Abstract
Colorectal adenocarcinoma is the second cause of cancer mortality in developed countries. Rac1 is a member of the family of Rho GTPases that regulates many intracellular signaling pathways, including those involved in tumorigenesis, invasion, and metastasis. We have investigated the role of Rac1 in colorectal tumor progression by genetic modification of the human colorectal adenocarcinoma cell line SW620 to either overexpress Rac1 or lack Rac1 expression. Tumor behavior was studied by orthotopic injection of stably modified cell lines into the cecal wall of athymic nude mice, a model that replicates the histopathological appearance and clinical behavior of human colorectal adenocarcinoma in humans. While overexpression of Rac1 resulted in an accelerated tumorigenic process, inducing a faster mortality rate, inhibition of Rac1 completely suppressed tumor formation. These results suggest that Rac1 plays a major role in colorectal adenocarcinoma progression. Finally, interference with Rac1 function may provide an important tool to block the malignant phenotype of colorectal adenocarcinoma cells.
Colorectal adenocarcinoma is currently the second most common cause of death due to malignancies in western countries,1 and its incidence is increasing in the rest of the world.2 Colorectal adenocarcinoma results from the progressive accumulation of genetic and epigenetic alterations that lead to the transformation of non-neoplastic colonic epithelial cells into adenoma and, later, adenocarcinoma cells.3,4 Recently, the most relevant genetic alterations in colorectal tumors have been identified, and the implication of Ras GTPases in the tumorigenic process has been described previously.5
The small guanosine triphosphatases (GTPases) Rho proteins compose an important subgroup within the Ras superfamily. To date, 22 Rho family members have been identified, divided into six groups based in homology and/or function.6 The three prototypic Rho GTPases, RhoA, Rac1, and Cdc42, are known for their effects on the actin cytoskeleton7,8,9,10,11 and their regulatory role on adhesion, motility, cell cycle, apoptosis, transcriptional regulation, and membrane trafficking. These functions are mediated through several signaling pathways, like c-Jun NH2-terminal kinase, p38, and phosphatidylinositol 3-kinase, as well as transcription factors, such as serum response factor and nuclear factor-κB.12,13,14 Their ability to act as general modulators of several cellular processes results in important roles in tumor biology and other human diseases, including cancer.15,16,17
Rho GTPases influence a variety of processes in cancer, including cell transformation, survival, invasion, metastasis, and angiogenesis.15,16 Accumulating evidence indicates that Rac1-dependent cell signaling is important for malignant transformation. Thus, Rac1 overexpression has been found in breast carcinoma, gastric carcinoma, oral squamous cell carcinoma, non-small cell lung carcinoma, and testicular germ cell tumors.16 Rac1 plays an important role in leptin-mediated invasiveness of colonic epithelial cells18 and in colorectal tumors truncated APC proteins stimulate the activities of Asef, a Rac-specific guanine exchange factor.19 Cross-talk between Rac1 and the dysregulated Wnt signaling pathway in colon adenocarcinoma cells has been found.20,21,22
We have investigated whether Rac1 plays a role in the tumorigenic properties of human colorectal adenocarcinoma cells using an orthotopic model that closely reproduces the clinical behavior of human colorectal adenocarcinoma.23 Overexpression of Rac1 in SW620 cells, derived from a lymph node metastasis of a primary human colorectal adenocarcinoma, resulted in the acceleration of the tumorigenic process. By contrast, specific inhibition of Rac1 expression by RNA interference completely suppressed tumor formation. These results demonstrate a decisive role of Rac1 in colorectal tumorigenesis.
Materials and Methods
Synthesis of Short Hairpin RNA Constructs
Short hairpin RNA (shRNA) against human RAC1 was designed according to Agami and co-workers.24 The 64-nucleotide DNA template oligonucleotides were engineered to produce a 19-nucleotide sequence derived from the target transcript flanked with AA at the 5′ end and TT at the 3′ end and comprising more than 30% GC content. Candidate sequences were submitted to a BLAST search against the human genome sequence to ensure that only one gene of the human genome was targeted. The oligonucleotide pair was designed to produce restriction site overhangs on annealing (BglII at the 5′ end and HindIII at the 3′ end) for cloning into vector pSUPER-neo (sense primer, 5′-GATCCCCTATATCCCTACTGTCTTTGTTCAAGAGACAAAGACAGTAGGGATATATTTTTGGAAA-3′; and antisense primer, 5′-AGCTTTTCCAAAAATATATCCCTACTGTCTTTGTCTCTTGAACAAAGACAGTAGGGATATAG-GG-3′).24 The underlined characters indicate the mRNA target sequences, and the bold characters refer to the 9-bp hairpin loop and restriction site overhangs.
Cell Culture, Constructs, and Transfection
The human colorectal adenocarcinoma cell line SW620 was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, and 100 U/ml penicillin-streptomycin (Gibco-BRL Life Technologies, Paisley, UK) at 37°C, in 95% humidity and 5% CO2 pressure.
Construction of pCDNA3B-neo directing expression of wild-type (WT) Rac1 protein and conferring neomycin resistance has been previously described.25 For stable transfection, SW620 cells were plated in six-well dishes at 60 to 70% confluence. Transfections were performed with Lipofectamine Plus (Invitrogen, Carlsbad, CA) as directed by the manufacturer. The amount of plasmid DNA was kept constant at 3 μg per dish with the corresponding empty vector. After transfection, cells were cultured for 24 hours in fresh medium before selection in medium containing 2.5 mg/ml neomycin (Sigma Chemical, St. Louis, MO) for 12 days. Two independent clones were selected for each shRNA vector (i1Clone and i2Clone) or GTPase expression vector (wt1Clone and wt2Clone).
Immunoblotting and Antibodies
For detection of Rac1, whole-cell lysates were prepared in lysis buffer (50 mmol/L NaF,10 mmol/L Na4P2O7, 5 mmol/L EDTA, 50 mmol/L Tris-HCl, pH 7.5, 0.5% Triton X-100, 10 mmol/L NaPPi, and 0.5% sodium deoxycholate) containing protease and phosphatase inhibitors: 200 μmol/L orthovanadate, 1 mmol/L dithiothreitol, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 2 μg/ml pepstatin, and 400 μmol/L phenylmethylsulphonyl fluoride. Ten to 20 μg of total protein was analyzed by SDS-electrophoresis on 15% polyacrylamide gels (BioRad Laboratories, Hercules, CA). After transfer of proteins to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, MA), the blots were incubated with the corresponding antibodies. Immunocomplexes were visualized by enhanced chemiluminiscence detection (Amersham Pharmacia Biotech, Buckinghamshire, UK) using an anti-mouse antibody conjugated to peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). Mouse monoclonal anti-Rac1 was purchased from Upstate Biotechnology (Charlottesville, VA), and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase was purchased from Chemicon (Temecula, CA). A quantification of Rac1 protein was carried out by densitometry analysis performed by NIH Image software 1.62 (public domain image processing and analysis program for the Apple Macintosh developed at the U.S. National Institutes of Health).
In Vivo Tumorigenesis by Orthotopic Cell Microinjection
Stably transfected tumor cells (1 × 106) were resuspended in 50 μl of culture medium (Dulbecco’s modified Eagle’s medium) and injected directly into the cecal wall of Swiss Nu/Nu male mice, using the orthotopic cell microinjection procedure, as previously described.23 We studied oncogenesis in five animal groups: the SW620 parental cell line (n = 9), the two Rac1 overexpressor clones [wt1Clone (n = 8) and wt2Clone (n = 8)] and the two Rac1-interfered clones [i1Clone (n = 8) and i2Clone (n = 8)]. We recorded tumor take rate and survival time. Mice were monitored every week, and animals died naturally or were sacrificed when signs of advanced disease were detected.
Pathology Procedures
Complete necropsy examinations of each animal were performed. Any external anomaly and the weight of each animal were recorded. The cervical, thoracic, abdominal, and pelvic organs were extracted en bloc, and the presence of tumor was assessed macroscopically in every organ. All tumor foci were measured, and the presence of any other macroscopic anomalies was recorded. Small samples of tumor were obtained and frozen for biochemical studies (see below). The whole bloc of organs was fixed in 4% formaldehyde in phosphate buffer solution for 24 hours. The brain was also extracted and fixed in the same solution. After fixation, the organs were dissected from the whole bloc, and appropriate photographs of the tumor and the present anomalies were taken with a Nikon Coolpix 5700 digital camera. The tumor and every organ were paraffin embedded in toto. Six-micrometer-thick sections from each paraffin bloc were obtained and stained with H&E. The sections were examined microscopically to describe the tumor and detect its presence in organs in which it was not seen macroscopically. The micrographs shown in Figures 3 to 5 were made using a digital camera (AxioCam HRc; Zeiss, Oberkochen, Germany) attached to a Zeiss Axiophot microscope. The tumor was staged in each animal as follows: local tumor growth at the site of injection was stage I; stage I plus foci of tumor in the visceral and parietal peritoneum was stage II; stage I or II plus mesenteric lymph node and/or pancreatic metastasis was stage III; stage I, II, or III plus hepatic and/or lung metastasis was stage IV.23 Any other microscopic anomalies were recorded. These anomalies, together with the macroscopic ones, served to establish the cause of death in each animal. Sections from selected blocs containing the tumor were also stained with periodic acid-Schiff reaction and immunohistochemistry with the following antibodies: anti-Rac1 (Upstate Biotechnology); anti-AE1/AE3, anti-cytokeratin 7, anti-cytokeratin 20, anti-cytokeratin high molecular weight, anti-epithelial membrane antigen, and anti-vimentin (Progen Biotechnik GMBH, Heidelberg, Germany); anti-cytokeratin low molecular weight (Enzo Life Sciences, New York, NY); and anti-carcinoembryonic antigen (Novocastra, Newcastle, UK). Negative controls were achieved by substituting the primary antibodies with preimmune sera from the species in which they had been raised.
Figure 3.
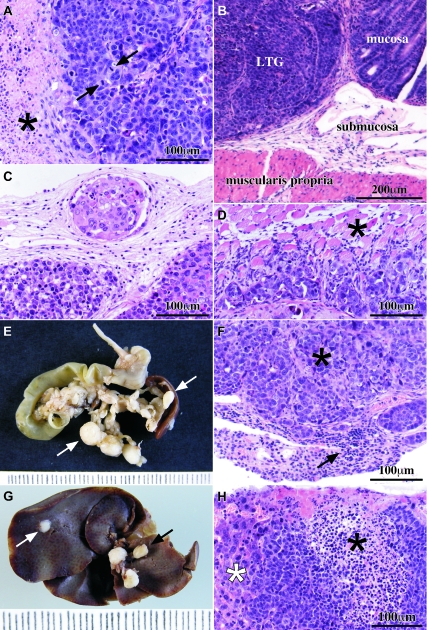
Pathology findings and tumor spread. A: Microscopically, the tumor had the features of a poorly differentiated adenocarcinoma with cells arranged in sheets and extended necrotic areas (black asterisk). Very few glandular lumens were seen. Highly atypical nuclei and frequent mitoses were observed (black arrows). B: Local tumor growth (LTG) at the injection site began at the submucosa and extended through all of the layers of the cecal wall. C: Tumor cells were found inside lymphatic and blood vessels adjacent to the tumor at the injection site in the cecum. The lymphatic depicted here was located in the subseroral connective tissue under the local tumor growth. D: Tumor cells were found infiltrating the peritoneal surface of the diaphragm and reaching the muscle fibers (black asterisk). E: Mesenteric lymph node and pancreatic metastases were seen as white and rounded masses (white arrows). F: Lymphocytes (black arrow) in metastasized lymph nodes were displaced to the periphery of the node by the tumor deposit (black asterisk). G: Macroscopic view of the liver surface infiltrated by tumor cells. Lymph nodes from the hepatic hilium (black arrow) were metastasized in some cases. Tumor deposits at the liver were always seen under the hepatic capsule (white arrow). H: Liver parenchyma (white asterisk) infiltrated by tumor cells (black asterisk). A, B, C, D, F, and H are H&E-stained sections.
Figure 4.
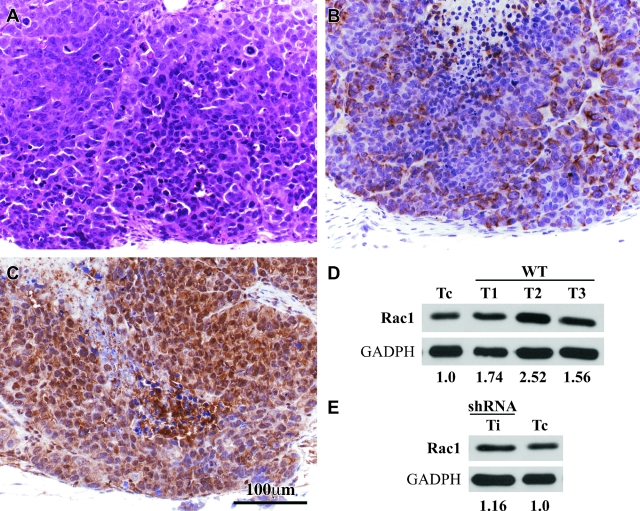
Expression of Rac1 in tumors samples. A: An adjacent section of C was stained with H&E to show the microscopic features of the tumor. B: An adjacent section of C was stained with antibodies against vimentin showing cytoplasmic immunostaining of many tumor cells. C: A tumor section stained with antibodies against Rac1. The antibody stained the cytoplasm of all tumor cells in all of the cases studied. D: Western blot analysis of Rac1 protein in three tumor samples from necropsy of mice. Quantification by densitometry analysis showed overexpression of Rac1 protein. E: The tumor sample corresponding to the iRac1-induced tumor showed re-expression of Rac1. Quantification of Rac1 protein was performed by densitometry analysis. Calibration bar in C also applies to A and B.
Figure 5.
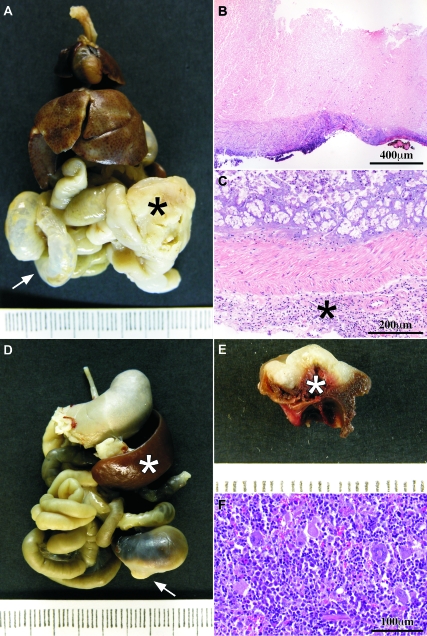
Causes of death. A: Macroscopic view of the thoracic and abdominal organs en bloc; there is a tumor at the cecum (black asterisk) whose growth has obstructed the intestinal traffic and dilated the small bowel (white arrow). B: Obstructive tumor showed areas of complete necrosis through the whole tumor thickness. C: Microscopic view of the large bowel from a case with intestinal obstruction; there are many inflammatory cells in the subserosal connective tissue (asterisk). D: Macroscopic view of the thoracic and abdominal organs en bloc; there is a white tumor (arrow) that has been bleeding; the spleen (white asterisk) is enlarged (compare with A) due to hematopoietic hyperplasia in response to chronic blood loss. E: On the cut surface, the white tumor showed a hemorrhagic surface. The intestinal lumen was filled with blood (white asterisk). F: The hematopoietic tissue of the spleen was hyperplasic in those cases with tumor bleeding. B, C, and F are H&E-stained sections.
Preparation of Tissue Extracts
The frozen tumor samples from mice were homogenized and lysed with detergent solution (1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.3 mol/L NaCl, 25 mmol/L HEPES, 20 mmol/L β-glycero-phosphate, and 0.1% Triton X-100) containing protease and phosphatase inhibitors mentioned above. The extracts were treated with 1.5 μl (375 U) of Benzonase from Calbiochem (San Diego, CA), for degradation of nucleic acids, which leads to higher resolution in 2DE-gels.
Statistical Analysis
Statistical significance in tumor take rate or survival time between a control group (parental SW620) and each of the genetically modified groups (stably transfected SW620 cells) was evaluated. The Exact Fisher Test was used to statistically analyze the differences in tumor take rates. The likelihood of survival among groups was estimated according to the method of Kaplan and Meier, and survival distributions were determined using the Log Rank Test. P < 0.05 was considered to have statistical significance.
Results
Characterization of Rac1 Expression through Gene Targeting
To overexpress Rac1, a eukaryotic expression vector containing the cDNA of the wild-type gene was used (pcDNA-RAC1wt), and the small interference RNA technique was used to specifically knockdown gene expression of RAC1. Two neomycin-resistant stable clones from each modulation were selected, wt1Clone and wt2Clone, and i1Clone and i2Clone, respectively, according to the total Rac1 protein levels, determined by Western blot (Figure 1).
Figure 1.
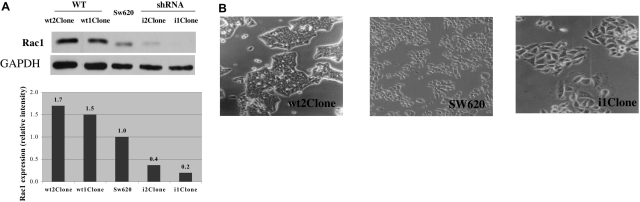
Effect of genetically induced modulation of Rac1 expression in SW620 cells. A: SW620 cells were stably transfected with pcDNA-RAC1wt or anti-RAC1 shRNA. Two independent clones were selected and analyzed by Western blot for the expression of Rac1. In the bottom panel, densitometric analysis of the blot is shown. Results shown are representative from three determinations with similar results. B: The morphological changes induced by overexpression or depletion of Rac1 are shown in phase-contrast images (magnification, ×10) of SW620 control and transfected cells. While cells overexpressing Rac1 were smaller and more compact than control cells, with a major adhesion capacity, interfered cells were bigger and extended, supporting the evidence of its role in the organization of the actin cytoskeleton and cell motility.
Transfection of RAC1-directed shRNA dramatically reduced Rac1 protein expression and fell to 20% in comparison with SW620 control cells. Overexpression of Rac1wt was increased by 1.7-fold (Figure 1A). The modest increase in Rac1wt expression in stable clones is likely due to high intrinsic Rac1 levels in the parental cells. Overexpression or inhibition of Rac1 induced strong phenotypic changes in cells (Figure 1B). Furthermore, the apparently moderate levels of overexpression were sufficient to induce genetic changes determined by their transcriptomes.22
Tumorigenic Process Acceleration Induced by Rac1wt
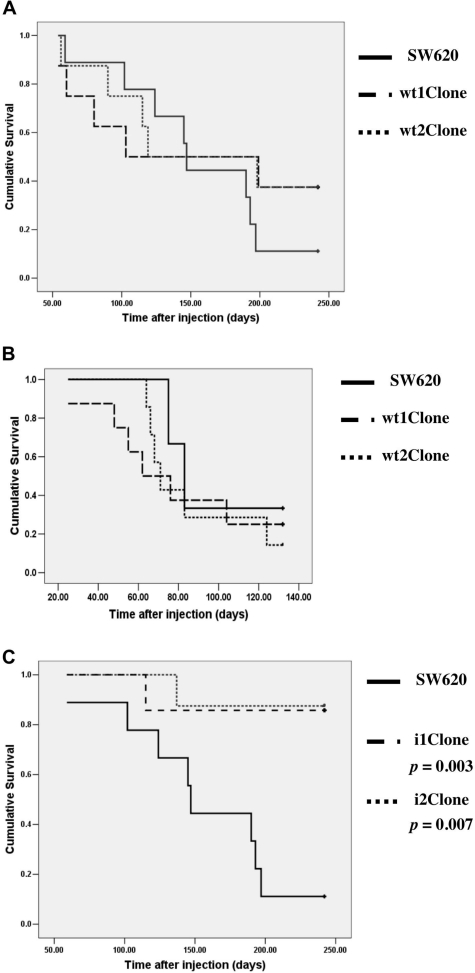
To examine the effect of increased or decreased Rac1 protein levels on tumor growth, 1 × 106 cells of the indicated stable cell lines (control SW620 and RAC1wt- and iRAC1-transfected clones) resuspended in 50 μl of Dulbecco’s modified Eagle’s medium were injected orthotopically into the submucosa of the cecal wall of athymic nude mice. Injection of cells resulted in tumor development, as previously described.23 As shown in Table 1, local tumor growth was confirmed in 63% of the mice bearing SW620/Rac1wt and 89% of the mice bearing control tumors (SW620). Injection of RAC1wt-transformed cells was highly lethal (Figure 2A). Four months after injection, 50% of RAC1wt-injected mice were dead, accelerating the tumorigenic process compared with the parental cells. Although not statistically significant due to the small sample size, as determined by the log rank test (with values of P = 0.44 and 0.42 for wt1Clone and wt2Clone, respectively), there was a clear trend toward a shorter survival in animals bearing tumors derived from Rac1 overexpressor clones. To confirm these results, the orthotopic experiment was repeated, with a similar outcome (Figure 2B), and the tendency of Rac1 aggressiveness was confirmed (with values of P = 0.63 and 0.71 for wt1Clone and wt2Clone, respectively). These results indicated that cell lines that overexpress of Rac1wt are more aggressive than the parental cell line SW620.
Table 1.
Take Rates after Orthotopic Injection of Control, RAC1wt, or iRAC1 Cell Lines
| Cell line | Gene | Cell no. | No. of mice | Local tumor growth take rate [n (%)] |
|---|---|---|---|---|
| SW620 | None | 1.0 × 106 | 9 | 8/9 (89) |
| wt1Clone | RAC1wt | 1.0 × 106 | 8 | 5/8 (63) |
| wt2Clone | RAC1wt | 1.0 × 106 | 8 | 5/8 (63) |
| i1Clone | iRAC1 | 1.0 × 106 | 8 | 1/8 (13)* |
| i2Clone | iRAC1 | 1.0 × 106 | 8 | 1/7 (14)* |
P = 0.003.
Figure 2.
Survival curve of mice bearing tumors derived from RAC1wt- or iRAC1-transformed cell lines. Control cells (SW620) and modified cell lines (wt1Clone, wt2Clone, i1Clone, and i2Clone) were injected into the cecal wall of Swiss Nu/Nu male mice. A: The survival time, estimated using the method of Kaplan and Meier, was shorter in mice inoculated with wt1Clone or wt2Clone than the control group. B: 2.0 × 106 cells were injected in mice, and the survival time estimated as described previously.23 Mice inoculated with wt1Clone or wt2Clone showed shorter survival than the control group. C: Estimation of survival time by the method of Kaplan and Meier. Cells expressing the anti-RAC1 shRNA showed significantly longer survival times than the control group.
Reduction of in Vivo Tumor Growth by Depletion of Rac1
In keeping with the above results, shRNA-mediated depletion of Rac1 strongly inhibited the local tumor growth on injected cells. Although orthotopic injection was successfully performed, only 13 to 14% of the mice injected with the iRAC1 cells died at the end of the experiment, 242 days after injection, and only these mice presented local tumor growth (Table 1). Finally, the cumulative survival after orthotopic injections of iRAC1-transformed cells and parental cells demonstrated the suppression of tumorigenesis as measured by the take rate, leading to an increased survival time (Figure 2C). Survival time was found statistically significant by the log rank test with values of P = 0.003 and 0.007 for i1Clone and i2Clone, respectively. Data were also statistically significant for the take rate when the exact Fisher test was applied, with values of P = 0.003 for both i1 and i2 clones.
Pathology Findings
The macroscopic features of the tumors generated at the injection site by SW620, wt1Clone, wt2Clone, and the two derived from the i1Clone and i2Clone, were similar in all of the animals. These tumors were solid and whitish with some necrotic foci and extended longitudinally through the cecum. When the cecum was transversally open, the tumor occupied and expanded the cecal wall narrowing the cecal lumen, closing it completely in some animals. The foci of tumor at other locations showed the same macroscopic features. The microscopic features of the tumor at the injection site were also the same in all of the animals. It consisted of a poorly differentiated adenocarcinoma made of sheets of cells with highly atypical nuclei that occasionally formed glands. Some tumor cells were multinucleated, and others had an intracytoplasmic lumen that gave them the appearance of a signet ring. The mitotic rate was higher than 5 mitoses per 10 high-power fields, and many necrotic areas were seen (Figure 3A). The tumor cells were periodic acid-Schiff negative and were stained focally in the cytoplasm with antibodies against AE1/AE3, low-molecular-weight cytokeratins, and epithelial membrane antigen, whereas they were not stained with antibodies against cytokeratin 20, 7 and high-molecular-weight cytokeratins. Vimentin was also positive in the cytoplasm of many tumor cells, but carcinoembryonic antigen was negative, due possibly to the poorly differentiated characteristics of the adenocarcinoma. In keeping with these results, Hewitt et al26 had previously described a weak staining for carcinoembryonic antigen in SW620, and the American Type Culture Collection indicates that these cells synthesize only small quantities of carcinoembryonic antigen.
The tumor expanded from its initial location at the submucosa through all of the intestinal wall layers ulcerating the mucosa and reaching the serosal surface in all of the animals (Figure 3B). The tumor was also found in all of the animals inside the lumen of lymphatic vessels of the mucosa, the submucosa, the muscular layers, and the serosa (Figure 3C). Some animals also showed tumor foci in the visceral and parietal peritoneum that infiltrated the adjacent organs, such as the diaphragm (Figure 3D). Others also showed metastasis to mesenteric lymph nodes and pancreas (Figure 3, E and F). Tumor foci in the liver were always found in continuity with the peritoneal surface of the organ (Figure 3, G and H) and were never seen inside vascular lumens, so we considered these tumor foci as stage II. Tumor deposits in the lungs or in the brain were not found. The data of the tumor staging of the animals belonging to each group are summarized in Table 2. The microscopic and immunohistochemical features of the metastases of the tumor were the same than those found at the injection site.
Table 2.
Tumor Spreading Produced by Local Tumor Growth and/or Metastasis Induced by Orthotopic Injection of Cells Transformed by Either RAC1wt or iRAC1 Compared with Control Cells SW620
| Cell line | Gene | No. of mice bearing tumor | Metastasis findings in tumor-bearing mice [n (%)]
|
|||
|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | |||
| SW620 | None | 8 | 2 (25) | 0 (0) | 6 (75) | 0 (0) |
| wt1Clone | RAC1wt | 5 | 2 (40) | 1 (20) | 2 (40) | 0 (0) |
| wt2Clone | RAC1wt | 5 | 0 (0) | 0 (0) | 5 (100) | 0 (0) |
| i1Clone | iRAC1 | 1 | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| i2Clone | iRAC1 | 1 | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
The tumor was staged in each animal as follows: stage I, local tumor site at the site of injection; stage II, stage I plus foci of tumor in the visceral and parietal peritoneum; stage III, stage I or II plus mesenteric lymph node and/or pancreatic metastasis; stage IV, stage I, II, or III plus hepatic and/or lung metastasis.
Unexpectedly, there were no statistically significant differences among groups in macroscopic sizes and volumes and in microscopic findings. To confirm that the tumors arose from the injected cells, tumor sections from cecum of the mice injected with wt1Clone and wt2Clone and SW620 as a control were stained for the anti-Rac1 antibody with their corresponding controls (Figure 4, A–C). Rac1 staining was positive in all of the cases and was found mainly in the cytoplasm of the tumor cells. Frozen tumor samples from the cecum of three mice were also analyzed by Western blot to confirm Rac1 protein overexpression (Figure 4D).
Immunohistochemistry analysis was also performed in tumor sections from the two tumors grown under interference conditions (data not shown). These tumors showed the same microscopic characteristics as the tumors generated by Rac1wt overexpression. The frozen tumor samples were also analyzed by Western blot. Re-expression of Rac1 was observed in both cases as shown in Figure 4E. This finding indicates that tumor growth was the result of instability of shRNA in vivo, and therefore the lack of interference after injection allowed the re-expression of the protein.
The macroscopic and microscopic anomalies found at the necropsy considered as a whole served to establish the cause of death of each animal. Some animals showed a very narrow lumen of the cecum caused by the tumor and dilation of the small bowel (Figure 5A). Microscopically, the intestinal wall had been completely replaced in some areas by necrotic tumor (Figure 5B). There were abundant inflammatory cells at the serosal surface of the small and large bowel (Figure 5C). The death of these animals was attributed to peritonitis due to intestinal obstruction caused by the tumor. Other animals showed the large bowel filled with blood while the rest of the organs showed an intense pallor and the spleen was enlarged (Figure 5D). On the cut surface, part of the cecal wall had been replaced by the local tumor growth, of which the surface was hemorrhagic (Figure 5E). Microscopically, the tumor surface was also hemorrhagic. The death of these animals was attributed to intestinal hemorrhage originated in the tumor. This hemorrhage was chronic in many animals because they showed compensatory hyperplasia of the hematopoietic tissue of the spleen (Figure 5F). Other less frequent causes of death were sepsis and obstruction of the urinary flow due to tumor compression of pelvic structures. The main causes of death of the animals belonging to each group are summarized in Table 3.
Table 3.
Cause of Death Produced by Tumor Growth Induced by Orthotopic Injection of Cells Transformed by Either RAC1wt or iRAC1 Compared with Control Cells SW620
| Cell line | Gene | No. of mice bearing tumor | Cause of death [n (%)]
|
|
|---|---|---|---|---|
| Hemorrhage | Intestinal blockage | |||
| SW620 | None | 8 | 2/8 (25) | 4/8 (50) |
| wt1Clone | RAC1wt | 5 | 2/5 (40) | 1/5 (20) |
| wt2Clone | RAC1wt | 5 | 0/5 (0) | 3/5 (60) |
| i1Clone | iRAC1 | 1 | 1/1 (100) | 0/1 (0) |
| i2Clone | iRAC1 | 1 | 0/1 (0) | 0/1 (0) |
Discussion
Although significant progress has been achieved in deciphering molecular events associated to the onset of colorectal adenocarcinoma, the specific signal transduction pathways involved have not been fully characterized.4,27 Increased Rho proteins, including Rac1, may be involved in the proliferation of cancer cells and/or response to death signals.28,29,30,31,32
Rac1 is involved in membrane ruffling, morphological and growth transformation, and signaling to a variety of downstream transcription factors, including c-Jun, SRF, and nuclear factor-κB.13,33,34,35 Also, Rac1 is involved in cell adhesion and migration, and its activation is associated with increased tumor invasiveness.36 Rac1 is overexpressed in different types of cancers. Rac1-dependent cell signaling may be relevant for malignant transformation16 and may respond to external tissue-specific clues that signal toward promoting proliferation, adhesion, or motility.37 It is now clear that Rac1 is involved in multiple stages of the tumorigenic process, raising the possibilities that Rac1 and its effectors are useful anticancer targets. Thus, interference with Rac1 function might be explored as an attractive and novel anticancer strategy.15
Several evidences support the importance of Rac1 in colorectal adenocarcinoma. Extensive studies into the molecular etiology of cancer have established a central role for the Wnt signal transduction pathway. Mutations in the canonical Wnt signaling pathway leading to its activation are known to cause the majority of intestinal tumors. Recent studies have suggested that Rac1 could modulate β-catenin function in the context of the canonical Wnt signaling pathway and that the activation of Rac1 may represent a common step of colorectal tumorigenesis.20,21,22,38
It has been suggested that Rac1 and MAPK p38 are potentially involved in key mechanisms leading to malignant transformation in the colonic crypt.39 Moreover, the α6β4 integrin may mediate motile events required for invasion of colon carcinoma cells through Rac1 and phosphatidylinositol 3-kinase signaling.40 However, there is little evidence so far from animal studies to define how Rac1 contributes to colon cancer cell proliferation, survival, invasion, and metastasis, and we therefore focused on this system. We genetically modulated the expression levels of the human RAC1 gene, by either up- or down-regulation. Characterization of the effects of Rac1 modulation in colorectal carcinoma cell line-derived tumors suggests a critical role of Rac1 in colorectal adenocarcinoma progression.
Animal models for human colorectal cancer can provide insight into the mechanisms that underlie the development and pathogenesis of this type of tumor. The ideal animal model should therefore faithfully replicate all aspects of tumor development in man. These include the sequential acquisition of genetic alterations with consequent changes in cell behavior and tumor biology.41 Moreover, many of the most-used mouse models form adenomas primarily in the small bowel, with relatively few lesions in the descending and sigmoid colon.42
Here, we demonstrate that Rac1 is critically involved in the pathogenesis of human colorectal cancer cells. The colorectal adenocarcinoma-derived cell line SW620 had acquired the sequential mutation of APC, TP53, and SMAD4 and activation of the proto-oncogene KRAS43. The ectopic expression of Rac1 is sufficient to induce cell transformation.44 To evaluate the in vivo effects of this GTPase, RAC1wt-overexpressing and RAC1-interfering cancer cells were injected orthotopically into the mouse submucosal layer of the cecal wall, and tumor growth, progression, dissemination, and metastasis of the generated colorectal adenocarcinoma were analyzed.
A major advantage of the orthotopic cell microinjection model is the fidelity of emulating human disease. It allows the possibility to modify the experimental conditions to get new methods for cancer diagnosis and prognosis as well as novel strategies to develop new selective drugs. Another advantage of the orthotopic cell microinjection model is the high incidence of the tumor take rate and that the microenvironment is optimal to influence the tumor behavior. Furthermore, this model of colorectal adenocarcinoma in mouse resembles the behavior of human colorectal adenocarcinoma and the cause of death of patients who have not undergone surgical or chemotherapeutic interventions.23 We observed a reduction of survival time in mice with RAC1wt-induced colorectal tumors when compared with control mice, suggesting the high lethality produced by this protein.
Metastasis is the most common complication in human tumors leading to patient death. The variety of cellular functions attributed to Rho proteins includes cell morphology, formation of stress fibers and focal adhesions, membrane ruffling, cell motility, and invasion.45 Activation or overexpression of Rho proteins has been associated with enhanced invasion and motility.36,40,46,47 We have previously reported that cells overexpressing Rac1 acquire metastatic properties in vivo.29 In keeping with this, we have found tumoral cell spreading in the visceral and parietal peritoneum, metastasis to the pancreas and to mesenteric lymph nodes, and tumor foci in the liver. As previously described,26 SW620 cells metastasize to the liver, but metastases were only present on the peritoneal surface of the liver and not within the parenchyma. Because SW620 cells were derived from a lymph node metastasis, the cells may have acquired the ability for lymphatic metastasis but not the ability for hematogenous metastasis to the liver parenchyma. It may be relevant that SW620 cells are epidermal growth factor receptor-negative,48 because there is evidence that epidermal growth factor receptor expression is necessary for colon cancer metastasis to the liver parenchyma.49
Even though no sustained differences in colonized organs or number of metastasis appeared between the control and modified groups (probably tumors killed the animals before metastasis grew further due to the aggressiveness of Rac1 overexpression), there is increasing evidence of the role of Rac1 or downstream effectors in invasion and metastasis.29,50 In addition, most studies have been performed with activated mutants, which presented a more profound effect in adhesion, spreading, and membrane ruffling of colon cancer cells,51 in agreement with our previous findings that although both wild-type and activated mutants of Rho proteins are tumorigenic, the mutated version is more potent.28
Short hairpin RNAs can be used in vitro to produce sequence-specific gene silencing of mammalian cells,52,53 resulting in the effective in vivo suppression of gene expression in adult mice.54,55,56 Several studies have shown the efficacy of interfering Rac1 expression in different cancer cell lines.57,58 SW620 expresses relatively high levels of Rac1, but when its expression was inhibited, tumor growth in mice was completely abolished. In keeping with this observation, Malliri et al59 reported that Tiam1-null mice have a reduction in Ras-induced skin tumors, suggesting that Rac1 contributes primarily to tumor development by promoting cell proliferation.
Our data suggest that Rac1 plays a crucial role in local growth of colorectal adenocarcinoma and has a causal role in promoting tumor progression. Furthermore, interference of its expression or activity may provide a positive clinical benefit for patients with colorectal carcinoma. Recently, inhibition of Rac1 activity has been evaluated as a possible method for cancer treatment.60,61,62,63,64 Specific Rac1 inhibition in colorectal adenocarcinoma may represent a powerful tool for the in vivo inhibition of tumor growth and may have potential therapeutic value.
Acknowledgments
We thank the Photography Unit of the Instituto de Investigaciones Biomédicas “Alberto Sols” (CSIC, Madrid) and Dr. Salvador Aznar Benitah for his assistance in designing the shRNA of RAC1.
Footnotes
Address reprint requests to Prof. Juan Carlos Lacal, Centro Nacional de Biotecnología, C/ Darwin 3, Campus de Cantoblanco, 28049 Madrid, Spain. E-mail: jclacal@cnb.uam.es.
Supported by grants to J.C.L. from Ministerio de Educación y Ciencia (SAF2004-0577 and SAF2005-06195-C02-01), Comunidad de Madrid (S-BIO/0280/2006), Ministerio de Sanidad (RTICC RD06/0020/0016), and TCD Pharma SL and by Ministerio de EducaciónyCíencia (SAF03-07437) and Agĕncia de Géstió d’Ajuts de Recerca (SGR05-1050) to R.M. C.E. is a fellow from Ministerio de Educación y Ciencia.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it). J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 2002;16:1032–1054. doi: 10.1101/gad.978802. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal JC. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- Murga C, Zohar M, Teramoto H, Gutkind JS. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kappaB. Oncogene. 2002;21:207–216. doi: 10.1038/sj.onc.1205036. [DOI] [PubMed] [Google Scholar]
- Aznar S, Fernandez-Valeron P, Espina C, Lacal JC. Rho GTPases: potential candidates for anticancer therapy. Cancer Lett. 2004;206:181–191. doi: 10.1016/j.canlet.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Gómez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27:602–613. doi: 10.1002/bies.20238. [DOI] [PubMed] [Google Scholar]
- Boettner B, Van Aelst L. The role of Rho GTPases in disease development. Gene. 2002;286:155–174. doi: 10.1016/s0378-1119(02)00426-2. [DOI] [PubMed] [Google Scholar]
- Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, Gespach C. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, Higuchi O, Akiyama T. Asef, a link between the tumor suppressor APC and G-protein signaling. Science. 2000;289:1194–1197. doi: 10.1126/science.289.5482.1194. [DOI] [PubMed] [Google Scholar]
- Esufali S, Bapat B. Cross-talk between Rac1 GTPase and dysregulated Wnt signaling pathway leads to cellular redistribution of beta-catenin and TCF/LEF-mediated transcriptional activation. Oncogene. 2004;23:8260–8271. doi: 10.1038/sj.onc.1208007. [DOI] [PubMed] [Google Scholar]
- Malliri A, Rygiel TP, van der Kammen RA, Song JY, Engers R, Hurlstone AF, Clevers H, Collard JG. The rac activator Tiam1 is a Wnt-responsive gene that modifies intestinal tumor development. J Biol Chem. 2006;281:543–548. doi: 10.1074/jbc.M507582200. [DOI] [PubMed] [Google Scholar]
- Gomez del Pulgar T, Bandres E, Espina C, Valdes-Mora F, Perez-Palacios R, Garcia-Amigot F, Garcia-Foncillas J, Lacal JC. Differential expression of Rac1 identifies its target genes and its contribution to progression of colorectal cancer. Int J Biochem Cell Biol. 2007;39:2289-2302. doi: 10.1016/j.biocel.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Céspedes MV, Espina C, Garcia-Cabezas MA, Trias M, Boluda A, del Pulgar MT, Sancho FJ, Nistal M, Lacal JC, Mangues R. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am J Pathol. 2007;170:1077–1085. doi: 10.2353/ajpath.2007.060773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Aznar S, Valeron PF, del Rincon SV, Perez LF, Perona R, Lacal JC. Simultaneous tyrosine and serine phosphorylation of STAT3 transcription factor is involved in Rho A GTPase oncogenic transformation. Mol Biol Cell. 2001;12:3282–3294. doi: 10.1091/mbc.12.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, Stamp GW, Stetler-Stevenson WG. Validation of a model of colon cancer progression. J Pathol. 2000;192:446–454. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Oving IM, Clevers HC. Molecular causes of colon cancer. Eur J Clin Invest. 2002;32:448–457. doi: 10.1046/j.1365-2362.2002.01004.x. [DOI] [PubMed] [Google Scholar]
- Perona R, Esteve P, Jimenez B, Ballestero RP, Ramon y Cajal S, Lacal JC. Tumorigenic activity of rho genes from Aplysia californica. Oncogene. 1993;8:1285–1292. [PubMed] [Google Scholar]
- del Peso L, Hernandez-Alcoceba R, Embade N, Carnero A, Esteve P, Paje C, Lacal JC. Rho proteins induce metastatic properties in vivo. Oncogene. 1997;15:3047–3057. doi: 10.1038/sj.onc.1201499. [DOI] [PubMed] [Google Scholar]
- Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- Jiménez B, Arends M, Esteve P, Perona R, Sanchez R, Ramon y Cajal S, Wyllie A, Lacal JC. Induction of apoptosis in NIH3T3 cells after serum deprivation by overexpression of rho-p21, a GTPase protein of the ras superfamily. Oncogene. 1995;10:811–816. [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Pruitt K, Der CJ. Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett. 2001;171:1–10. doi: 10.1016/s0304-3835(01)00528-6. [DOI] [PubMed] [Google Scholar]
- Benitah SA, Valeron PF, van Aelst L, Marshall CJ, Lacal JC. Rho GTPases in human cancer: an unresolved link to upstream and downstream transcriptional regulation. Biochim Biophys Acta. 2004;1705:121–132. doi: 10.1016/j.bbcan.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Lechner S, Muller-Ladner U, Renke B, Scholmerich J, Ruschoff J, Kullmann F. Gene expression pattern of laser microdissected colonic crypts of adenomas with low grade dysplasia. Gut. 2003;52:1148–1153. doi: 10.1136/gut.52.8.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Heijstek MW, Kranenburg O, Borel Rinkes IH. Mouse models of colorectal cancer and liver metastases. Dig Surg. 2005;22:16–25. doi: 10.1159/000085342. [DOI] [PubMed] [Google Scholar]
- Alencar H, King R, Funovics M, Stout C, Weissleder R, Mahmood U. A novel mouse model for segmental orthotopic colon cancer. Int J Cancer. 2005;117:335–339. doi: 10.1002/ijc.21185. [DOI] [PubMed] [Google Scholar]
- Flatmark K, Maelandsmo GM, Martinsen M, Rasmussen H, Fodstad O. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur J Cancer. 2004;40:1593–1598. doi: 10.1016/j.ejca.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LD, Valverius EM, Tsokos M, Mickley LA, Rosen N, Bates SE. Modulation of EGF receptor expression by differentiating agents in human colon carcinoma cell lines. Cancer Commun. 1990;2:345–355. doi: 10.3727/095535490820874092. [DOI] [PubMed] [Google Scholar]
- Radinsky R, Risin S, Fan D, Dong Z, Bielenberg D, Bucana CD, Fidler IJ. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- Carter JH, Douglass LE, Deddens JA, Colligan BM, Bhatt TR, Pemberton JO, Konicek S, Hom J, Marshall M, Graff JR. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res. 2004;10:3448–3456. doi: 10.1158/1078-0432.CCR-03-0210. [DOI] [PubMed] [Google Scholar]
- Mohri T, Adachi Y, Ikehara S, Hioki K, Tokunaga R, Taketani S. Activated Rac1 selectively up-regulates the expression of integrin alpha6beta4 and induces cell adhesion and membrane ruffles of nonadherent colon cancer Colo201 cells. Exp Cell Res. 1999;253:533–540. doi: 10.1006/excr.1999.4720. [DOI] [PubMed] [Google Scholar]
- McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291–1300. [PubMed] [Google Scholar]
- Yang G, Thompson JA, Fang B, Liu J. Silencing of H-ras gene expression by retrovirus-mediated siRNA decreases transformation efficiency and tumor growth in a model of human ovarian cancer. Oncogene. 2003;22:5694–5701. doi: 10.1038/sj.onc.1206858. [DOI] [PubMed] [Google Scholar]
- Xue Y, Bi F, Zhang X, Pan Y, Liu N, Zheng Y, Fan D. Inhibition of endothelial cell proliferation by targeting Rac1 GTPase with small interference RNA in tumor cells. Biochem Biophys Res Commun. 2004;320:1309–1315. doi: 10.1016/j.bbrc.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Chan AY, Coniglio SJ, Chuang YY, Michaelson D, Knaus UG, Philips MR, Symons M. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24:7821–7829. doi: 10.1038/sj.onc.1208909. [DOI] [PubMed] [Google Scholar]
- Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002;417:867–871. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- Joyce PL, Cox AD. Rac1 and Rac3 are targets for geranylgeranyltransferase I inhibitor-mediated inhibition of signaling, transformation, and membrane ruffling. Cancer Res. 2003;63:7959–7967. [PubMed] [Google Scholar]
- Menna PL, Skilton G, Leskow FC, Alonso DF, Gomez DE, Kazanietz MG. Inhibition of aggressiveness of metastatic mouse mammary carcinoma cells by the beta2-chimaerin GAP domain. Cancer Res. 2003;63:2284–2291. [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Man K, Ho JW, Wang XH, Poon RT, Xu Y, Ng KT, Chu AC, Sun CK, Ng IO, Sun HC, Tang ZY, Xu R, Fan ST. FTY720: a promising agent for treatment of metastatic hepatocellular carcinoma. Clin Cancer Res. 2005;11:8458–8466. doi: 10.1158/1078-0432.CCR-05-0447. [DOI] [PubMed] [Google Scholar]
- Shan D, Chen L, Njardarson JT, Gaul C, Ma X, Danishefsky SJ, Huang XY. Synthetic analogues of migrastatin that inhibit mammary tumor metastasis in mice. Proc Natl Acad Sci USA. 2005;102:3772–3776. doi: 10.1073/pnas.0500658102. [DOI] [PMC free article] [PubMed] [Google Scholar]