Abstract
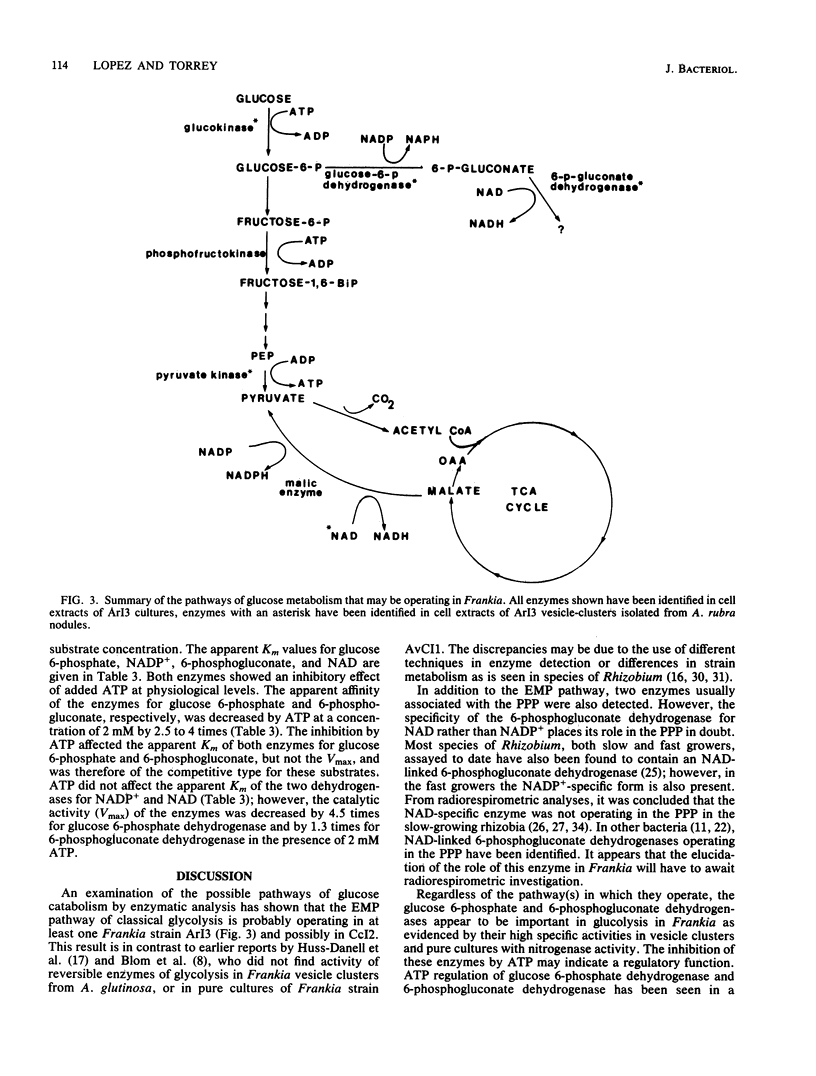
Enzymes of glucose metabolism were assayed in crude cell extracts of Frankia strains HFPArI3 and HFPCcI2 as well as in isolated vesicle clusters from Alnus rubra root nodules. Activities of the Embden-Meyerhof-Parnas pathway enzymes glucokinase, phosphofructokinase, and pyruvate kinase were found in Frankia strain HFPArI3 and glucokinase and pyruvate kinase were found in Frankia strain HFPCcI2 and in the vesicle clusters. An NADP+-linked glucose 6-phosphate dehydrogenase and an NAD-linked 6-phosphogluconate dehydrogenase were found in all of the extracts, although the role of these enzymes is unclear. No NADP+-linked 6-phosphogluconate dehydrogenase was found. Both dehydrogenases were inhibited by adenosine 5-triphosphate, and the apparent Km's for glucose 6-phosphate and 6-phosphogluconate were 6.86 X 10(-4) and 7.0 X 10(-5) M, respectively. In addition to the enzymes mentioned above, an NADP+-linked malic enzyme was detected in the pure cultures but not in the vesicle clusters. In contrast, however, the vesicle clusters had activity of an NAD-linked malic enzyme. The possibility that this enzyme resulted from contamination from plant mitochondria trapped in the vesicle clusters could not be discounted. None of the extracts showed activities of the Entner-Doudoroff enzymes or the gluconate metabolism enzymes gluconate dehydrogenase or gluconokinase. Propionate- versus trehalose-grown cultures of strain HFPArI3 showed similar activities of most enzymes except malic enzyme, which was higher in the cultures grown on the organic acid. Nitrogen-fixing cultures of strain HFPArI3 showed higher specific activities of glucose 6-phosphate and 6-phosphogluconate dehydrogenases and phosphofructokinase than ammonia-grown cultures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigad G. Inhibition of glucose 6-phosphate dehydrogenase by adenosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1543–1547. doi: 10.1073/pnas.56.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. R. Isolation of frankia strains from alder actinorhizal root nodules. Appl Environ Microbiol. 1982 Aug;44(2):461–465. doi: 10.1128/aem.44.2.461-465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Induction and regulation of a nicotinamide adenine dinucleotide-specific 6-phosphogluconate dehydrogenase in Streptococcus faecalis. J Bacteriol. 1972 Jan;109(1):106–115. doi: 10.1128/jb.109.1.106-115.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaham D., Deltredici P., Torrey J. G. Isolation and Cultivation in vitro of the Actinomycete Causing Root Nodulation in Comptonia. Science. 1978 Feb 24;199(4331):899–902. doi: 10.1126/science.199.4331.899. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Gluconate catabolism in Rhizobium japonicum. J Bacteriol. 1970 Mar;101(3):698–704. doi: 10.1128/jb.101.3.698-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Glucose catabolism in Rhizobium japonicum. J Bacteriol. 1969 Mar;97(3):1184–1191. doi: 10.1128/jb.97.3.1184-1191.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Wyk J. C. Multiple forms of Pseudomonas multivorans glucose-6-phosphate and 6-phosphogluconate dehydrogenases: differences in size, pyridine nucleotide specificity, and susceptibility to inhibition by adenosine 5'-triphosphate. J Bacteriol. 1972 Jun;110(3):1107–1117. doi: 10.1128/jb.110.3.1107-1117.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T., Neidhardt F. C. Adenosine triphosphate-linked control of Pseudomonas aeruginosa glucose-6-phosphate dehydrogenase. J Bacteriol. 1967 Apr;93(4):1337–1345. doi: 10.1128/jb.93.4.1337-1345.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Drets G., Gardiol A., Arias A. 6-Phospho-D-gluconate:NAD+ 2-oxidoreductase (decarboxylating) from slow-growing Rhizobia. J Bacteriol. 1977 Jun;130(3):1139–1143. doi: 10.1128/jb.130.3.1139-1143.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulongoy K., Elkan G. H. Glucose catabolism in two derivatives of a Rhizobium japonicum strain differing in nitrogen-fixing efficiency. J Bacteriol. 1977 Jul;131(1):179–187. doi: 10.1128/jb.131.1.179-187.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulongoy K., Elkan G. H. The role of 6-phosphogluconate dehydrogenase in Rhizobium. Can J Microbiol. 1977 Sep;23(9):1293–1298. doi: 10.1139/m77-193. [DOI] [PubMed] [Google Scholar]
- Schindler J., Schlegel H. G. Regulation der Glucose-6-phosphat-Dehydrogenase aus verschiedenen Bakterienarten durch ATP. Arch Mikrobiol. 1969;66(1):69–78. [PubMed] [Google Scholar]
- Tjepkema J. D., Ormerod W., Torrey J. G. Factors affecting vesicle formation and acetylene reduction (nitrogenase activity) in Frankia sp. CpI1. Can J Microbiol. 1981 Aug;27(8):815–823. doi: 10.1139/m81-126. [DOI] [PubMed] [Google Scholar]
- Zobel R. W., Del Tredici P., Torrey J. G. Method for growing plants aeroponically. Plant Physiol. 1976 Mar;57(3):344–346. doi: 10.1104/pp.57.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]