 10.1006/geno.1997.4744. [DOI] [PubMed] [Google Scholar]
- Giri S, Jatana M, Rattan R, Won JS, Singh I, Singh AK. Galactosylsphingosine (psychosine)-induced expression of cytokine-mediated inducible nitric oxide synthases via AP-1 and C/EBP: implications for Krabbe disease. FASEB J. 2002;16:661–672. doi: 10.1096/fj.01-0798com. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Basaraba RJ, Mazzaferro EM, Gould DH. Globoid cell-like leukodystrophy in a domestic longhaired cat. Vet Pathol. 2002;39:494–496. doi: 10.1354/vp.39-4-494. [DOI] [PubMed] [Google Scholar]
- D’Agostino AN, Sayre GP, Hayles AB. Krabbe’s disease. Globoid cell type of leukodystrophy, Arch Neurol. 1963;8:82–96. doi: 10.1001/archneur.1963.00460010098012. [DOI] [PubMed] [Google Scholar]
- Gullotta F, Pavone L, Mollica F, Grasso S, Valenti C. Krabbe’s disease with unusual clinical and morphological features. Neuropadiatrie. 1979;10:395–400. doi: 10.1055/s-0028-1085341. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Sourander P, Svennerholm L. Diagnosis of Krabbe’s infantile leucodystrophy. J Neurol Neurosurg Psychiatry. 1963;26:195–198. doi: 10.1136/jnnp.26.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Suzuki K. The twitcher mouse. A model of human globoid cell leukodystrophy (Krabbe’s disease). Am J Pathol. 1983;111:394–397. [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Suzuki Y. Galactosylceramide lipidosis: Globoid cell leukodystrophy (Krabbe disease). Stanbury JW, Wyngaarden JB, Fredrickson DS, Goldstein JI, Brown MS, editors. New York: McGraw Hill,; The Metabolic Basis of Inherited Disease. 1983:pp 857–880. [Google Scholar]
- Suzuki K. Genetic galactosylceramidase deficiency (globoid cell leukodystrophy). Krabbe disease in different mammalian species. Neurochem Pathol. 1985;3:53–68. doi: 10.1007/BF02834075. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Chiu FC, Katayama M, Sacchi RS, Suzuki K. Expression of glial fibrillary acidic protein in the CNS and PNS of murine globoid cell leukodystrophy, the twitcher. Am J Pathol. 1986;125:227–243. [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. The twitcher mouse: a model for Krabbe disease and for experimental therapies. Brain Pathol. 1995;5:249–258. doi: 10.1111/j.1750-3639.1995.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Baskin GB, Ratterree M, Davison BB, Falkenstein KP, Clarke MR, England JD, Vanier MT, Luzi P, Rafi MA, Wenger DA. Genetic galactocerebrosidase deficiency (globoid cell leukodystrophy, Krabbe disease) in rhesus monkeys (Macaca mulatta). Lab Anim Sci. 1998;48:476–482. [PubMed] [Google Scholar]
- Victoria T, Rafi MA, Wenger DA. Cloning of the canine GALC cDNA and identification of the mutation causing globoid cell leukodystrophy in West Highland White and Cairn terriers. Genomics. 1996;33:457–462. doi: 10.1006/geno.1996.0220. [DOI] [PubMed] [Google Scholar]
- Weimer MB, Gutierrez A, Baskin GB, Borda JT, Veazey RS, Myers L, Phillippi-Falkenstein KM, Bunnell BA, Ratterree MS, England JD. Serial electrophysiologic studies in rhesus monkeys with Krabbe disease. Muscle Nerve. 2005;32:185–190. doi: 10.1002/mus.20350. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain, Alzheimer Dis Assoc Disord. 2000;14 Suppl 1:S47–S53. doi: 10.1097/00002093-200000001-00008. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Harrison JK. Chemokines and Alzheimer’s disease. Neurobiol Aging. 2001;22:909–913. doi: 10.1016/s0197-4580(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Liddle AC. Microglial signalling cascades in neurodegenerative disease. Prog Brain Res. 2001;132:555–565. doi: 10.1016/S0079-6123(01)32103-9. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Williams K, Bar-Or A, Ulvestad E, Olivier A, Antel JP, Yong VW. Biology of adult human microglia in culture: comparisons with peripheral blood monocytes and astrocytes. J Neuropathol Exp Neurol. 1992;51:538–549. doi: 10.1097/00005072-199209000-00009. [DOI] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Mork S, Antel J, Nyland H. Phenotypic differences between human monocytes/macrophages and microglial cells studied in situ and in vitro. J Neuropathol Exp Neurol. 1994;53:492–501. doi: 10.1097/00005072-199409000-00008. [DOI] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Bjerkvig R, Tiekotter K, Antel J, Matre R. Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukoc Biol. 1994;56:732–740. doi: 10.1002/jlb.56.6.732. [DOI] [PubMed] [Google Scholar]
- Becher B, Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18:1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system, Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux M, DeGregorio G, Schneider ML, Suomi SJ. Inanimate environmental enrichment for group-housed rhesus macaque infants. Am J Primatol. 1990;22:61–67. doi: 10.1002/ajp.1350220104. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Suomi SJ. The effect of mild stress during pregnancy on birthweight and neuromotor maturation in rhesus monkey infants (M. mulatta). Infant Behav Dev. 1992;15:389–403. [Google Scholar]
- Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behav Dev. 1992;15:155–177. [Google Scholar]
- Borda JT, Alvarez X, Kondova I, Aye P, Simon MA, Desrosiers RC, Lackner AA. Cell tropism of simian immunodeficiency virus in culture is not predictive of in vivo tropism or pathogenesis. Am J Pathol. 2004;165:2111–2122. doi: 10.1016/S0002-9440(10)63261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock AM, Standing D, Bullshields K, Schwartz E, Paden CM, Poulsen DJ. In vivo inhibition of hippocampal Ca2+/calmodulin-dependent protein kinase II by RNA interference. Mol Ther. 2005;11:899–905. doi: 10.1016/j.ymthe.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Mathew JS, Westmoreland S, Alvarez X, Simon MA, Pauley DR, MacKey JJ, Lackner AA. Expression of peripherin in the brain of macaques (Macaca mulatta and M. fascicularis) occurs in astrocytes rather than neurons and is associated with encephalitis. Neuropathol Appl Neurobiol. 2001;27:434–443. doi: 10.1046/j.1365-2990.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- Williams K, Schwartz A, Corey S, Orandle M, Kennedy W, Thompson B, Alvarez X, Brown C, Gartner S, Lackner A. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, Sasaki A, Taguchi N, Maeda M, Tsukagoshi H, Sato K, Yamaguchi H. Human GLUT5 immunolabeling is useful for evaluating microglial status in neuropathological study using paraffin sections. Acta Neuropathol (Berl) 2003;105:157–162. doi: 10.1007/s00401-002-0627-4. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, Grisham MB. Role of nitric oxide in inflammation. Acta Physiol Scand. 2001;173:113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroy J, Warren CD, Raghavan SS, Kolodny EH. Animal models for lysosomal storage diseases: their past and future contribution. Hum Pathol. 1989;20:823–826. doi: 10.1016/0046-8177(89)90091-9. [DOI] [PubMed] [Google Scholar]
- Itoh M, Hayashi M, Fujioka Y, Nagashima K, Morimatsu Y, Matsuyama H. Immunohistological study of globoid cell leukodystrophy. Brain Dev. 2002;24:284–290. doi: 10.1016/s0387-7604(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Nguyen T, O’Dowd BF, Lynch KR. Identification of a molecular target of psychosine and its role in globoid cell formation. J Cell Biol. 2001;153:429–434. doi: 10.1083/jcb.153.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle EA, McGeer PL. Cellular immune response in multiple sclerosis plaques. Am J Pathol. 1990;137:575–584. [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Vedeler C, Antel J, Nyland H, Mork S, Matre R. Reactive microglia in multiple sclerosis lesions have an increased expression of receptors for the Fc part of IgG. J Neurol Sci. 1994;121:125–131. doi: 10.1016/0022-510x(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Williams K, Ulvestad E, Waage A, Antel JP, McLaurin J. Activation of adult human derived microglia by myelin phagocytosis in vitro. J Neurosci Res. 1994;38:433–443. doi: 10.1002/jnr.490380409. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol Aging. 1999;20:395–405. doi: 10.1016/s0197-4580(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine SM, Brown DC. IL-6 and TNFalpha expression in brains of twitcher, quaking and normal mice. J Neuroimmunol. 1997;73:47–56. doi: 10.1016/s0165-5728(96)00166-x. [DOI] [PubMed] [Google Scholar]
- Pedchenko TV, LeVine SM. IL-6 deficiency causes enhanced pathology in Twitcher (globoid cell leukodystrophy) mice. Exp Neurol. 1999;158:459–468. doi: 10.1006/exnr.1999.7125. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merrill JE. Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience. 1994;61:575–585. doi: 10.1016/0306-4522(94)90435-9. [DOI] [PubMed] [Google Scholar]
- Mitrovic B, Ignarro LJ, Vinters HV, Akers MA, Schmid I, Uittenbogaart C, Merrill JE. Nitric oxide induces necrotic but not apoptotic cell death in oligodendrocytes. Neuroscience. 1995;65:531–539. doi: 10.1016/0306-4522(94)00491-m. [DOI] [PubMed] [Google Scholar]
- Hisahara S, Shoji S, Okano H, Miura M. ICE/CED-3 family executes oligodendrocyte apoptosis by tumor necrosis factor. J Neurochem. 1997;69:10–20. doi: 10.1046/j.1471-4159.1997.69010010.x. [DOI] [PubMed] [Google Scholar]
- Louis JC, Magal E, Takayama S, Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Wing MG, Zajicek J, Seilly DJ, Compston DA, Lachmann PJ. Oligodendrocytes lack glycolipid anchored proteins which protect them against complement lysis. Restoration of resistance to lysis by incorporation of CD59, Immunology. 1992;76:140–145. [PMC free article] [PubMed] [Google Scholar]
- Suarez I, Bodega G, Rubio M, Felipo V, Fernandez B. Neuronal and inducible nitric oxide synthase expression in the rat cerebellum following portacaval anastomosis. Brain Res. 2005;1047:205–213. doi: 10.1016/j.brainres.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Suarez I, Bodega G, Arilla E, Felipo V, Fernandez B. The expression of nNOS, iNOS and nitrotyrosine is increased in the rat cerebral cortex in experimental hepatic encephalopathy. Neuropathol Appl Neurobiol. 2006;32:594–604. doi: 10.1111/j.1365-2990.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- Boje KM, Jaworowicz D, Jr, Raybon JJ. Neuroinflammatory role of prostaglandins during experimental meningitis: evidence suggestive of an in vivo relationship between nitric oxide and prostaglandins. J Pharmacol Exp Ther. 2003;304:319–325. doi: 10.1124/jpet.102.041533. [DOI] [PubMed] [Google Scholar]
- Murphy S, Simmons ML, Agullo L, Garcia A, Feinstein DL, Galea E, Reis DJ, Minc-Golomb D, Schwartz JP. Synthesis of nitric oxide in CNS glial cells. Trends Neurosci. 1993;16:323–328. doi: 10.1016/0166-2236(93)90109-y. [DOI] [PubMed] [Google Scholar]
- Dubowitz DJ, Bernheim KA, Chen DY, Bradley WG, Jr, Andersen RA. Enhancing fMRI contrast in awake-behaving primates using intravascular magnetite dextran nanopartieles. Neuroreport. 2001;12:2335–2340. doi: 10.1097/00001756-200108080-00011. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Preuss TM, Kaas JH. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol. 2001;441:197–222. doi: 10.1002/cne.1407. [DOI] [PubMed] [Google Scholar]